User login
Remaining connected
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Cyclic vomiting syndrome: A GI primer
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS
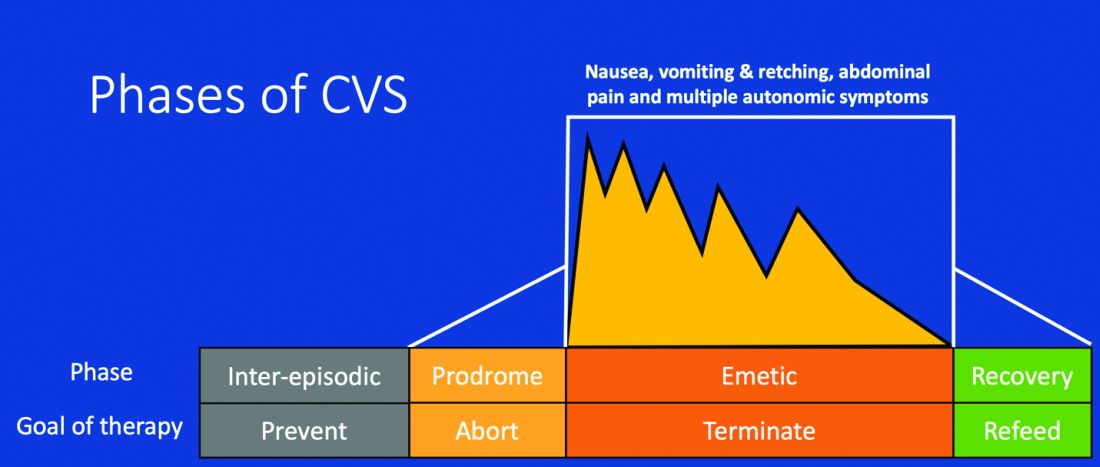
Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22
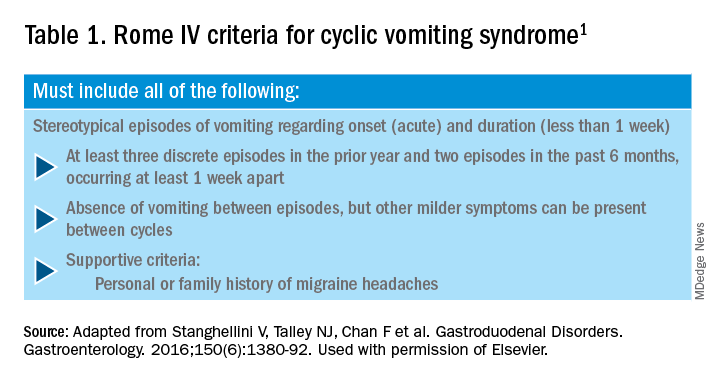
CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.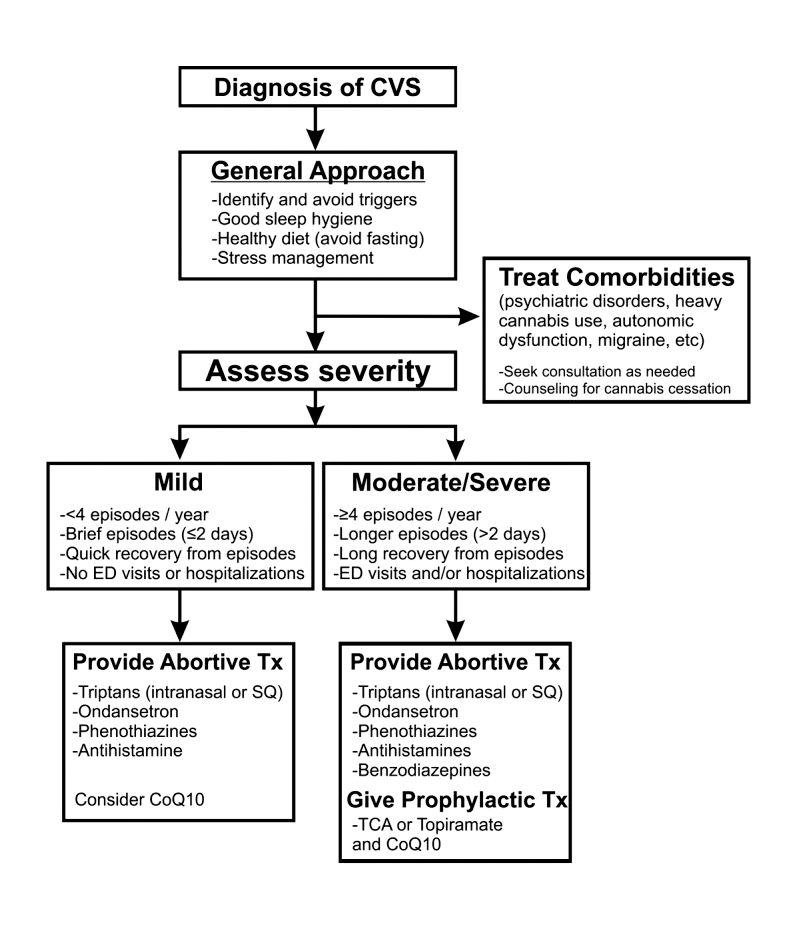
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
EMBRACA shows no overall survival benefit with talazoparib
Talazoparib did not confer an overall survival benefit over chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer, according to a final analysis of the phase 3 EMBRACA trial.
The progression-free survival benefit previously seen with talazoparib did not translate to an overall survival benefit. However, patient-reported quality of life continued to favor talazoparib in the final analysis, Jennifer K. Litton, MD, of the University of Texas MD Anderson Cancer Center in Houston, reported at the AACR virtual meeting I.
The EMBRACA trial enrolled adults with HER2-negative locally advanced or metastatic breast cancer and a deleterious or suspected deleterious germline BRCA mutation. They were randomized to talazoparib at 1 mg daily (n = 287) or to physician’s choice of single-agent chemotherapy (n = 144).
In the primary analysis, talazoparib was associated with significantly improved progression-free survival. The median progression-free survival was 8.6 months in the talazoparib arm and 5.6 months in the chemotherapy arm (hazard ratio, 0.54).
“At the time of the primary analysis, the overall survival data were immature, and the hazard ratio for the interim overall survival was 0.761, which was not statistically significant,” Dr. Litton said.
However, patient-reported outcomes favored talazoparib in the primary analysis, with patients in that arm showing “significant overall improvements with a significant delay in time to clinically meaningful deterioration in multiple cancer-related and breast cancer–specific symptoms, functions, quality of life, and global health,” Dr. Litton said.
Final overall survival
At the final analysis, the median follow-up was 44.9 months for the talazoparib arm and 36.8 months for the chemotherapy arm.
The median overall survival was 19.3 months in the talazoparib arm and 19.5 months in the chemotherapy arm (HR, 0.848; P = .17)
The results were “generally consistent” across patient subgroups,” Dr. Litton said, adding that “the effect of treatment with talazoparib was also similar irrespective of BRCA status, as well as triple-negative or hormone-receptor-positive subtypes.”
Of note, most patients received poststudy therapies. These included PARP inhibitors in 4.5% of patients in the talazoparib arm and 32.6% of patients in the chemotherapy arm, and platinum drugs in 46.3% and 41.7%, respectively.
Patients who received chemotherapy on study but did not receive a subsequent PARP inhibitor or platinum therapy had both shorter total treatment duration and shorter overall survival, compared with patients who did receive subsequent treatment.
In the talazoparib arm, outcomes were similar whether or not patients received a subsequent PARP inhibitor or platinum therapy.
“Interpretation of the overall survival results may have been confounded by subsequent treatment, so two sensitivity analyses accounting for subsequent PARP inhibition or platinum therapy were carried out,” Dr. Litton said.
She noted that adjustment for poststudy treatment lowered the hazard ratio, but there was still no significant difference between the talazoparib and chemotherapy arms. These results suggest “the primary overall survival analysis underestimated the treatment benefit of talazoparib,” Dr. Litton said. She also noted that a longer platinum-free interval prior to study entry was generally associated with a longer duration of survival, particularly in the talazoparib arm.
Quality of life and safety
Patient-reported outcomes continued to favor talazoparib with extended follow-up and were consistent with the initial analysis, Dr. Litton noted.
The updated analysis revealed “a significant improvement in estimated overall change from baseline in the global health quality of life scores for those patients receiving talazoparib, while a significant deterioration was observed in patients receiving chemotherapy,” she said.
The estimated overall change in score was a 2.1-point increase in the talazoparib arm and a 5.7-point decrease in the chemotherapy arm (P = .001). The median time to clinically meaningful deterioration in global health quality of life scores was 26.3 months in the talazoparib arm and 6.7 months in the chemotherapy arm (HR, 0.385).
At the final analysis, the overall safety profile was consistent with that reported previously. Talazoparib was generally well tolerated, and no new safety signals were identified.
Grade 3/4 serious adverse events occurred in 28.3% of patients in the talazoparib arm and 27% of those in the chemotherapy arm. Adverse events led to treatment discontinuation in 7.7% and 9.5% of patients, respectively.
Most grade 3/4 adverse events were hematologic, and most were successfully managed by supportive care, including transfusions and dose modifications, Dr. Litton said.
She noted that one patient in the chemotherapy arm assigned to receive capecitabine had been diagnosed with acute myeloid leukemia at the time of the first analysis. “And now we report an additional case of [acute myeloid leukemia] in a patient who was randomized to the talazoparib arm,” Dr. Litton said.
Jury’s still out
Based on existing data, including from EMBRACA, the jury is still out on whether PARP inhibition is associated with an overall survival benefit in this setting, said invited discussant Susan Domcheck, MD, of the University of Pennsylvania in Philadelphia.
She suggested that could change with ongoing efforts to identify biomarkers for treatment response and new approaches to treatment, such as earlier lines of therapy and combinations.
“At this time, germline BRCA 1 and 2 pathogenic variants are the best predictor of PARP inhibitor sensitivity in breast cancer,” Dr. Domcheck said. “Not all the tumors are sensitive, but this is true of [estrogen receptor–positive] breast cancer and hormonal therapy, and HER2-positive breast cancer as well.”
Studies investigating approaches to improve survival are “incredibly important, because the progression-free survival is not as long as we would like it to be and there’s not an overwhelming overall survival benefit, for sure,” she said.
The EMBRACA trial was funded by Medivation (Pfizer). Dr. Litton and colleagues disclosed numerous relationships with pharmaceutical companies and other organizations. Dr. Domcheck disclosed relationships with AstraZeneca, Clovis, and Bristol Myers Squibb.
SOURCE: Litton J et al., AACR 20, Abstract CT071.
Talazoparib did not confer an overall survival benefit over chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer, according to a final analysis of the phase 3 EMBRACA trial.
The progression-free survival benefit previously seen with talazoparib did not translate to an overall survival benefit. However, patient-reported quality of life continued to favor talazoparib in the final analysis, Jennifer K. Litton, MD, of the University of Texas MD Anderson Cancer Center in Houston, reported at the AACR virtual meeting I.
The EMBRACA trial enrolled adults with HER2-negative locally advanced or metastatic breast cancer and a deleterious or suspected deleterious germline BRCA mutation. They were randomized to talazoparib at 1 mg daily (n = 287) or to physician’s choice of single-agent chemotherapy (n = 144).
In the primary analysis, talazoparib was associated with significantly improved progression-free survival. The median progression-free survival was 8.6 months in the talazoparib arm and 5.6 months in the chemotherapy arm (hazard ratio, 0.54).
“At the time of the primary analysis, the overall survival data were immature, and the hazard ratio for the interim overall survival was 0.761, which was not statistically significant,” Dr. Litton said.
However, patient-reported outcomes favored talazoparib in the primary analysis, with patients in that arm showing “significant overall improvements with a significant delay in time to clinically meaningful deterioration in multiple cancer-related and breast cancer–specific symptoms, functions, quality of life, and global health,” Dr. Litton said.
Final overall survival
At the final analysis, the median follow-up was 44.9 months for the talazoparib arm and 36.8 months for the chemotherapy arm.
The median overall survival was 19.3 months in the talazoparib arm and 19.5 months in the chemotherapy arm (HR, 0.848; P = .17)
The results were “generally consistent” across patient subgroups,” Dr. Litton said, adding that “the effect of treatment with talazoparib was also similar irrespective of BRCA status, as well as triple-negative or hormone-receptor-positive subtypes.”
Of note, most patients received poststudy therapies. These included PARP inhibitors in 4.5% of patients in the talazoparib arm and 32.6% of patients in the chemotherapy arm, and platinum drugs in 46.3% and 41.7%, respectively.
Patients who received chemotherapy on study but did not receive a subsequent PARP inhibitor or platinum therapy had both shorter total treatment duration and shorter overall survival, compared with patients who did receive subsequent treatment.
In the talazoparib arm, outcomes were similar whether or not patients received a subsequent PARP inhibitor or platinum therapy.
“Interpretation of the overall survival results may have been confounded by subsequent treatment, so two sensitivity analyses accounting for subsequent PARP inhibition or platinum therapy were carried out,” Dr. Litton said.
She noted that adjustment for poststudy treatment lowered the hazard ratio, but there was still no significant difference between the talazoparib and chemotherapy arms. These results suggest “the primary overall survival analysis underestimated the treatment benefit of talazoparib,” Dr. Litton said. She also noted that a longer platinum-free interval prior to study entry was generally associated with a longer duration of survival, particularly in the talazoparib arm.
Quality of life and safety
Patient-reported outcomes continued to favor talazoparib with extended follow-up and were consistent with the initial analysis, Dr. Litton noted.
The updated analysis revealed “a significant improvement in estimated overall change from baseline in the global health quality of life scores for those patients receiving talazoparib, while a significant deterioration was observed in patients receiving chemotherapy,” she said.
The estimated overall change in score was a 2.1-point increase in the talazoparib arm and a 5.7-point decrease in the chemotherapy arm (P = .001). The median time to clinically meaningful deterioration in global health quality of life scores was 26.3 months in the talazoparib arm and 6.7 months in the chemotherapy arm (HR, 0.385).
At the final analysis, the overall safety profile was consistent with that reported previously. Talazoparib was generally well tolerated, and no new safety signals were identified.
Grade 3/4 serious adverse events occurred in 28.3% of patients in the talazoparib arm and 27% of those in the chemotherapy arm. Adverse events led to treatment discontinuation in 7.7% and 9.5% of patients, respectively.
Most grade 3/4 adverse events were hematologic, and most were successfully managed by supportive care, including transfusions and dose modifications, Dr. Litton said.
She noted that one patient in the chemotherapy arm assigned to receive capecitabine had been diagnosed with acute myeloid leukemia at the time of the first analysis. “And now we report an additional case of [acute myeloid leukemia] in a patient who was randomized to the talazoparib arm,” Dr. Litton said.
Jury’s still out
Based on existing data, including from EMBRACA, the jury is still out on whether PARP inhibition is associated with an overall survival benefit in this setting, said invited discussant Susan Domcheck, MD, of the University of Pennsylvania in Philadelphia.
She suggested that could change with ongoing efforts to identify biomarkers for treatment response and new approaches to treatment, such as earlier lines of therapy and combinations.
“At this time, germline BRCA 1 and 2 pathogenic variants are the best predictor of PARP inhibitor sensitivity in breast cancer,” Dr. Domcheck said. “Not all the tumors are sensitive, but this is true of [estrogen receptor–positive] breast cancer and hormonal therapy, and HER2-positive breast cancer as well.”
Studies investigating approaches to improve survival are “incredibly important, because the progression-free survival is not as long as we would like it to be and there’s not an overwhelming overall survival benefit, for sure,” she said.
The EMBRACA trial was funded by Medivation (Pfizer). Dr. Litton and colleagues disclosed numerous relationships with pharmaceutical companies and other organizations. Dr. Domcheck disclosed relationships with AstraZeneca, Clovis, and Bristol Myers Squibb.
SOURCE: Litton J et al., AACR 20, Abstract CT071.
Talazoparib did not confer an overall survival benefit over chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer, according to a final analysis of the phase 3 EMBRACA trial.
The progression-free survival benefit previously seen with talazoparib did not translate to an overall survival benefit. However, patient-reported quality of life continued to favor talazoparib in the final analysis, Jennifer K. Litton, MD, of the University of Texas MD Anderson Cancer Center in Houston, reported at the AACR virtual meeting I.
The EMBRACA trial enrolled adults with HER2-negative locally advanced or metastatic breast cancer and a deleterious or suspected deleterious germline BRCA mutation. They were randomized to talazoparib at 1 mg daily (n = 287) or to physician’s choice of single-agent chemotherapy (n = 144).
In the primary analysis, talazoparib was associated with significantly improved progression-free survival. The median progression-free survival was 8.6 months in the talazoparib arm and 5.6 months in the chemotherapy arm (hazard ratio, 0.54).
“At the time of the primary analysis, the overall survival data were immature, and the hazard ratio for the interim overall survival was 0.761, which was not statistically significant,” Dr. Litton said.
However, patient-reported outcomes favored talazoparib in the primary analysis, with patients in that arm showing “significant overall improvements with a significant delay in time to clinically meaningful deterioration in multiple cancer-related and breast cancer–specific symptoms, functions, quality of life, and global health,” Dr. Litton said.
Final overall survival
At the final analysis, the median follow-up was 44.9 months for the talazoparib arm and 36.8 months for the chemotherapy arm.
The median overall survival was 19.3 months in the talazoparib arm and 19.5 months in the chemotherapy arm (HR, 0.848; P = .17)
The results were “generally consistent” across patient subgroups,” Dr. Litton said, adding that “the effect of treatment with talazoparib was also similar irrespective of BRCA status, as well as triple-negative or hormone-receptor-positive subtypes.”
Of note, most patients received poststudy therapies. These included PARP inhibitors in 4.5% of patients in the talazoparib arm and 32.6% of patients in the chemotherapy arm, and platinum drugs in 46.3% and 41.7%, respectively.
Patients who received chemotherapy on study but did not receive a subsequent PARP inhibitor or platinum therapy had both shorter total treatment duration and shorter overall survival, compared with patients who did receive subsequent treatment.
In the talazoparib arm, outcomes were similar whether or not patients received a subsequent PARP inhibitor or platinum therapy.
“Interpretation of the overall survival results may have been confounded by subsequent treatment, so two sensitivity analyses accounting for subsequent PARP inhibition or platinum therapy were carried out,” Dr. Litton said.
She noted that adjustment for poststudy treatment lowered the hazard ratio, but there was still no significant difference between the talazoparib and chemotherapy arms. These results suggest “the primary overall survival analysis underestimated the treatment benefit of talazoparib,” Dr. Litton said. She also noted that a longer platinum-free interval prior to study entry was generally associated with a longer duration of survival, particularly in the talazoparib arm.
Quality of life and safety
Patient-reported outcomes continued to favor talazoparib with extended follow-up and were consistent with the initial analysis, Dr. Litton noted.
The updated analysis revealed “a significant improvement in estimated overall change from baseline in the global health quality of life scores for those patients receiving talazoparib, while a significant deterioration was observed in patients receiving chemotherapy,” she said.
The estimated overall change in score was a 2.1-point increase in the talazoparib arm and a 5.7-point decrease in the chemotherapy arm (P = .001). The median time to clinically meaningful deterioration in global health quality of life scores was 26.3 months in the talazoparib arm and 6.7 months in the chemotherapy arm (HR, 0.385).
At the final analysis, the overall safety profile was consistent with that reported previously. Talazoparib was generally well tolerated, and no new safety signals were identified.
Grade 3/4 serious adverse events occurred in 28.3% of patients in the talazoparib arm and 27% of those in the chemotherapy arm. Adverse events led to treatment discontinuation in 7.7% and 9.5% of patients, respectively.
Most grade 3/4 adverse events were hematologic, and most were successfully managed by supportive care, including transfusions and dose modifications, Dr. Litton said.
She noted that one patient in the chemotherapy arm assigned to receive capecitabine had been diagnosed with acute myeloid leukemia at the time of the first analysis. “And now we report an additional case of [acute myeloid leukemia] in a patient who was randomized to the talazoparib arm,” Dr. Litton said.
Jury’s still out
Based on existing data, including from EMBRACA, the jury is still out on whether PARP inhibition is associated with an overall survival benefit in this setting, said invited discussant Susan Domcheck, MD, of the University of Pennsylvania in Philadelphia.
She suggested that could change with ongoing efforts to identify biomarkers for treatment response and new approaches to treatment, such as earlier lines of therapy and combinations.
“At this time, germline BRCA 1 and 2 pathogenic variants are the best predictor of PARP inhibitor sensitivity in breast cancer,” Dr. Domcheck said. “Not all the tumors are sensitive, but this is true of [estrogen receptor–positive] breast cancer and hormonal therapy, and HER2-positive breast cancer as well.”
Studies investigating approaches to improve survival are “incredibly important, because the progression-free survival is not as long as we would like it to be and there’s not an overwhelming overall survival benefit, for sure,” she said.
The EMBRACA trial was funded by Medivation (Pfizer). Dr. Litton and colleagues disclosed numerous relationships with pharmaceutical companies and other organizations. Dr. Domcheck disclosed relationships with AstraZeneca, Clovis, and Bristol Myers Squibb.
SOURCE: Litton J et al., AACR 20, Abstract CT071.
FROM AACR 2020
Standing orders for vaccines may improve pediatric vaccination rates
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
FROM PEDIATRICS
Two rare neurologic conditions linked to COVID-19
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
Survey: Hydroxychloroquine use fairly common in COVID-19
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
COVID-19: No U.S. spike expected in pandemic-related suicidal ideation
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
FROM AAS 2020
Crohn’s: Novel biomarker predicts early responses to anti-TNF therapy
A novel biomarker may predict early responses to anti–tumor necrosis factor (TNF) therapy in patients with Crohn’s disease, according to investigators.
After 1 week of therapy with adalimumab, serum levels of citrullinated and matrix metalloproteinase-degraded vimentin (VICM) predicted early responses to treatment with an odds ratio of 42.5, reported lead author Joachim H. Mortensen, PhD, of Nordic Bioscience, Herlev, Denmark, and colleagues.
“VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients,” the investigators wrote in the Journal of Clinical Gastroenterology.This is an area of particular need, the investigators explained because 10%-40% of patients with Crohn’s disease have an inadequate response to anti-TNF therapy, yet early detection of treatment failure remains challenging.
According to the investigators, C-reactive protein (CRP) may serve as a reliable biomarker for acute inflammation, but intestinal tissue remodeling in Crohn’s disease is largely caused by chronic inflammation, which is driven by proteases. As these proteases degrade intestinal tissue, protein fragments called neo-epitopes are released into circulation, offering a potential biomarker of disease activity. Previous research showed that VICM, a neo-epitope that correlates with macrophage activity, was highly associated with Crohn’s disease.
“Because anti-TNF reduces the number of activated macrophages, we hypothesized that changes in VICM levels can be applied to monitor the outcome of early response to anti-TNF treatment in Crohn’s disease,” the investigators wrote.
To test this hypothesis, the investigators retrospectively analyzed clinical data and serum samples from 42 patients with Crohn’s disease, of whom 21 were treated with adalimumab at University Medical Center Groningen (the Netherlands) and 21 were treated with infliximab at Aarhus (Denmark) University Hospital. In both cohorts, disease activity was measured by the Harvey-Bradshaw Index, with responders defined by a score of less than 5, which indicates clinical remission. Harvey-Bradshaw Index scores were evaluated at week 8 and week 14 for the adalimumab and infliximab groups, respectively. In the adalimumab group, serum VICM and CRP levels were evaluated at baseline, then at weeks 1 and 8. In the infliximab group, VICM and CRP were measured at baseline, then at weeks 2, 6, and 14.
Patients responding to adalimumab had significantly lower levels of VICM than did nonresponders at week 1 (P = .007) and week 8 (P = .048). Although infliximab responders showed numerical differences in VICM, compared with nonresponders, at week 2 (P = .48), these differences lacked statistical significance until week 6 (P = .046), then lost significance again at week 14 (P = .23). No significant differences in CRP levels between responders and nonresponders were detected at any timepoints.
Using a receiver operating characteristic–curve analysis, the investigators identified a clinically relevant cutoff value for VICM at 12.5 ng/mL. With this cutoff, responders were identifiable as early as week 6 in the infliximab group with an area under the curve (AUC) of 0.89 (specificity, 75%; sensitivity, 88%; P less than .01). In the adalimumab group, responders were identifiable as early as week 1, with an AUC of 0.91 (specificity, 87%; sensitivity, 100%; P less than .01).
In the infliximab group, patients with a serum VICM level less than 12.5 ng/mL at week 6 were 22.5 times more likely to be responders (odds ratio, 22.5). Patients treated with adalimumab were 42 times more likely to be responders if their VICM level fell below the cutoff at week 1. A similar analysis involving CRP had no predictive value.
“In conclusion, the reduction in VICM serum levels, but not reduction in CRP levels, was associated with early response to [anti–TNF-alpha] treatment in patients with Crohn’s disease,” the investigators concluded. “Thus, VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients.”
The study was funded by the Danish Research Foundation. The investigators disclosed additional relationships with Nordic Bioscience.
SOURCE: Mortensen JH et al. J Clin Gastroenterol. 2020 Apr 14. doi: 10.1097/MCG.0000000000001341.
A novel biomarker may predict early responses to anti–tumor necrosis factor (TNF) therapy in patients with Crohn’s disease, according to investigators.
After 1 week of therapy with adalimumab, serum levels of citrullinated and matrix metalloproteinase-degraded vimentin (VICM) predicted early responses to treatment with an odds ratio of 42.5, reported lead author Joachim H. Mortensen, PhD, of Nordic Bioscience, Herlev, Denmark, and colleagues.
“VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients,” the investigators wrote in the Journal of Clinical Gastroenterology.This is an area of particular need, the investigators explained because 10%-40% of patients with Crohn’s disease have an inadequate response to anti-TNF therapy, yet early detection of treatment failure remains challenging.
According to the investigators, C-reactive protein (CRP) may serve as a reliable biomarker for acute inflammation, but intestinal tissue remodeling in Crohn’s disease is largely caused by chronic inflammation, which is driven by proteases. As these proteases degrade intestinal tissue, protein fragments called neo-epitopes are released into circulation, offering a potential biomarker of disease activity. Previous research showed that VICM, a neo-epitope that correlates with macrophage activity, was highly associated with Crohn’s disease.
“Because anti-TNF reduces the number of activated macrophages, we hypothesized that changes in VICM levels can be applied to monitor the outcome of early response to anti-TNF treatment in Crohn’s disease,” the investigators wrote.
To test this hypothesis, the investigators retrospectively analyzed clinical data and serum samples from 42 patients with Crohn’s disease, of whom 21 were treated with adalimumab at University Medical Center Groningen (the Netherlands) and 21 were treated with infliximab at Aarhus (Denmark) University Hospital. In both cohorts, disease activity was measured by the Harvey-Bradshaw Index, with responders defined by a score of less than 5, which indicates clinical remission. Harvey-Bradshaw Index scores were evaluated at week 8 and week 14 for the adalimumab and infliximab groups, respectively. In the adalimumab group, serum VICM and CRP levels were evaluated at baseline, then at weeks 1 and 8. In the infliximab group, VICM and CRP were measured at baseline, then at weeks 2, 6, and 14.
Patients responding to adalimumab had significantly lower levels of VICM than did nonresponders at week 1 (P = .007) and week 8 (P = .048). Although infliximab responders showed numerical differences in VICM, compared with nonresponders, at week 2 (P = .48), these differences lacked statistical significance until week 6 (P = .046), then lost significance again at week 14 (P = .23). No significant differences in CRP levels between responders and nonresponders were detected at any timepoints.
Using a receiver operating characteristic–curve analysis, the investigators identified a clinically relevant cutoff value for VICM at 12.5 ng/mL. With this cutoff, responders were identifiable as early as week 6 in the infliximab group with an area under the curve (AUC) of 0.89 (specificity, 75%; sensitivity, 88%; P less than .01). In the adalimumab group, responders were identifiable as early as week 1, with an AUC of 0.91 (specificity, 87%; sensitivity, 100%; P less than .01).
In the infliximab group, patients with a serum VICM level less than 12.5 ng/mL at week 6 were 22.5 times more likely to be responders (odds ratio, 22.5). Patients treated with adalimumab were 42 times more likely to be responders if their VICM level fell below the cutoff at week 1. A similar analysis involving CRP had no predictive value.
“In conclusion, the reduction in VICM serum levels, but not reduction in CRP levels, was associated with early response to [anti–TNF-alpha] treatment in patients with Crohn’s disease,” the investigators concluded. “Thus, VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients.”
The study was funded by the Danish Research Foundation. The investigators disclosed additional relationships with Nordic Bioscience.
SOURCE: Mortensen JH et al. J Clin Gastroenterol. 2020 Apr 14. doi: 10.1097/MCG.0000000000001341.
A novel biomarker may predict early responses to anti–tumor necrosis factor (TNF) therapy in patients with Crohn’s disease, according to investigators.
After 1 week of therapy with adalimumab, serum levels of citrullinated and matrix metalloproteinase-degraded vimentin (VICM) predicted early responses to treatment with an odds ratio of 42.5, reported lead author Joachim H. Mortensen, PhD, of Nordic Bioscience, Herlev, Denmark, and colleagues.
“VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients,” the investigators wrote in the Journal of Clinical Gastroenterology.This is an area of particular need, the investigators explained because 10%-40% of patients with Crohn’s disease have an inadequate response to anti-TNF therapy, yet early detection of treatment failure remains challenging.
According to the investigators, C-reactive protein (CRP) may serve as a reliable biomarker for acute inflammation, but intestinal tissue remodeling in Crohn’s disease is largely caused by chronic inflammation, which is driven by proteases. As these proteases degrade intestinal tissue, protein fragments called neo-epitopes are released into circulation, offering a potential biomarker of disease activity. Previous research showed that VICM, a neo-epitope that correlates with macrophage activity, was highly associated with Crohn’s disease.
“Because anti-TNF reduces the number of activated macrophages, we hypothesized that changes in VICM levels can be applied to monitor the outcome of early response to anti-TNF treatment in Crohn’s disease,” the investigators wrote.
To test this hypothesis, the investigators retrospectively analyzed clinical data and serum samples from 42 patients with Crohn’s disease, of whom 21 were treated with adalimumab at University Medical Center Groningen (the Netherlands) and 21 were treated with infliximab at Aarhus (Denmark) University Hospital. In both cohorts, disease activity was measured by the Harvey-Bradshaw Index, with responders defined by a score of less than 5, which indicates clinical remission. Harvey-Bradshaw Index scores were evaluated at week 8 and week 14 for the adalimumab and infliximab groups, respectively. In the adalimumab group, serum VICM and CRP levels were evaluated at baseline, then at weeks 1 and 8. In the infliximab group, VICM and CRP were measured at baseline, then at weeks 2, 6, and 14.
Patients responding to adalimumab had significantly lower levels of VICM than did nonresponders at week 1 (P = .007) and week 8 (P = .048). Although infliximab responders showed numerical differences in VICM, compared with nonresponders, at week 2 (P = .48), these differences lacked statistical significance until week 6 (P = .046), then lost significance again at week 14 (P = .23). No significant differences in CRP levels between responders and nonresponders were detected at any timepoints.
Using a receiver operating characteristic–curve analysis, the investigators identified a clinically relevant cutoff value for VICM at 12.5 ng/mL. With this cutoff, responders were identifiable as early as week 6 in the infliximab group with an area under the curve (AUC) of 0.89 (specificity, 75%; sensitivity, 88%; P less than .01). In the adalimumab group, responders were identifiable as early as week 1, with an AUC of 0.91 (specificity, 87%; sensitivity, 100%; P less than .01).
In the infliximab group, patients with a serum VICM level less than 12.5 ng/mL at week 6 were 22.5 times more likely to be responders (odds ratio, 22.5). Patients treated with adalimumab were 42 times more likely to be responders if their VICM level fell below the cutoff at week 1. A similar analysis involving CRP had no predictive value.
“In conclusion, the reduction in VICM serum levels, but not reduction in CRP levels, was associated with early response to [anti–TNF-alpha] treatment in patients with Crohn’s disease,” the investigators concluded. “Thus, VICM may help to facilitate early decision-making of the best possible treatment option for Crohn’s disease patients.”
The study was funded by the Danish Research Foundation. The investigators disclosed additional relationships with Nordic Bioscience.
SOURCE: Mortensen JH et al. J Clin Gastroenterol. 2020 Apr 14. doi: 10.1097/MCG.0000000000001341.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
COVID-19: Calls to NYC crisis hotline soar
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Acetaminophen plus ibuprofen cut patient-controlled morphine after total hip arthroplasty
Background: The use of multimodal non-opioid analgesics is a common practice to minimize postoperative pain and opioid analgesic use. There is limited high-quality evidence to confirm the synergistic effect and safety of acetaminophen and ibuprofen in the peripostoperative setting. The Paracetamol and NSAID in combination (PANSAID) trial investigated the analgesic efficacy and safety of four multimodal analgesic regimens after total hip arthroplasty.
Study design: Multicenter, randomized, blinded trial.
Setting: A total of six hospitals in Denmark, which represented regional and large university settings.
Synopsis: A total of 559 patients who underwent total hip arthroplasty were randomized to receive one of the following oral regimens: acetaminophen (1,000 mg) and ibuprofen (400 mg), acetaminophen (1,000 mg) and placebo, ibuprofen (400 mg) and placebo, and half-strength acetaminophen (500 mg) and ibuprofen (200 mg). One of the regimens was initiated 1 hour before surgery and continued every 6 hours for a total of 4 doses on the first postoperative day. The mean age was 67 years, and half of the patients were women.
The median morphine consumption in the 24 hours after surgery was significantly lower with full-strength acetaminophen-ibuprofen compared with acetaminophen monotherapy (20 mg vs. 36 mg, 99.6% confidence interval, 6.5-24; P < .001), which exceeded the prespecified 10-mg threshold for a minimal clinically important difference (MCID). The difference between acetaminophen-ibuprofen and ibuprofen monotherapy (20 mg vs. 26 mg) did not exceed the MCID, and was not clinically meaningful. The differences in morphine consumption with full-strength acetaminophen-ibuprofen compared to half-strength acetaminophen-ibuprofen (28 mg) and ibuprofen compared to acetaminophen monotherapy were not statistically significant.
Serious adverse events, the other primary outcome, within 90 days after surgery (15% in the ibuprofen group and 11% in the acetaminophen group, relative risk, 1.44; 97.5% CI, 0.79-2.64; P = .18) did not differ between acetaminophen monotherapy and ibuprofen monotherapy. Secondary outcomes included statistically significant analgesia (lower pain scores) at rest and with mobilization at 24 hours in the acetaminophen-ibuprofen group compared to the other groups.
An interesting observation was that acetaminophen-ibuprofen did not exceed the MCID compared to ibuprofen, which suggests that ibuprofen monotherapy may be a reasonable option for early postoperative analgesia.
Bottom line: Acetaminophen-ibuprofen reduced postoperative morphine use and had improved analgesia 24 hours after total hip arthroplasty, and was not associated with an increased 3-month risk of serious adverse events.
Citation: Thybo KH et al. Effect of combination of paracetamol (acetaminophen) and ibuprofen vs. either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty. The PANSAID randomized clinical trial. JAMA. 2019;321(6):562-71.
Dr. Lambert is a hospital medicine clinician and addiction medicine specialist in the division of hospital medicine at Massachusetts General Hospital.
Background: The use of multimodal non-opioid analgesics is a common practice to minimize postoperative pain and opioid analgesic use. There is limited high-quality evidence to confirm the synergistic effect and safety of acetaminophen and ibuprofen in the peripostoperative setting. The Paracetamol and NSAID in combination (PANSAID) trial investigated the analgesic efficacy and safety of four multimodal analgesic regimens after total hip arthroplasty.
Study design: Multicenter, randomized, blinded trial.
Setting: A total of six hospitals in Denmark, which represented regional and large university settings.
Synopsis: A total of 559 patients who underwent total hip arthroplasty were randomized to receive one of the following oral regimens: acetaminophen (1,000 mg) and ibuprofen (400 mg), acetaminophen (1,000 mg) and placebo, ibuprofen (400 mg) and placebo, and half-strength acetaminophen (500 mg) and ibuprofen (200 mg). One of the regimens was initiated 1 hour before surgery and continued every 6 hours for a total of 4 doses on the first postoperative day. The mean age was 67 years, and half of the patients were women.
The median morphine consumption in the 24 hours after surgery was significantly lower with full-strength acetaminophen-ibuprofen compared with acetaminophen monotherapy (20 mg vs. 36 mg, 99.6% confidence interval, 6.5-24; P < .001), which exceeded the prespecified 10-mg threshold for a minimal clinically important difference (MCID). The difference between acetaminophen-ibuprofen and ibuprofen monotherapy (20 mg vs. 26 mg) did not exceed the MCID, and was not clinically meaningful. The differences in morphine consumption with full-strength acetaminophen-ibuprofen compared to half-strength acetaminophen-ibuprofen (28 mg) and ibuprofen compared to acetaminophen monotherapy were not statistically significant.
Serious adverse events, the other primary outcome, within 90 days after surgery (15% in the ibuprofen group and 11% in the acetaminophen group, relative risk, 1.44; 97.5% CI, 0.79-2.64; P = .18) did not differ between acetaminophen monotherapy and ibuprofen monotherapy. Secondary outcomes included statistically significant analgesia (lower pain scores) at rest and with mobilization at 24 hours in the acetaminophen-ibuprofen group compared to the other groups.
An interesting observation was that acetaminophen-ibuprofen did not exceed the MCID compared to ibuprofen, which suggests that ibuprofen monotherapy may be a reasonable option for early postoperative analgesia.
Bottom line: Acetaminophen-ibuprofen reduced postoperative morphine use and had improved analgesia 24 hours after total hip arthroplasty, and was not associated with an increased 3-month risk of serious adverse events.
Citation: Thybo KH et al. Effect of combination of paracetamol (acetaminophen) and ibuprofen vs. either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty. The PANSAID randomized clinical trial. JAMA. 2019;321(6):562-71.
Dr. Lambert is a hospital medicine clinician and addiction medicine specialist in the division of hospital medicine at Massachusetts General Hospital.
Background: The use of multimodal non-opioid analgesics is a common practice to minimize postoperative pain and opioid analgesic use. There is limited high-quality evidence to confirm the synergistic effect and safety of acetaminophen and ibuprofen in the peripostoperative setting. The Paracetamol and NSAID in combination (PANSAID) trial investigated the analgesic efficacy and safety of four multimodal analgesic regimens after total hip arthroplasty.
Study design: Multicenter, randomized, blinded trial.
Setting: A total of six hospitals in Denmark, which represented regional and large university settings.
Synopsis: A total of 559 patients who underwent total hip arthroplasty were randomized to receive one of the following oral regimens: acetaminophen (1,000 mg) and ibuprofen (400 mg), acetaminophen (1,000 mg) and placebo, ibuprofen (400 mg) and placebo, and half-strength acetaminophen (500 mg) and ibuprofen (200 mg). One of the regimens was initiated 1 hour before surgery and continued every 6 hours for a total of 4 doses on the first postoperative day. The mean age was 67 years, and half of the patients were women.
The median morphine consumption in the 24 hours after surgery was significantly lower with full-strength acetaminophen-ibuprofen compared with acetaminophen monotherapy (20 mg vs. 36 mg, 99.6% confidence interval, 6.5-24; P < .001), which exceeded the prespecified 10-mg threshold for a minimal clinically important difference (MCID). The difference between acetaminophen-ibuprofen and ibuprofen monotherapy (20 mg vs. 26 mg) did not exceed the MCID, and was not clinically meaningful. The differences in morphine consumption with full-strength acetaminophen-ibuprofen compared to half-strength acetaminophen-ibuprofen (28 mg) and ibuprofen compared to acetaminophen monotherapy were not statistically significant.
Serious adverse events, the other primary outcome, within 90 days after surgery (15% in the ibuprofen group and 11% in the acetaminophen group, relative risk, 1.44; 97.5% CI, 0.79-2.64; P = .18) did not differ between acetaminophen monotherapy and ibuprofen monotherapy. Secondary outcomes included statistically significant analgesia (lower pain scores) at rest and with mobilization at 24 hours in the acetaminophen-ibuprofen group compared to the other groups.
An interesting observation was that acetaminophen-ibuprofen did not exceed the MCID compared to ibuprofen, which suggests that ibuprofen monotherapy may be a reasonable option for early postoperative analgesia.
Bottom line: Acetaminophen-ibuprofen reduced postoperative morphine use and had improved analgesia 24 hours after total hip arthroplasty, and was not associated with an increased 3-month risk of serious adverse events.
Citation: Thybo KH et al. Effect of combination of paracetamol (acetaminophen) and ibuprofen vs. either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty. The PANSAID randomized clinical trial. JAMA. 2019;321(6):562-71.
Dr. Lambert is a hospital medicine clinician and addiction medicine specialist in the division of hospital medicine at Massachusetts General Hospital.