User login
Postcesarean recovery protocols reduce opioid use
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
REPORTING FROM ACOG 2020
Survey: Supportive oncodermatology program improves QOL for cancer patients
according to a cross-sectional survey of participants.
The average quality of life score prior to program participation in 34 adult patients enrolled in the George Washington University Supportive Oncodermatology Clinic who responded to the survey was 6.5, indicating a moderate effect of their dermatologic symptoms on quality of life. After the beginning of treatment, the average score declined significantly to 3.8, indicating a small effect of the symptoms on quality of life, Leora Aizman, a medical student at George Washington University, Washington, and colleagues reported in the Journal of Drugs in Dermatology.
“On average, [quality of life] total scores were significantly reduced by 2.7 points after joining the supportive oncodermatology clinic,” the authors wrote.
Decreases were seen across all quality of life categories, including physical symptoms, embarrassment, clothes, social/leisure, work/school, and close relationships; the only score that didn’t decrease significantly was for physical symptoms of itch, pain, or soreness (1.43 vs. 1.1 before and after therapy), whereas the category that showed the greatest difference was embarrassment about the dermatologic condition (1.57 vs. 0.83 before and after therapy).
As for satisfaction with the program, the average participant satisfaction score was 4.15, indicating satisfaction with the program. The lowest – an average of 3.67, indicating neutral to satisfied – was related to the effects of the program on treatment adherence.
Survey respondents were adults aged over 18 years who received dermatologic care between the opening of the clinic in May 2017 and Nov. 1, 2019. The online survey included questions adapted from the Dermatology Life Quality Index and Patient Satisfaction Questionnaire.
The findings, though limited by the potential for recall bias and other factors inherent in a survey-based study, suggest that participation in a comprehensive, supportive program could be of benefit for cancer patients experiencing dermatologic conditions from cancer treatment, the authors wrote, explaining that such conditions can be disabling and are associated with negative psychosocial effects. In fact, more than half of all cancer patients experience treatment interruption because of such events.
The findings also underscore the importance of a close partnerships between dermatologists and oncologists as nearly 90% of the surveyed patients were referred to the clinic by their oncologist, the authors wrote. However, the uncertainty that survey respondents experienced with respect to the effects of program participation on treatment adherence highlights a need for further study.
“Our results highlight that supportive oncodermatology interventions improve the psychosocial wellness of patients but require further research on evidence-based preventive and active management strategies,” they wrote.
Additionally, more work is needed to “optimize treatment of secondary toxicities and allow for the continuation of life-prolonging anticancer therapy,” they noted, adding that “prospective, multicenter studies on the management of [dermatologic adverse events] are critical to better understand the effectiveness of these clinics.”
This study was funded by a La Roche–Posay grant. Ms. Aizman reported having no disclosures. One coauthor reported relationships involving consulting and/or honoraria with several companies, including La Roche–Posay.
SOURCE: Aizman L et al. J Drugs Dermatol. 2020 Apr 17. doi: 10.36849/JDD.2020.5040.
according to a cross-sectional survey of participants.
The average quality of life score prior to program participation in 34 adult patients enrolled in the George Washington University Supportive Oncodermatology Clinic who responded to the survey was 6.5, indicating a moderate effect of their dermatologic symptoms on quality of life. After the beginning of treatment, the average score declined significantly to 3.8, indicating a small effect of the symptoms on quality of life, Leora Aizman, a medical student at George Washington University, Washington, and colleagues reported in the Journal of Drugs in Dermatology.
“On average, [quality of life] total scores were significantly reduced by 2.7 points after joining the supportive oncodermatology clinic,” the authors wrote.
Decreases were seen across all quality of life categories, including physical symptoms, embarrassment, clothes, social/leisure, work/school, and close relationships; the only score that didn’t decrease significantly was for physical symptoms of itch, pain, or soreness (1.43 vs. 1.1 before and after therapy), whereas the category that showed the greatest difference was embarrassment about the dermatologic condition (1.57 vs. 0.83 before and after therapy).
As for satisfaction with the program, the average participant satisfaction score was 4.15, indicating satisfaction with the program. The lowest – an average of 3.67, indicating neutral to satisfied – was related to the effects of the program on treatment adherence.
Survey respondents were adults aged over 18 years who received dermatologic care between the opening of the clinic in May 2017 and Nov. 1, 2019. The online survey included questions adapted from the Dermatology Life Quality Index and Patient Satisfaction Questionnaire.
The findings, though limited by the potential for recall bias and other factors inherent in a survey-based study, suggest that participation in a comprehensive, supportive program could be of benefit for cancer patients experiencing dermatologic conditions from cancer treatment, the authors wrote, explaining that such conditions can be disabling and are associated with negative psychosocial effects. In fact, more than half of all cancer patients experience treatment interruption because of such events.
The findings also underscore the importance of a close partnerships between dermatologists and oncologists as nearly 90% of the surveyed patients were referred to the clinic by their oncologist, the authors wrote. However, the uncertainty that survey respondents experienced with respect to the effects of program participation on treatment adherence highlights a need for further study.
“Our results highlight that supportive oncodermatology interventions improve the psychosocial wellness of patients but require further research on evidence-based preventive and active management strategies,” they wrote.
Additionally, more work is needed to “optimize treatment of secondary toxicities and allow for the continuation of life-prolonging anticancer therapy,” they noted, adding that “prospective, multicenter studies on the management of [dermatologic adverse events] are critical to better understand the effectiveness of these clinics.”
This study was funded by a La Roche–Posay grant. Ms. Aizman reported having no disclosures. One coauthor reported relationships involving consulting and/or honoraria with several companies, including La Roche–Posay.
SOURCE: Aizman L et al. J Drugs Dermatol. 2020 Apr 17. doi: 10.36849/JDD.2020.5040.
according to a cross-sectional survey of participants.
The average quality of life score prior to program participation in 34 adult patients enrolled in the George Washington University Supportive Oncodermatology Clinic who responded to the survey was 6.5, indicating a moderate effect of their dermatologic symptoms on quality of life. After the beginning of treatment, the average score declined significantly to 3.8, indicating a small effect of the symptoms on quality of life, Leora Aizman, a medical student at George Washington University, Washington, and colleagues reported in the Journal of Drugs in Dermatology.
“On average, [quality of life] total scores were significantly reduced by 2.7 points after joining the supportive oncodermatology clinic,” the authors wrote.
Decreases were seen across all quality of life categories, including physical symptoms, embarrassment, clothes, social/leisure, work/school, and close relationships; the only score that didn’t decrease significantly was for physical symptoms of itch, pain, or soreness (1.43 vs. 1.1 before and after therapy), whereas the category that showed the greatest difference was embarrassment about the dermatologic condition (1.57 vs. 0.83 before and after therapy).
As for satisfaction with the program, the average participant satisfaction score was 4.15, indicating satisfaction with the program. The lowest – an average of 3.67, indicating neutral to satisfied – was related to the effects of the program on treatment adherence.
Survey respondents were adults aged over 18 years who received dermatologic care between the opening of the clinic in May 2017 and Nov. 1, 2019. The online survey included questions adapted from the Dermatology Life Quality Index and Patient Satisfaction Questionnaire.
The findings, though limited by the potential for recall bias and other factors inherent in a survey-based study, suggest that participation in a comprehensive, supportive program could be of benefit for cancer patients experiencing dermatologic conditions from cancer treatment, the authors wrote, explaining that such conditions can be disabling and are associated with negative psychosocial effects. In fact, more than half of all cancer patients experience treatment interruption because of such events.
The findings also underscore the importance of a close partnerships between dermatologists and oncologists as nearly 90% of the surveyed patients were referred to the clinic by their oncologist, the authors wrote. However, the uncertainty that survey respondents experienced with respect to the effects of program participation on treatment adherence highlights a need for further study.
“Our results highlight that supportive oncodermatology interventions improve the psychosocial wellness of patients but require further research on evidence-based preventive and active management strategies,” they wrote.
Additionally, more work is needed to “optimize treatment of secondary toxicities and allow for the continuation of life-prolonging anticancer therapy,” they noted, adding that “prospective, multicenter studies on the management of [dermatologic adverse events] are critical to better understand the effectiveness of these clinics.”
This study was funded by a La Roche–Posay grant. Ms. Aizman reported having no disclosures. One coauthor reported relationships involving consulting and/or honoraria with several companies, including La Roche–Posay.
SOURCE: Aizman L et al. J Drugs Dermatol. 2020 Apr 17. doi: 10.36849/JDD.2020.5040.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
The Changing Landscape of Acute Migraine
Acute migraine onset results in 1.2 million emergency room visits and costs Americans $36 billion in health care expenditures annually, with the additional financial toll of 157 million lost workdays and unknown damage incurred by associated psychologic comorbidities.
In this supplement, Robert Cowan, MD discusses the latest understanding of acute migraine etiology, as well as updates in diagnostics, clinical trial design, and treatment options.
Acute migraine onset results in 1.2 million emergency room visits and costs Americans $36 billion in health care expenditures annually, with the additional financial toll of 157 million lost workdays and unknown damage incurred by associated psychologic comorbidities.
In this supplement, Robert Cowan, MD discusses the latest understanding of acute migraine etiology, as well as updates in diagnostics, clinical trial design, and treatment options.
Acute migraine onset results in 1.2 million emergency room visits and costs Americans $36 billion in health care expenditures annually, with the additional financial toll of 157 million lost workdays and unknown damage incurred by associated psychologic comorbidities.
In this supplement, Robert Cowan, MD discusses the latest understanding of acute migraine etiology, as well as updates in diagnostics, clinical trial design, and treatment options.
Vaginal cleansing at cesarean delivery works in practice
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
REPORTING FROM ACOG 2020
Lichen Planopilaris in a Patient Treated With Bexarotene for Lymphomatoid Papulosis
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.
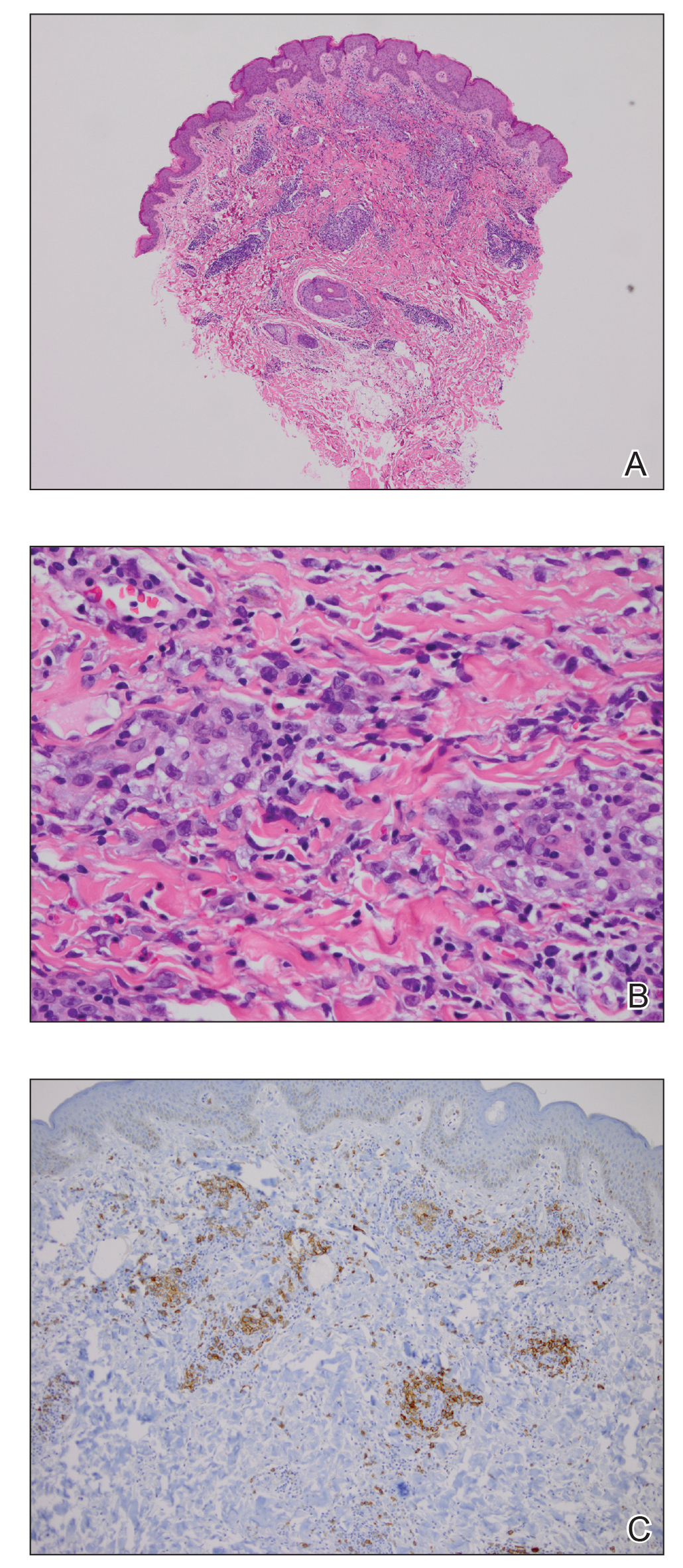
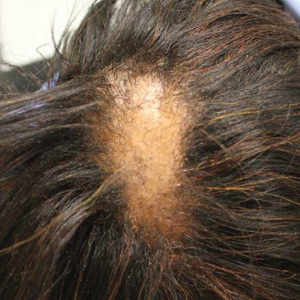
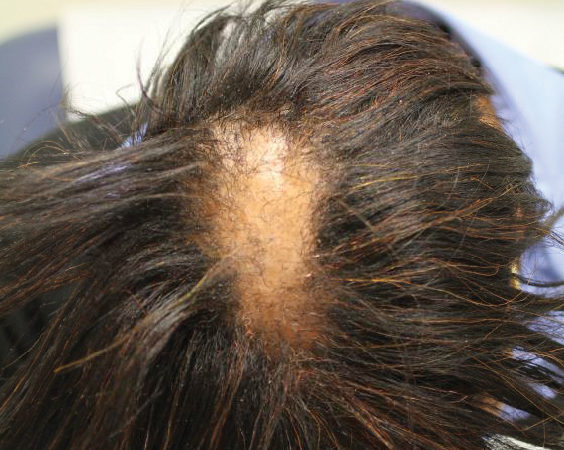
Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.
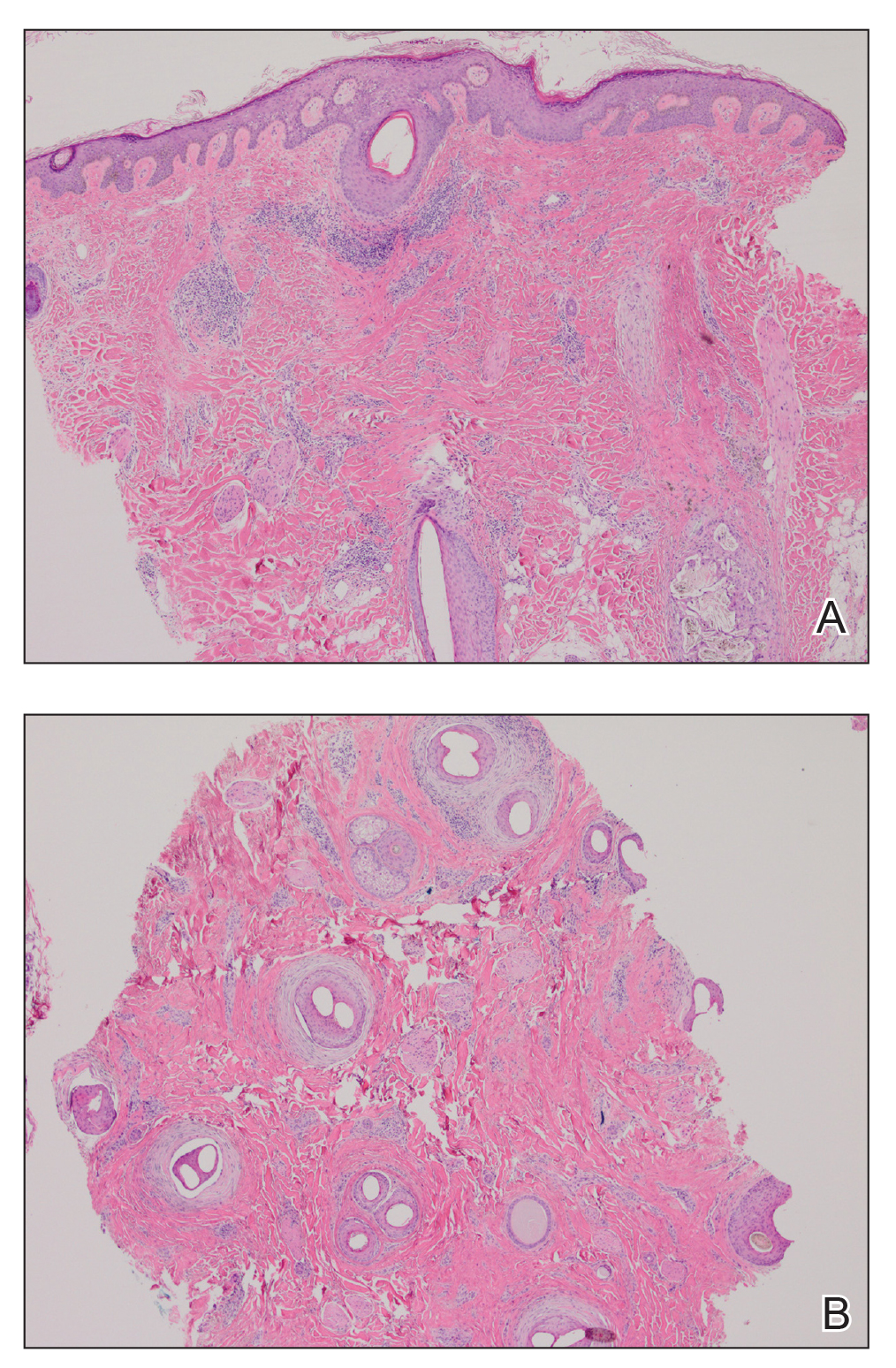
Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.

Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.


Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.

Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.


Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
Practice Points
- Oral retinoids may be associated with development of lichen planopilaris (LPP).
- Hypertriglyceridemia may be associated with onset of LPP.
Rural ICU capacity could be strained by COVID-19
The nonmetropolitan, largely rural, areas of the United States have fewer ICU beds than do urban areas, but their populations may be at higher risk for COVID-19 complications, according to the Kaiser Family Foundation.
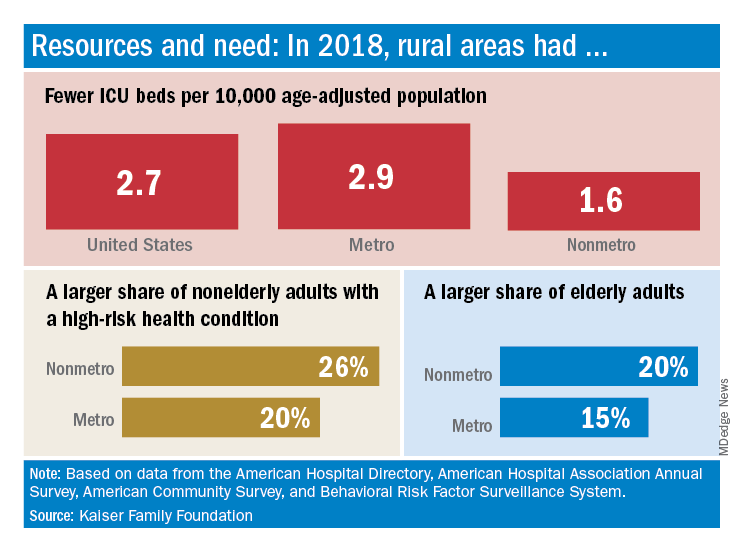
In 2018, the United States had 2.7 ICU beds per 10,000 age-adjusted population, but that number drops to 1.6 beds per 10,000 in nonmetro America and rises to 2.9 per 10,000 in metro areas. Counts for all hospital beds were much closer: 21.6 per 10,000 (rural) and 23.9 per 10,000 (urban), Kaiser investigators reported.
“The novel coronavirus was slower to spread to rural areas in the U.S., but that appears to be changing, with new outbreaks becoming evident in less densely populated parts of the country,” Kendal Orgera and associates said in a recent analysis.
Those rural areas have COVID-19 issues beyond ICU bed counts. Populations in nonmetro areas are less healthy – 26% of adults under age 65 years had a preexisting medical condition in 2018, compared with 20% in metro areas – and older – 20% of people are 65 and older, versus 15% in metro areas, they said.
“If coronavirus continues to spread in rural communities across the U.S., it is possible many [nonmetro] areas will face shortages of ICU beds with limited options to adapt. Patients in rural areas experiencing more severe illnesses may be transferred to hospitals with greater capacity, but if nearby urban areas are also overwhelmed, transfer may not be an option,” Ms. Orgera and associates wrote.
They defined nonmetro counties as those with rural towns of fewer than 2,500 people and/or “urban areas with populations ranging from 2,500 to 49,999 that are not part of larger labor market areas.” The Kaiser analysis involved 2018 data from the American Hospital Association, American Hospital Directory, American Community Survey, and the Behavioral Risk Factor Surveillance System.
The nonmetropolitan, largely rural, areas of the United States have fewer ICU beds than do urban areas, but their populations may be at higher risk for COVID-19 complications, according to the Kaiser Family Foundation.

In 2018, the United States had 2.7 ICU beds per 10,000 age-adjusted population, but that number drops to 1.6 beds per 10,000 in nonmetro America and rises to 2.9 per 10,000 in metro areas. Counts for all hospital beds were much closer: 21.6 per 10,000 (rural) and 23.9 per 10,000 (urban), Kaiser investigators reported.
“The novel coronavirus was slower to spread to rural areas in the U.S., but that appears to be changing, with new outbreaks becoming evident in less densely populated parts of the country,” Kendal Orgera and associates said in a recent analysis.
Those rural areas have COVID-19 issues beyond ICU bed counts. Populations in nonmetro areas are less healthy – 26% of adults under age 65 years had a preexisting medical condition in 2018, compared with 20% in metro areas – and older – 20% of people are 65 and older, versus 15% in metro areas, they said.
“If coronavirus continues to spread in rural communities across the U.S., it is possible many [nonmetro] areas will face shortages of ICU beds with limited options to adapt. Patients in rural areas experiencing more severe illnesses may be transferred to hospitals with greater capacity, but if nearby urban areas are also overwhelmed, transfer may not be an option,” Ms. Orgera and associates wrote.
They defined nonmetro counties as those with rural towns of fewer than 2,500 people and/or “urban areas with populations ranging from 2,500 to 49,999 that are not part of larger labor market areas.” The Kaiser analysis involved 2018 data from the American Hospital Association, American Hospital Directory, American Community Survey, and the Behavioral Risk Factor Surveillance System.
The nonmetropolitan, largely rural, areas of the United States have fewer ICU beds than do urban areas, but their populations may be at higher risk for COVID-19 complications, according to the Kaiser Family Foundation.

In 2018, the United States had 2.7 ICU beds per 10,000 age-adjusted population, but that number drops to 1.6 beds per 10,000 in nonmetro America and rises to 2.9 per 10,000 in metro areas. Counts for all hospital beds were much closer: 21.6 per 10,000 (rural) and 23.9 per 10,000 (urban), Kaiser investigators reported.
“The novel coronavirus was slower to spread to rural areas in the U.S., but that appears to be changing, with new outbreaks becoming evident in less densely populated parts of the country,” Kendal Orgera and associates said in a recent analysis.
Those rural areas have COVID-19 issues beyond ICU bed counts. Populations in nonmetro areas are less healthy – 26% of adults under age 65 years had a preexisting medical condition in 2018, compared with 20% in metro areas – and older – 20% of people are 65 and older, versus 15% in metro areas, they said.
“If coronavirus continues to spread in rural communities across the U.S., it is possible many [nonmetro] areas will face shortages of ICU beds with limited options to adapt. Patients in rural areas experiencing more severe illnesses may be transferred to hospitals with greater capacity, but if nearby urban areas are also overwhelmed, transfer may not be an option,” Ms. Orgera and associates wrote.
They defined nonmetro counties as those with rural towns of fewer than 2,500 people and/or “urban areas with populations ranging from 2,500 to 49,999 that are not part of larger labor market areas.” The Kaiser analysis involved 2018 data from the American Hospital Association, American Hospital Directory, American Community Survey, and the Behavioral Risk Factor Surveillance System.
Price increases for RA biologics keep out-of-pocket costs high for Medicare patients
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
FROM JAMA NETWORK OPEN
Things We Do For No Reason™: Treatment of Infection-Related Fever in Hospitalized Patients

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.
© 2020 Society of Hospital Medicine
Improving Hand Hygiene Adherence in Healthcare Workers Before Patient Contact: A Multimodal Intervention in Four Tertiary Care Hospitals in Japan
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
© 2020 Society of Hospital Medicine
Things We Do For No Reason™: Routine Overnight Vital Sign Checks

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
© 2020 Society of Hospital Medicine