User login
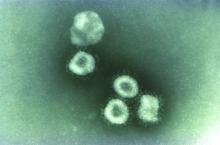
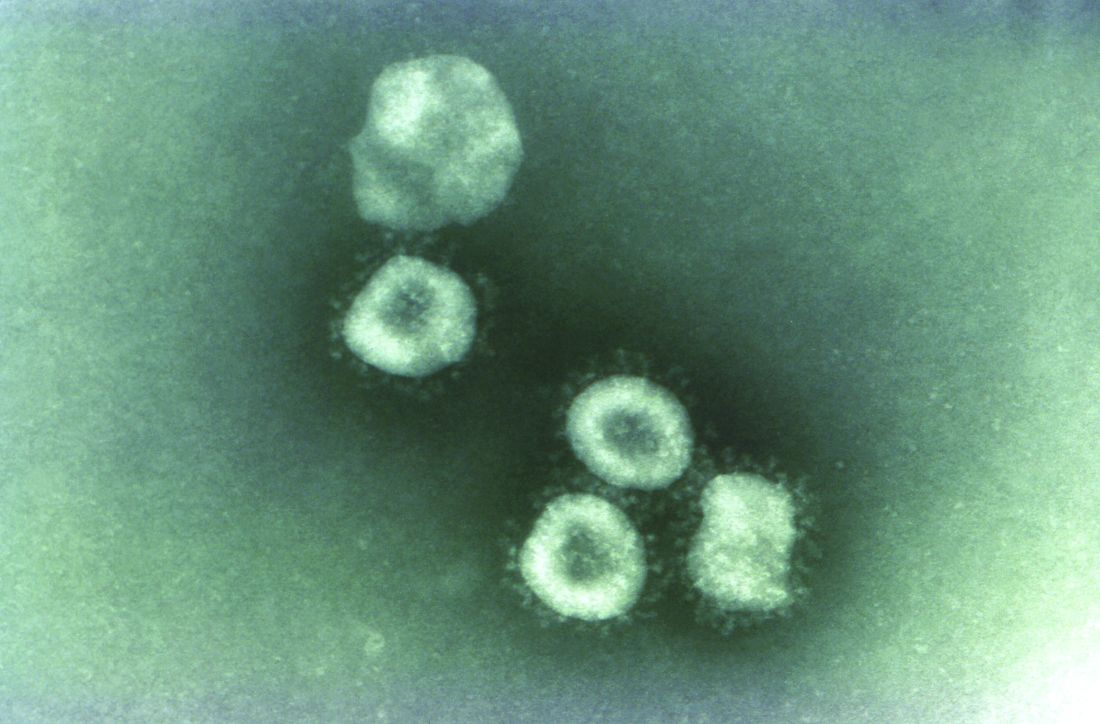
Contact tracing, isolation have impact, study shows
A far-reaching surveillance initiative was implemented in Shenzhen, China, to isolate and contact trace people suspected of having the COVID-19 coronavirus. This initiative led to faster confirmation of new cases and reduced the window of time during which people were infectious in the community. This potentially reduced the number of new infections that arose from each case, according to a study of patients and contacts over 4 weeks (Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099[20]30287-5).
“The experience of COVID-19 in the city of Shenzhen may demonstrate the huge scale of testing and contact tracing that’s needed to reduce the virus spreading,” said study coauthor Ting Ma, PhD, of Harbin Institute of Technology at Shenzhen.
Dr. Ma acknowledged that some of the measures the program used, such as isolating people outside their homes, may be difficult to impose in other countries, “but we urge governments to consider our findings in the global response to COVID-19.”
The study followed 391 coronavirus cases and 1,286 close contacts identified by the Shenzhen Center for Disease Control and Prevention from Jan. 14 to Feb. 12 this year. The study showed that contact tracing led to confirming new diagnoses within 3.2 days on average vs. 5.5 for symptom-based surveillance, and reduced the time it took to isolate newly infected people by 2 days, from an average of 4.6 to 2.7 days. Eighty-seven people were diagnosed with COVID-19 after they were contact traced and tested. Twenty percent of them had no symptoms, and 29% had no fever. Three deaths occurred in the group during the study period.
The surveillance program was comprehensive and intense. On Jan. 8, the Shenzhen CDC started monitoring travelers from Hubei province, of which Wuhan is the capital, for symptoms of COVID-19. Shenzhen is a city of about 12.5 million people in southeastern China, near Hong Kong, and is about 560 miles south of Wuhan. Over the next 2 weeks, the Shenzhen CDC expanded that surveillance program to all travelers from Hubei regardless of symptoms, along with local hospital patients and people detected by fever screenings at area clinics.
Suspected cases and close contacts underwent nasal-swab testing at 40 different locations in the city. The program identified close contacts through contact tracing, and included anyone who lived in the same dwelling, shared a meal, traveled, or had a social interaction with an index 2 days before symptoms appeared. Casual contacts and some close contacts, such as clinic nurses, who wore masks during the encounters were excluded.
“To achieve similar results, other countries might be able to combine near-universal testing and intensive contact tracing with social distancing and partial lockdowns,” said Dr. Ma. “Although no lockdown measures were introduced in Shenzhen until the end of our study period, Wuhan’s lockdown could have significantly restricted the spread of coronavirus to Shenzhen.”
The researchers noted that children are as susceptible to the virus as are adults, even though their symptoms are not as severe as those of adults. The rate of infection in children 10 and younger was similar to the overall infection rate, 7.4% vs. 6.6%, so the researchers noted that surveillance measures should target them as well.
“This study to me confirms a lot of what we’ve already known,” Aaron E. Glatt, MD, chairman of medicine and an epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “It’s an elegant study, but at the same time it sends us a message that we’re at a critical point of time for us to intervene and prevent cases at the very beginning.”
He acknowledged that the Shenzhen effort was intense. “It’s always a resource-intense requirement to do such extensive contact tracing, but that doesn’t mean it shouldn’t be done to the best of your ability to do so,” he said. He was struck by the low relative rate of infection among contacts in the study – around 7%. “There are differences obviously in infection rates in every outbreak,” he said. “Every individual has their own particular infection rate. While we can take ranges and statistical guesses for every individual patient, it could be very high or very low, and that’s most critical to nip it in the bud.”
Lead author Qifang Bi and study coauthors had no financial relationships to disclose.
SOURCE: Bi Q et al. Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099(20)30287-5.
A far-reaching surveillance initiative was implemented in Shenzhen, China, to isolate and contact trace people suspected of having the COVID-19 coronavirus. This initiative led to faster confirmation of new cases and reduced the window of time during which people were infectious in the community. This potentially reduced the number of new infections that arose from each case, according to a study of patients and contacts over 4 weeks (Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099[20]30287-5).
“The experience of COVID-19 in the city of Shenzhen may demonstrate the huge scale of testing and contact tracing that’s needed to reduce the virus spreading,” said study coauthor Ting Ma, PhD, of Harbin Institute of Technology at Shenzhen.
Dr. Ma acknowledged that some of the measures the program used, such as isolating people outside their homes, may be difficult to impose in other countries, “but we urge governments to consider our findings in the global response to COVID-19.”
The study followed 391 coronavirus cases and 1,286 close contacts identified by the Shenzhen Center for Disease Control and Prevention from Jan. 14 to Feb. 12 this year. The study showed that contact tracing led to confirming new diagnoses within 3.2 days on average vs. 5.5 for symptom-based surveillance, and reduced the time it took to isolate newly infected people by 2 days, from an average of 4.6 to 2.7 days. Eighty-seven people were diagnosed with COVID-19 after they were contact traced and tested. Twenty percent of them had no symptoms, and 29% had no fever. Three deaths occurred in the group during the study period.
The surveillance program was comprehensive and intense. On Jan. 8, the Shenzhen CDC started monitoring travelers from Hubei province, of which Wuhan is the capital, for symptoms of COVID-19. Shenzhen is a city of about 12.5 million people in southeastern China, near Hong Kong, and is about 560 miles south of Wuhan. Over the next 2 weeks, the Shenzhen CDC expanded that surveillance program to all travelers from Hubei regardless of symptoms, along with local hospital patients and people detected by fever screenings at area clinics.
Suspected cases and close contacts underwent nasal-swab testing at 40 different locations in the city. The program identified close contacts through contact tracing, and included anyone who lived in the same dwelling, shared a meal, traveled, or had a social interaction with an index 2 days before symptoms appeared. Casual contacts and some close contacts, such as clinic nurses, who wore masks during the encounters were excluded.
“To achieve similar results, other countries might be able to combine near-universal testing and intensive contact tracing with social distancing and partial lockdowns,” said Dr. Ma. “Although no lockdown measures were introduced in Shenzhen until the end of our study period, Wuhan’s lockdown could have significantly restricted the spread of coronavirus to Shenzhen.”
The researchers noted that children are as susceptible to the virus as are adults, even though their symptoms are not as severe as those of adults. The rate of infection in children 10 and younger was similar to the overall infection rate, 7.4% vs. 6.6%, so the researchers noted that surveillance measures should target them as well.
“This study to me confirms a lot of what we’ve already known,” Aaron E. Glatt, MD, chairman of medicine and an epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “It’s an elegant study, but at the same time it sends us a message that we’re at a critical point of time for us to intervene and prevent cases at the very beginning.”
He acknowledged that the Shenzhen effort was intense. “It’s always a resource-intense requirement to do such extensive contact tracing, but that doesn’t mean it shouldn’t be done to the best of your ability to do so,” he said. He was struck by the low relative rate of infection among contacts in the study – around 7%. “There are differences obviously in infection rates in every outbreak,” he said. “Every individual has their own particular infection rate. While we can take ranges and statistical guesses for every individual patient, it could be very high or very low, and that’s most critical to nip it in the bud.”
Lead author Qifang Bi and study coauthors had no financial relationships to disclose.
SOURCE: Bi Q et al. Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099(20)30287-5.
A far-reaching surveillance initiative was implemented in Shenzhen, China, to isolate and contact trace people suspected of having the COVID-19 coronavirus. This initiative led to faster confirmation of new cases and reduced the window of time during which people were infectious in the community. This potentially reduced the number of new infections that arose from each case, according to a study of patients and contacts over 4 weeks (Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099[20]30287-5).
“The experience of COVID-19 in the city of Shenzhen may demonstrate the huge scale of testing and contact tracing that’s needed to reduce the virus spreading,” said study coauthor Ting Ma, PhD, of Harbin Institute of Technology at Shenzhen.
Dr. Ma acknowledged that some of the measures the program used, such as isolating people outside their homes, may be difficult to impose in other countries, “but we urge governments to consider our findings in the global response to COVID-19.”
The study followed 391 coronavirus cases and 1,286 close contacts identified by the Shenzhen Center for Disease Control and Prevention from Jan. 14 to Feb. 12 this year. The study showed that contact tracing led to confirming new diagnoses within 3.2 days on average vs. 5.5 for symptom-based surveillance, and reduced the time it took to isolate newly infected people by 2 days, from an average of 4.6 to 2.7 days. Eighty-seven people were diagnosed with COVID-19 after they were contact traced and tested. Twenty percent of them had no symptoms, and 29% had no fever. Three deaths occurred in the group during the study period.
The surveillance program was comprehensive and intense. On Jan. 8, the Shenzhen CDC started monitoring travelers from Hubei province, of which Wuhan is the capital, for symptoms of COVID-19. Shenzhen is a city of about 12.5 million people in southeastern China, near Hong Kong, and is about 560 miles south of Wuhan. Over the next 2 weeks, the Shenzhen CDC expanded that surveillance program to all travelers from Hubei regardless of symptoms, along with local hospital patients and people detected by fever screenings at area clinics.
Suspected cases and close contacts underwent nasal-swab testing at 40 different locations in the city. The program identified close contacts through contact tracing, and included anyone who lived in the same dwelling, shared a meal, traveled, or had a social interaction with an index 2 days before symptoms appeared. Casual contacts and some close contacts, such as clinic nurses, who wore masks during the encounters were excluded.
“To achieve similar results, other countries might be able to combine near-universal testing and intensive contact tracing with social distancing and partial lockdowns,” said Dr. Ma. “Although no lockdown measures were introduced in Shenzhen until the end of our study period, Wuhan’s lockdown could have significantly restricted the spread of coronavirus to Shenzhen.”
The researchers noted that children are as susceptible to the virus as are adults, even though their symptoms are not as severe as those of adults. The rate of infection in children 10 and younger was similar to the overall infection rate, 7.4% vs. 6.6%, so the researchers noted that surveillance measures should target them as well.
“This study to me confirms a lot of what we’ve already known,” Aaron E. Glatt, MD, chairman of medicine and an epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “It’s an elegant study, but at the same time it sends us a message that we’re at a critical point of time for us to intervene and prevent cases at the very beginning.”
He acknowledged that the Shenzhen effort was intense. “It’s always a resource-intense requirement to do such extensive contact tracing, but that doesn’t mean it shouldn’t be done to the best of your ability to do so,” he said. He was struck by the low relative rate of infection among contacts in the study – around 7%. “There are differences obviously in infection rates in every outbreak,” he said. “Every individual has their own particular infection rate. While we can take ranges and statistical guesses for every individual patient, it could be very high or very low, and that’s most critical to nip it in the bud.”
Lead author Qifang Bi and study coauthors had no financial relationships to disclose.
SOURCE: Bi Q et al. Lancet Infect Dis. 2020 Apr 27. doi: 10.1016/S1473-3099(20)30287-5.
FROM LANCET INFECTIOUS DISEASE
Acute kidney injury in children hospitalized with diarrheal illness in the U.S.
Clinical question: To determine the incidence and consequences of acute kidney injury among children hospitalized with diarrheal illness in the United States.
Background: Diarrheal illness is the fourth leading cause of death for children younger than 5 years and the fifth leading cause of years of life lost globally. In the United States, diarrheal illness remains a leading cause of hospital admission among young children. Complications of severe diarrheal illness include hypovolemic acute kidney injury (AKI). Hospitalized children who develop AKI experience longer hospital stays and higher mortality. Additionally, children who experience AKI are at increased risk for chronic kidney disease (CKD), hypertension, and proteinuria.
Study design: Retrospective cohort study.
Setting: Kids’ Inpatient Database (KID) from 2009 and 2012. The authors used secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnoses of AKI to identify patients.
Synopsis: The authors reviewed all patients with diarrhea and found that the incidence of AKI in children hospitalized was 0.8%. Those with infectious diarrhea had an incidence of 1% and with noninfectious diarrhea had an incidence of 0.6%. There was a higher incidence of dialysis-requiring AKI in patients with infectious diarrhea. The odds of developing AKI increased with older age in both infectious and noninfectious diarrheal illnesses. As compared with noninfectious diarrheal illness, infectious diarrheal illness was associated with higher odds of AKI (odds ratio, 2.1; 95% confidence interval, 1.7-2.7). Irrespective of diarrhea type, hematologic and rheumatologic conditions, solid organ transplant, CKD, and hypertension were associated with higher odds of developing AKI. AKI in infectious diarrheal illness was also associated with other renal or genitourinary abnormalities, whereas AKI in noninfectious diarrheal illness was associated with diabetes, cardiovascular, and neurologic conditions.
Hospitalizations for diarrheal illness complicated by AKI were associated with higher mortality, prolonged LOS, and higher hospital cost with odds of death increased eightfold with AKI, mean hospital stay was prolonged by 3 days, and costs increased by greater than $9,000 per hospital stay. The development of AKI in hospitalized diarrheal illness was associated with an up to 11-fold increase in the odds of in-hospital mortality for infectious (OR, 10.8; 95% CI, 3.4-34.3) and noninfectious diarrheal illness (OR, 7.0; 95% CI, 3.1-15.7).
The strengths of this study include broad representation of hospitals caring for children across the United States. The study was limited by its use of ICD-9 codes which may misidentify AKI. The authors were unable to determine if identifying AKI could improve outcomes for patients with diarrheal illness.
Bottom line: AKI in diarrhea illnesses is relatively rare. Close attention should be given to AKI in patients with certain serious comorbid illnesses.
Article citation: Bradshaw C, Han J, Chertow GM, Long J, Sutherland SM, Anand S. Acute Kidney Injury in Children Hospitalized With Diarrheal Illness in the United States. Hosp Pediatr. 2019 Dec;9(12):933-941.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, and serves as the pediatrics editor for The Hospitalist.
Clinical question: To determine the incidence and consequences of acute kidney injury among children hospitalized with diarrheal illness in the United States.
Background: Diarrheal illness is the fourth leading cause of death for children younger than 5 years and the fifth leading cause of years of life lost globally. In the United States, diarrheal illness remains a leading cause of hospital admission among young children. Complications of severe diarrheal illness include hypovolemic acute kidney injury (AKI). Hospitalized children who develop AKI experience longer hospital stays and higher mortality. Additionally, children who experience AKI are at increased risk for chronic kidney disease (CKD), hypertension, and proteinuria.
Study design: Retrospective cohort study.
Setting: Kids’ Inpatient Database (KID) from 2009 and 2012. The authors used secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnoses of AKI to identify patients.
Synopsis: The authors reviewed all patients with diarrhea and found that the incidence of AKI in children hospitalized was 0.8%. Those with infectious diarrhea had an incidence of 1% and with noninfectious diarrhea had an incidence of 0.6%. There was a higher incidence of dialysis-requiring AKI in patients with infectious diarrhea. The odds of developing AKI increased with older age in both infectious and noninfectious diarrheal illnesses. As compared with noninfectious diarrheal illness, infectious diarrheal illness was associated with higher odds of AKI (odds ratio, 2.1; 95% confidence interval, 1.7-2.7). Irrespective of diarrhea type, hematologic and rheumatologic conditions, solid organ transplant, CKD, and hypertension were associated with higher odds of developing AKI. AKI in infectious diarrheal illness was also associated with other renal or genitourinary abnormalities, whereas AKI in noninfectious diarrheal illness was associated with diabetes, cardiovascular, and neurologic conditions.
Hospitalizations for diarrheal illness complicated by AKI were associated with higher mortality, prolonged LOS, and higher hospital cost with odds of death increased eightfold with AKI, mean hospital stay was prolonged by 3 days, and costs increased by greater than $9,000 per hospital stay. The development of AKI in hospitalized diarrheal illness was associated with an up to 11-fold increase in the odds of in-hospital mortality for infectious (OR, 10.8; 95% CI, 3.4-34.3) and noninfectious diarrheal illness (OR, 7.0; 95% CI, 3.1-15.7).
The strengths of this study include broad representation of hospitals caring for children across the United States. The study was limited by its use of ICD-9 codes which may misidentify AKI. The authors were unable to determine if identifying AKI could improve outcomes for patients with diarrheal illness.
Bottom line: AKI in diarrhea illnesses is relatively rare. Close attention should be given to AKI in patients with certain serious comorbid illnesses.
Article citation: Bradshaw C, Han J, Chertow GM, Long J, Sutherland SM, Anand S. Acute Kidney Injury in Children Hospitalized With Diarrheal Illness in the United States. Hosp Pediatr. 2019 Dec;9(12):933-941.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, and serves as the pediatrics editor for The Hospitalist.
Clinical question: To determine the incidence and consequences of acute kidney injury among children hospitalized with diarrheal illness in the United States.
Background: Diarrheal illness is the fourth leading cause of death for children younger than 5 years and the fifth leading cause of years of life lost globally. In the United States, diarrheal illness remains a leading cause of hospital admission among young children. Complications of severe diarrheal illness include hypovolemic acute kidney injury (AKI). Hospitalized children who develop AKI experience longer hospital stays and higher mortality. Additionally, children who experience AKI are at increased risk for chronic kidney disease (CKD), hypertension, and proteinuria.
Study design: Retrospective cohort study.
Setting: Kids’ Inpatient Database (KID) from 2009 and 2012. The authors used secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnoses of AKI to identify patients.
Synopsis: The authors reviewed all patients with diarrhea and found that the incidence of AKI in children hospitalized was 0.8%. Those with infectious diarrhea had an incidence of 1% and with noninfectious diarrhea had an incidence of 0.6%. There was a higher incidence of dialysis-requiring AKI in patients with infectious diarrhea. The odds of developing AKI increased with older age in both infectious and noninfectious diarrheal illnesses. As compared with noninfectious diarrheal illness, infectious diarrheal illness was associated with higher odds of AKI (odds ratio, 2.1; 95% confidence interval, 1.7-2.7). Irrespective of diarrhea type, hematologic and rheumatologic conditions, solid organ transplant, CKD, and hypertension were associated with higher odds of developing AKI. AKI in infectious diarrheal illness was also associated with other renal or genitourinary abnormalities, whereas AKI in noninfectious diarrheal illness was associated with diabetes, cardiovascular, and neurologic conditions.
Hospitalizations for diarrheal illness complicated by AKI were associated with higher mortality, prolonged LOS, and higher hospital cost with odds of death increased eightfold with AKI, mean hospital stay was prolonged by 3 days, and costs increased by greater than $9,000 per hospital stay. The development of AKI in hospitalized diarrheal illness was associated with an up to 11-fold increase in the odds of in-hospital mortality for infectious (OR, 10.8; 95% CI, 3.4-34.3) and noninfectious diarrheal illness (OR, 7.0; 95% CI, 3.1-15.7).
The strengths of this study include broad representation of hospitals caring for children across the United States. The study was limited by its use of ICD-9 codes which may misidentify AKI. The authors were unable to determine if identifying AKI could improve outcomes for patients with diarrheal illness.
Bottom line: AKI in diarrhea illnesses is relatively rare. Close attention should be given to AKI in patients with certain serious comorbid illnesses.
Article citation: Bradshaw C, Han J, Chertow GM, Long J, Sutherland SM, Anand S. Acute Kidney Injury in Children Hospitalized With Diarrheal Illness in the United States. Hosp Pediatr. 2019 Dec;9(12):933-941.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, and serves as the pediatrics editor for The Hospitalist.
Supreme Court: Government owes more than $12 billion to health plans
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
REPLENISH: Oral estradiol/progesterone slowed bone turnover as it cut VMS
Menopausal women with an intact uterus treated for a year with a daily, oral, single-pill formulation of estradiol and progesterone for the primary goal of reducing moderate to severe vasomotor symptoms showed evidence of reduced bone turnover in a post hoc, subgroup analysis of 157 women enrolled in the REPLENISH trial.
These findings “provide support for a potential skeletal benefit” when menopausal women with an intact uterus regularly used the tested estradiol plus progesterone formulation to treat moderate to severe vasomotor symptoms (VMS), Risa Kagan, MD, and associates wrote in an abstract released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting, and released abstracts for press coverage.
The reductions reported for three different markers of bone turnover compared with placebo control after 6 and 12 months on treatment with a U.S.-marketed estradiol plus progesterone formulation were ”reassuring and in line with expectations” said Lubna Pal, MBBS, professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., who was not involved with the study. But the findings fell short of addressing the more clinically relevant issue of whether the tested regimen reduces the rate of bone fractures.
“Only longer-term data can tell us whether this magnitude of effect is sustainable,” which would need to happen to cut fracture incidence, said Dr. Pal, who directs the menopause program at her center. “The reduction in bone-turnover markers is adequate, but magnitude alone doesn’t entirely explain a reduction in fractures.”
The bone-marker findings came from a post hoc analysis of data collected in REPLENISH (Safety and Efficacy Study of the Combination Estradiol and Progesterone to Treat Vasomotor Symptoms in Postmenopausal Women With an Intact Uterus), a phase 3 randomized trial run at 119 U.S. sites that during 2013-2015 enrolled 1,845 menopausal women aged 65 years with an intact uterus and with moderate to severe VMS. The researchers randomized women to alternative daily treatment regimens with a single, oral pill that combined a bioidentical form of 17 beta-estradiol at dosages of 1.0 mg/day, 0.50 mg/day, or 0.25 mg/day, and dosages of bioidentical progesterone at dosages of 100 mg/day or 50 mg/day, or to a placebo control arm; not every possible dosage combination underwent testing.
The original study’s primary safety endpoint was the incidence of endometrial hyperplasia after 12 months on treatment, and the results showed no such events in any dosage arm. The primary efficacy endpoints assessed the frequency and severity of VMS after 4 and 12 weeks on treatment, and the results showed that all four tested formulations of estradiol plus progesterone had similar, statistically significant effects on decreasing VMS frequency, and the four formulations reduced severity in a dose-dependent way (Obstet Gynecol. 2018 Jul;132[1]:161-70). Based in part on results from this pivotal trial, the 1-mg estradiol/100 mg progesterone formulation (Bijuva) received Food and Drug Administration marketing approval in late 2018.
The new analysis of bone-turnover markers focused on a subgroup of women selected from two of the drug-treated arms and control patients who had at least 50 moderate to severe VMS events per week at baseline, were at least 5 years out from their last menstrual period, and had data recorded for three markers of bone turnover at baseline, and after 6 and 12 months on treatment, according to the abstract published in Obstetrics and Gynecology.
The analysis included 56 women treated with 1.0 mg estradiol and 100 mg progesterone daily, 56 who received 0.5 mg estradiol and 100 mg progesterone daily, and 45 women in the placebo arm. It assessed changes from baseline in the on-treatment compared with placebo groups using immunoassays for bone-specific alkaline phosphatase (BSAP), C-terminal telopeptide of type 1 collagen (CTX-1), and N-terminal propeptide of type 1 procollagen (PINP). For this analysis, the researchers combined the two active-treatment arms, and found that all three markers showed statistically significant drops from baseline with treatment, compared with placebo at both 6- and 12-month follow-up. For all three markers, the declines on active treatment grew larger with time, and after 12 months the mean drops from baseline compared with placebo were an 18% relative reduction in BSAP, a 41% relative reduction in CTX-1, and a 29% relative reduction in PINP, reported Dr. Kagan, a gynecologist based in Berkeley, Calif., who is affiliated with the University of California San Francisco.
In addition to not yet providing more clinically meaningful results on bone fracture rates, Dr. Pal cited additional issues that limit interpretation of both these findings and the broader REPLENISH results. First, the new analysis of bone-turnover markers as reported in the abstract does not allow assessment of a possible dose-dependent effect on bone turnover. More importantly, while the full REPLENISH trial provided reassuring data on endometrial protection, it has not yet reported data on the other major safety concern of hormone replacement: the effects of treatment on breast cancer rates. “They need to show no harm” in breast tissue, Dr. Pal said in an interview. In addition, the positive effect reported on markers of bone turnover is not unique to this formulation. Other formulations with estrogen at similar dosages would have similar effects, she said.
Other considerations when using an oral formulation include the reduced risk for prothrombotic effects from estradiol when delivered transdermally instead of orally, although some women have trouble getting insurance coverage for transdermal formulations, Dr. Pal said. Oral estrogen is appropriate only for women without an elevated clotting risk. Daily progesterone treatment also is more problematic than a cyclical dosage regimen, but noncontinuous progesterone results in monthly bleeding, something that some women prefer to avoid. “I’m not convinced that continuous progesterone is a physiologic approach,” said Dr. Pal, a member of the Ob.Gyn. News editorial advisory board. In addition, some women may find the formulation attractive because both the estradiol and progesterone are bioidentical and not synthetic molecules.
In short, the oral estradiol plus progesterone formulation tested in REPLENISH “is another tool” that’s an option for selected menopausal women with a uterus. “The bone findings are in line with expectations and are reassuring. Long-term data are keenly awaited to understand whether the bone marker changes lead to fewer fractures. The impact of the treatment on breast safety will be especially important.” For women who want a bioidentical, oral option and to not have bleeding, the tested formulation can be an effective option providing both symptom benefit and endometrial protection, Dr. Pal said.
REPLENISH was funded by TherapeuticsMD, the company that markets the tested estradiol plus progesterone formulation (Bijuva). Dr. Kagan has been a consultant and adviser to and speaker on behalf of Therapeutics MD, and she has also been a consultant or adviser to or has spoken on behalf of AMAG, Amgen, Astellas, Lupin, Radius, and Warner Chilcott/Allergan, and she has received research funding from Endoceutics. Dr. Pal has been a consultant to Flow Health.
SOURCE: Kagan R et al. Obstet Gynecol. 2020 May;135:62S. doi: 10.1097/01.AOG.0000665076.20231.a8.
Menopausal women with an intact uterus treated for a year with a daily, oral, single-pill formulation of estradiol and progesterone for the primary goal of reducing moderate to severe vasomotor symptoms showed evidence of reduced bone turnover in a post hoc, subgroup analysis of 157 women enrolled in the REPLENISH trial.
These findings “provide support for a potential skeletal benefit” when menopausal women with an intact uterus regularly used the tested estradiol plus progesterone formulation to treat moderate to severe vasomotor symptoms (VMS), Risa Kagan, MD, and associates wrote in an abstract released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting, and released abstracts for press coverage.
The reductions reported for three different markers of bone turnover compared with placebo control after 6 and 12 months on treatment with a U.S.-marketed estradiol plus progesterone formulation were ”reassuring and in line with expectations” said Lubna Pal, MBBS, professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., who was not involved with the study. But the findings fell short of addressing the more clinically relevant issue of whether the tested regimen reduces the rate of bone fractures.
“Only longer-term data can tell us whether this magnitude of effect is sustainable,” which would need to happen to cut fracture incidence, said Dr. Pal, who directs the menopause program at her center. “The reduction in bone-turnover markers is adequate, but magnitude alone doesn’t entirely explain a reduction in fractures.”
The bone-marker findings came from a post hoc analysis of data collected in REPLENISH (Safety and Efficacy Study of the Combination Estradiol and Progesterone to Treat Vasomotor Symptoms in Postmenopausal Women With an Intact Uterus), a phase 3 randomized trial run at 119 U.S. sites that during 2013-2015 enrolled 1,845 menopausal women aged 65 years with an intact uterus and with moderate to severe VMS. The researchers randomized women to alternative daily treatment regimens with a single, oral pill that combined a bioidentical form of 17 beta-estradiol at dosages of 1.0 mg/day, 0.50 mg/day, or 0.25 mg/day, and dosages of bioidentical progesterone at dosages of 100 mg/day or 50 mg/day, or to a placebo control arm; not every possible dosage combination underwent testing.
The original study’s primary safety endpoint was the incidence of endometrial hyperplasia after 12 months on treatment, and the results showed no such events in any dosage arm. The primary efficacy endpoints assessed the frequency and severity of VMS after 4 and 12 weeks on treatment, and the results showed that all four tested formulations of estradiol plus progesterone had similar, statistically significant effects on decreasing VMS frequency, and the four formulations reduced severity in a dose-dependent way (Obstet Gynecol. 2018 Jul;132[1]:161-70). Based in part on results from this pivotal trial, the 1-mg estradiol/100 mg progesterone formulation (Bijuva) received Food and Drug Administration marketing approval in late 2018.
The new analysis of bone-turnover markers focused on a subgroup of women selected from two of the drug-treated arms and control patients who had at least 50 moderate to severe VMS events per week at baseline, were at least 5 years out from their last menstrual period, and had data recorded for three markers of bone turnover at baseline, and after 6 and 12 months on treatment, according to the abstract published in Obstetrics and Gynecology.
The analysis included 56 women treated with 1.0 mg estradiol and 100 mg progesterone daily, 56 who received 0.5 mg estradiol and 100 mg progesterone daily, and 45 women in the placebo arm. It assessed changes from baseline in the on-treatment compared with placebo groups using immunoassays for bone-specific alkaline phosphatase (BSAP), C-terminal telopeptide of type 1 collagen (CTX-1), and N-terminal propeptide of type 1 procollagen (PINP). For this analysis, the researchers combined the two active-treatment arms, and found that all three markers showed statistically significant drops from baseline with treatment, compared with placebo at both 6- and 12-month follow-up. For all three markers, the declines on active treatment grew larger with time, and after 12 months the mean drops from baseline compared with placebo were an 18% relative reduction in BSAP, a 41% relative reduction in CTX-1, and a 29% relative reduction in PINP, reported Dr. Kagan, a gynecologist based in Berkeley, Calif., who is affiliated with the University of California San Francisco.
In addition to not yet providing more clinically meaningful results on bone fracture rates, Dr. Pal cited additional issues that limit interpretation of both these findings and the broader REPLENISH results. First, the new analysis of bone-turnover markers as reported in the abstract does not allow assessment of a possible dose-dependent effect on bone turnover. More importantly, while the full REPLENISH trial provided reassuring data on endometrial protection, it has not yet reported data on the other major safety concern of hormone replacement: the effects of treatment on breast cancer rates. “They need to show no harm” in breast tissue, Dr. Pal said in an interview. In addition, the positive effect reported on markers of bone turnover is not unique to this formulation. Other formulations with estrogen at similar dosages would have similar effects, she said.
Other considerations when using an oral formulation include the reduced risk for prothrombotic effects from estradiol when delivered transdermally instead of orally, although some women have trouble getting insurance coverage for transdermal formulations, Dr. Pal said. Oral estrogen is appropriate only for women without an elevated clotting risk. Daily progesterone treatment also is more problematic than a cyclical dosage regimen, but noncontinuous progesterone results in monthly bleeding, something that some women prefer to avoid. “I’m not convinced that continuous progesterone is a physiologic approach,” said Dr. Pal, a member of the Ob.Gyn. News editorial advisory board. In addition, some women may find the formulation attractive because both the estradiol and progesterone are bioidentical and not synthetic molecules.
In short, the oral estradiol plus progesterone formulation tested in REPLENISH “is another tool” that’s an option for selected menopausal women with a uterus. “The bone findings are in line with expectations and are reassuring. Long-term data are keenly awaited to understand whether the bone marker changes lead to fewer fractures. The impact of the treatment on breast safety will be especially important.” For women who want a bioidentical, oral option and to not have bleeding, the tested formulation can be an effective option providing both symptom benefit and endometrial protection, Dr. Pal said.
REPLENISH was funded by TherapeuticsMD, the company that markets the tested estradiol plus progesterone formulation (Bijuva). Dr. Kagan has been a consultant and adviser to and speaker on behalf of Therapeutics MD, and she has also been a consultant or adviser to or has spoken on behalf of AMAG, Amgen, Astellas, Lupin, Radius, and Warner Chilcott/Allergan, and she has received research funding from Endoceutics. Dr. Pal has been a consultant to Flow Health.
SOURCE: Kagan R et al. Obstet Gynecol. 2020 May;135:62S. doi: 10.1097/01.AOG.0000665076.20231.a8.
Menopausal women with an intact uterus treated for a year with a daily, oral, single-pill formulation of estradiol and progesterone for the primary goal of reducing moderate to severe vasomotor symptoms showed evidence of reduced bone turnover in a post hoc, subgroup analysis of 157 women enrolled in the REPLENISH trial.
These findings “provide support for a potential skeletal benefit” when menopausal women with an intact uterus regularly used the tested estradiol plus progesterone formulation to treat moderate to severe vasomotor symptoms (VMS), Risa Kagan, MD, and associates wrote in an abstract released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting, and released abstracts for press coverage.
The reductions reported for three different markers of bone turnover compared with placebo control after 6 and 12 months on treatment with a U.S.-marketed estradiol plus progesterone formulation were ”reassuring and in line with expectations” said Lubna Pal, MBBS, professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., who was not involved with the study. But the findings fell short of addressing the more clinically relevant issue of whether the tested regimen reduces the rate of bone fractures.
“Only longer-term data can tell us whether this magnitude of effect is sustainable,” which would need to happen to cut fracture incidence, said Dr. Pal, who directs the menopause program at her center. “The reduction in bone-turnover markers is adequate, but magnitude alone doesn’t entirely explain a reduction in fractures.”
The bone-marker findings came from a post hoc analysis of data collected in REPLENISH (Safety and Efficacy Study of the Combination Estradiol and Progesterone to Treat Vasomotor Symptoms in Postmenopausal Women With an Intact Uterus), a phase 3 randomized trial run at 119 U.S. sites that during 2013-2015 enrolled 1,845 menopausal women aged 65 years with an intact uterus and with moderate to severe VMS. The researchers randomized women to alternative daily treatment regimens with a single, oral pill that combined a bioidentical form of 17 beta-estradiol at dosages of 1.0 mg/day, 0.50 mg/day, or 0.25 mg/day, and dosages of bioidentical progesterone at dosages of 100 mg/day or 50 mg/day, or to a placebo control arm; not every possible dosage combination underwent testing.
The original study’s primary safety endpoint was the incidence of endometrial hyperplasia after 12 months on treatment, and the results showed no such events in any dosage arm. The primary efficacy endpoints assessed the frequency and severity of VMS after 4 and 12 weeks on treatment, and the results showed that all four tested formulations of estradiol plus progesterone had similar, statistically significant effects on decreasing VMS frequency, and the four formulations reduced severity in a dose-dependent way (Obstet Gynecol. 2018 Jul;132[1]:161-70). Based in part on results from this pivotal trial, the 1-mg estradiol/100 mg progesterone formulation (Bijuva) received Food and Drug Administration marketing approval in late 2018.
The new analysis of bone-turnover markers focused on a subgroup of women selected from two of the drug-treated arms and control patients who had at least 50 moderate to severe VMS events per week at baseline, were at least 5 years out from their last menstrual period, and had data recorded for three markers of bone turnover at baseline, and after 6 and 12 months on treatment, according to the abstract published in Obstetrics and Gynecology.
The analysis included 56 women treated with 1.0 mg estradiol and 100 mg progesterone daily, 56 who received 0.5 mg estradiol and 100 mg progesterone daily, and 45 women in the placebo arm. It assessed changes from baseline in the on-treatment compared with placebo groups using immunoassays for bone-specific alkaline phosphatase (BSAP), C-terminal telopeptide of type 1 collagen (CTX-1), and N-terminal propeptide of type 1 procollagen (PINP). For this analysis, the researchers combined the two active-treatment arms, and found that all three markers showed statistically significant drops from baseline with treatment, compared with placebo at both 6- and 12-month follow-up. For all three markers, the declines on active treatment grew larger with time, and after 12 months the mean drops from baseline compared with placebo were an 18% relative reduction in BSAP, a 41% relative reduction in CTX-1, and a 29% relative reduction in PINP, reported Dr. Kagan, a gynecologist based in Berkeley, Calif., who is affiliated with the University of California San Francisco.
In addition to not yet providing more clinically meaningful results on bone fracture rates, Dr. Pal cited additional issues that limit interpretation of both these findings and the broader REPLENISH results. First, the new analysis of bone-turnover markers as reported in the abstract does not allow assessment of a possible dose-dependent effect on bone turnover. More importantly, while the full REPLENISH trial provided reassuring data on endometrial protection, it has not yet reported data on the other major safety concern of hormone replacement: the effects of treatment on breast cancer rates. “They need to show no harm” in breast tissue, Dr. Pal said in an interview. In addition, the positive effect reported on markers of bone turnover is not unique to this formulation. Other formulations with estrogen at similar dosages would have similar effects, she said.
Other considerations when using an oral formulation include the reduced risk for prothrombotic effects from estradiol when delivered transdermally instead of orally, although some women have trouble getting insurance coverage for transdermal formulations, Dr. Pal said. Oral estrogen is appropriate only for women without an elevated clotting risk. Daily progesterone treatment also is more problematic than a cyclical dosage regimen, but noncontinuous progesterone results in monthly bleeding, something that some women prefer to avoid. “I’m not convinced that continuous progesterone is a physiologic approach,” said Dr. Pal, a member of the Ob.Gyn. News editorial advisory board. In addition, some women may find the formulation attractive because both the estradiol and progesterone are bioidentical and not synthetic molecules.
In short, the oral estradiol plus progesterone formulation tested in REPLENISH “is another tool” that’s an option for selected menopausal women with a uterus. “The bone findings are in line with expectations and are reassuring. Long-term data are keenly awaited to understand whether the bone marker changes lead to fewer fractures. The impact of the treatment on breast safety will be especially important.” For women who want a bioidentical, oral option and to not have bleeding, the tested formulation can be an effective option providing both symptom benefit and endometrial protection, Dr. Pal said.
REPLENISH was funded by TherapeuticsMD, the company that markets the tested estradiol plus progesterone formulation (Bijuva). Dr. Kagan has been a consultant and adviser to and speaker on behalf of Therapeutics MD, and she has also been a consultant or adviser to or has spoken on behalf of AMAG, Amgen, Astellas, Lupin, Radius, and Warner Chilcott/Allergan, and she has received research funding from Endoceutics. Dr. Pal has been a consultant to Flow Health.
SOURCE: Kagan R et al. Obstet Gynecol. 2020 May;135:62S. doi: 10.1097/01.AOG.0000665076.20231.a8.
FROM ACOG 2020
More doctors used digital tools in 2019
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.
Call for Immuno-oncology and Immunotherapy Manuscripts
Federal Practitioner is inviting hematology and oncology health care providers and researchers to contribute to the November 2020 special issue on immuno-oncology. The special issue is produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in new research, case studies, review articles, and patient care program descriptions.
Interested authors can send a brief 2 to 3 sentence abstract to fedprac@mdedge.com by May 29, 2020, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
Federal Practitioner is inviting hematology and oncology health care providers and researchers to contribute to the November 2020 special issue on immuno-oncology. The special issue is produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in new research, case studies, review articles, and patient care program descriptions.
Interested authors can send a brief 2 to 3 sentence abstract to fedprac@mdedge.com by May 29, 2020, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
Federal Practitioner is inviting hematology and oncology health care providers and researchers to contribute to the November 2020 special issue on immuno-oncology. The special issue is produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in new research, case studies, review articles, and patient care program descriptions.
Interested authors can send a brief 2 to 3 sentence abstract to fedprac@mdedge.com by May 29, 2020, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
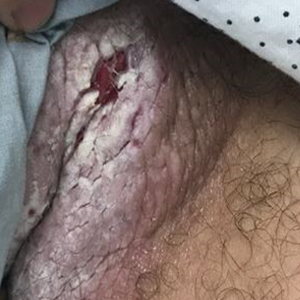
Tender White Lesions on the Groin
The Diagnosis: Candidal Intertrigo
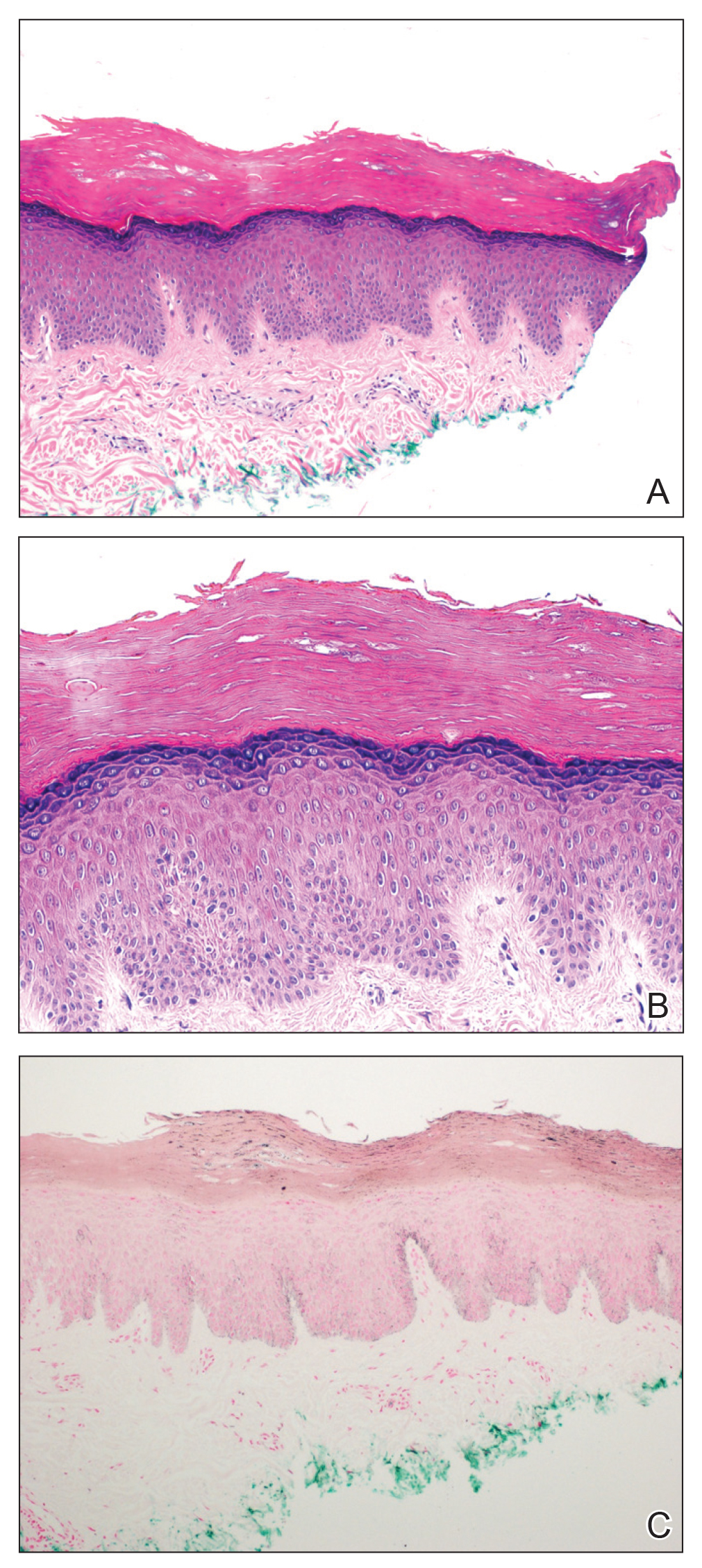
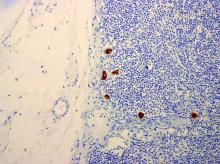
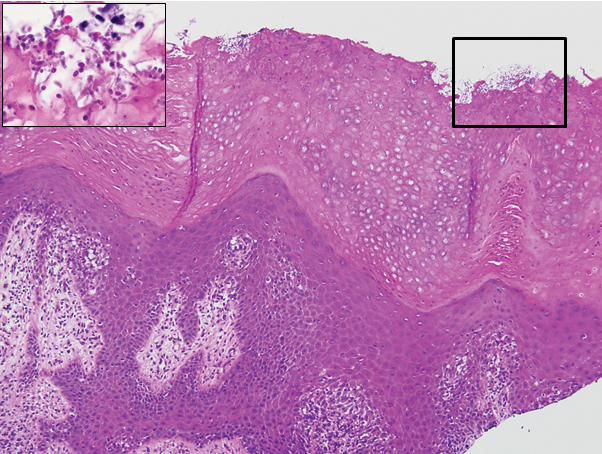
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).
Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1
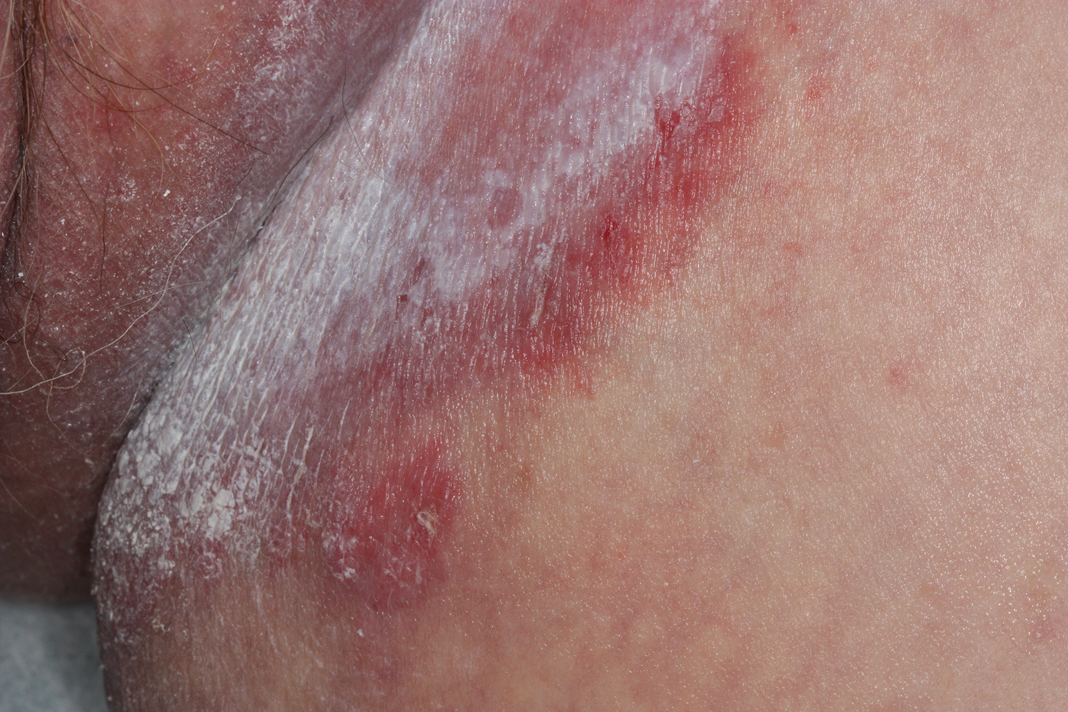
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
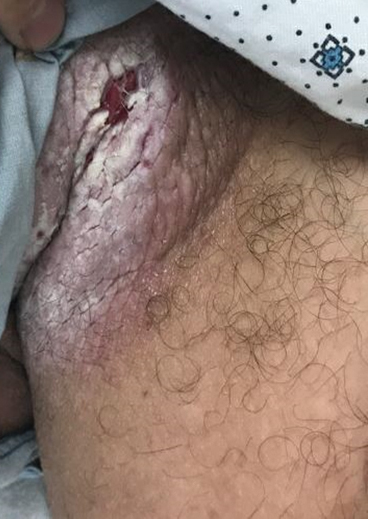
A 28-year-old man with a history of hyperimmunoglobulinemia E syndrome (previously known as Job syndrome), coarse facial features, and multiple skin and soft tissue infections presented to the university dermatology clinic with persistent white, macerated, fissured groin plaques that were present for months. The lesions were tender and pruritic with a burning sensation. Treatment with topical terbinafine and oral fluconazole was attempted without resolution of the eruption. A biopsy of the groin lesion was performed.
Sunless Tanner Caused Persistent Hyperpigmented Patches on the Hands
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
Practice Points
- Patient education on the benefits and risks associated with sunless tanners is critical when using these products.
- Sunless tanners containing dihydroxyacetone potentially can lead to persistent hyperpigmented patches on areas of contact.
- Skin biopsy showing hyperpigmented parakeratosis along with pigmentation of the stratum corneum can aid in diagnosis.
Pseudoepitheliomatous Hyperplasia Arising From Purple Tattoo Pigment
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.
Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.
Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.
Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
Practice Points
- Pseudoepitheliomatous hyperplasia (PEH) is a rare benign condition that can arise in response to multiple underlying triggers such as tattoo pigment.
- Histopathologic evaluation is essential for diagnosis and shows characteristic hyperplasia of the epidermis.
- Clinicians should consider intralesional steroids in the treatment of PEH once atypical mycobacterial and deep fungal infections have been ruled out.
What is the significance of isolated tumor cells in endometrial cancer?
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.
When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.