User login
Case reports illustrate heterogeneity of skin manifestations in COVID patients
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Reframing AUD as treatable may reduce stigma
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
FROM APA 2020
Gastroenterology groups map a return to elective endoscopy
Gastroenterologists can safely return to elective procedures when adequate personal protective equipment (PPE) is available, professional societies say.
Noting that some states have already lifted restrictions imposed to guard against COVID-19, the American Gastroenterological Association (AGA) and the Digestive Health Physicians Association (DHPA) on April 27 announced guidelines for resuming procedures delayed by the pandemic.
“Gastroenterologists are looking for some framework, however fluid it might be, to guide them in the next 2 to 4 weeks,” Paul Berggreen, MD, secretary of the DHPA, told Medscape Medical News.
The AGA and DHPA guidelines envision a return to elective procedures in areas where COVID-19 cases have been declining for at least 2 weeks and where they are permitted by government directives.
Decisions hinge on the availability of testing, Berggreen said. The guidelines recommend polymerase chain reaction (PCR) tests for COVID-19 infections prior to elective endoscopy. When these tests are not available, a daily temperature log for 10 days prior to the procedure may substitute, they say.
However, if no PCR test is done, the guidelines call on all procedure room personnel to use N95 masks or the equivalent. If these masks aren’t available, “consider delaying resumption of endoscopic procedures,” the guidelines say. The procedure should also be postponed or moved to an inpatient setting in the event of a positive test, according to the guidelines.
Only if the patient has a negative test result should the procedure go forward with the use of standard surgical masks rather than N95 masks or the equivalent, the guidelines say.
The mask recommendations differ slightly from a decision tree put forward during an American College of Gastroenterology (ACG) webinar on April 27, ACG President Mark Pochapin, MD, told Medscape Medical News.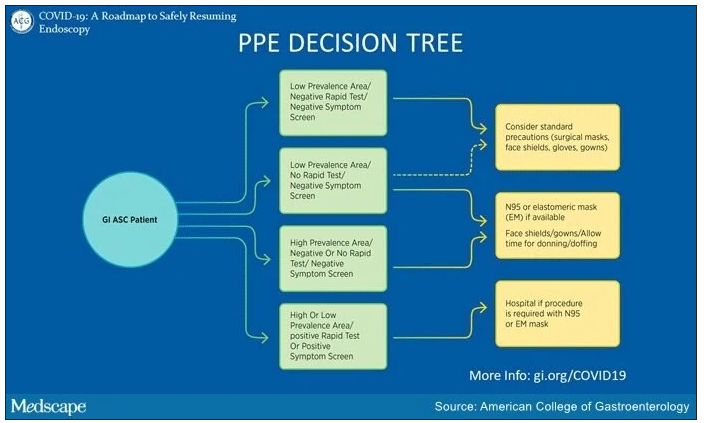
The ACG decision tree takes into consideration local prevalence of COVID-19. “In a low prevalence area if you have a negative test you can wear a regular surgical mask if the patient wears a surgical mask,” Pochapin said.
The ACG decision tree also envisions the possibility of endoscopy with surgical masks along with face shields in areas with low prevalence of COVID-19, even in the absence of testing, if a patient doesn’t have symptoms.
In contrast, the ACG decision tree calls for N95 or elastomeric masks in areas of high prevalence even with a negative test and a negative symptom screen. And it calls for a hospital procedure with an N95 or elastomeric mask in patients with either a positive symptom screen or a positive test.
In addition to masks, the AGA and DHPA guidelines recommend use of other PPE, such as water-resistant gowns, shoe covers, scrubs, double-gloving, and surgical head coverings.
They recommend daily screening of endoscopy center staff with temperature checks and surveys of COVID-19 symptoms and exposure.
Moreover, they call for social distancing of patients, visitors, and staff, and high-level disinfection of endoscopes. They recommend against endotracheal intubation of patients undergoing elective upper endoscopy.
The number of states allowing elective procedures is changing by the day, Berggreen said. In Arizona, where he practices, Gov. Doug Ducey removed all restrictions on elective procedures starting May 1. But other states, where caseloads have been higher, including New York and Massachusetts, have yet to follow suit.
“We are still in kind of a holding pattern,” Richard Hodin, MD, AGAF, chief of gastrointestinal surgery at Massachusetts General Hospital in Boston, told Medscape Medical News. Currently his facility is only doing procedures when a patient’s life is in danger.
The availability of testing also ranges widely from one practice to another. New York University, where Pochapin is director of gastroenterology, is able to do its own tests. Berggreen’s practice, Arizona Digestive Health in Phoenix, has assigned staff to swab patients in its parking lot and then send the samples to a lab by courier for analysis.
Berggreen said his practice has been essentially closed for the past month. In May, he expects his team will do about 30% of its normal volume. They will not start with purely elective procedures, such as following up on a polyp, but with semi-urgent cases, said Berggreen.
“Elective procedures can still be delayed a little bit longer,” he said. “But we’re trying to take care of our patients that are not purely elective: somebody with abdominal pain that you think is very likely a stomach ulcer, somebody with rectal bleeding or persistent diarrhea that›s really impacting their life and you›re thinking this could be an inflammatory condition of the colon.»
Berggreen said he is reassured by a recent survey of 968 healthcare workers in Northern Italy who conducted gastrointestinal endoscopy there during the COVID-19 outbreak. Only 4.3% of respondents tested positive for COVID-19, and 85.7% of these infections occurred before the introduction of PPE and measures to reduce cases. Results were similarly encouraging for patients.
Providing more endoscopy will relieve many patients, Berggreen said. “We all understand the need to limit the spread of the coronavirus but we also have patients who are going to start to have more struggles and potentially worse outcomes by sitting on a condition that requires endoscopy to diagnose and appropriately manage.”
Neither Berggreen, Pochapin, nor Hodin reported any relevant financial interests. The authors of the survey did not report a source of funding or any relevant financial interests.
This article first appeared on Medscape.com.
Gastroenterologists can safely return to elective procedures when adequate personal protective equipment (PPE) is available, professional societies say.
Noting that some states have already lifted restrictions imposed to guard against COVID-19, the American Gastroenterological Association (AGA) and the Digestive Health Physicians Association (DHPA) on April 27 announced guidelines for resuming procedures delayed by the pandemic.
“Gastroenterologists are looking for some framework, however fluid it might be, to guide them in the next 2 to 4 weeks,” Paul Berggreen, MD, secretary of the DHPA, told Medscape Medical News.
The AGA and DHPA guidelines envision a return to elective procedures in areas where COVID-19 cases have been declining for at least 2 weeks and where they are permitted by government directives.
Decisions hinge on the availability of testing, Berggreen said. The guidelines recommend polymerase chain reaction (PCR) tests for COVID-19 infections prior to elective endoscopy. When these tests are not available, a daily temperature log for 10 days prior to the procedure may substitute, they say.
However, if no PCR test is done, the guidelines call on all procedure room personnel to use N95 masks or the equivalent. If these masks aren’t available, “consider delaying resumption of endoscopic procedures,” the guidelines say. The procedure should also be postponed or moved to an inpatient setting in the event of a positive test, according to the guidelines.
Only if the patient has a negative test result should the procedure go forward with the use of standard surgical masks rather than N95 masks or the equivalent, the guidelines say.
The mask recommendations differ slightly from a decision tree put forward during an American College of Gastroenterology (ACG) webinar on April 27, ACG President Mark Pochapin, MD, told Medscape Medical News.
The ACG decision tree takes into consideration local prevalence of COVID-19. “In a low prevalence area if you have a negative test you can wear a regular surgical mask if the patient wears a surgical mask,” Pochapin said.
The ACG decision tree also envisions the possibility of endoscopy with surgical masks along with face shields in areas with low prevalence of COVID-19, even in the absence of testing, if a patient doesn’t have symptoms.
In contrast, the ACG decision tree calls for N95 or elastomeric masks in areas of high prevalence even with a negative test and a negative symptom screen. And it calls for a hospital procedure with an N95 or elastomeric mask in patients with either a positive symptom screen or a positive test.
In addition to masks, the AGA and DHPA guidelines recommend use of other PPE, such as water-resistant gowns, shoe covers, scrubs, double-gloving, and surgical head coverings.
They recommend daily screening of endoscopy center staff with temperature checks and surveys of COVID-19 symptoms and exposure.
Moreover, they call for social distancing of patients, visitors, and staff, and high-level disinfection of endoscopes. They recommend against endotracheal intubation of patients undergoing elective upper endoscopy.
The number of states allowing elective procedures is changing by the day, Berggreen said. In Arizona, where he practices, Gov. Doug Ducey removed all restrictions on elective procedures starting May 1. But other states, where caseloads have been higher, including New York and Massachusetts, have yet to follow suit.
“We are still in kind of a holding pattern,” Richard Hodin, MD, AGAF, chief of gastrointestinal surgery at Massachusetts General Hospital in Boston, told Medscape Medical News. Currently his facility is only doing procedures when a patient’s life is in danger.
The availability of testing also ranges widely from one practice to another. New York University, where Pochapin is director of gastroenterology, is able to do its own tests. Berggreen’s practice, Arizona Digestive Health in Phoenix, has assigned staff to swab patients in its parking lot and then send the samples to a lab by courier for analysis.
Berggreen said his practice has been essentially closed for the past month. In May, he expects his team will do about 30% of its normal volume. They will not start with purely elective procedures, such as following up on a polyp, but with semi-urgent cases, said Berggreen.
“Elective procedures can still be delayed a little bit longer,” he said. “But we’re trying to take care of our patients that are not purely elective: somebody with abdominal pain that you think is very likely a stomach ulcer, somebody with rectal bleeding or persistent diarrhea that›s really impacting their life and you›re thinking this could be an inflammatory condition of the colon.»
Berggreen said he is reassured by a recent survey of 968 healthcare workers in Northern Italy who conducted gastrointestinal endoscopy there during the COVID-19 outbreak. Only 4.3% of respondents tested positive for COVID-19, and 85.7% of these infections occurred before the introduction of PPE and measures to reduce cases. Results were similarly encouraging for patients.
Providing more endoscopy will relieve many patients, Berggreen said. “We all understand the need to limit the spread of the coronavirus but we also have patients who are going to start to have more struggles and potentially worse outcomes by sitting on a condition that requires endoscopy to diagnose and appropriately manage.”
Neither Berggreen, Pochapin, nor Hodin reported any relevant financial interests. The authors of the survey did not report a source of funding or any relevant financial interests.
This article first appeared on Medscape.com.
Gastroenterologists can safely return to elective procedures when adequate personal protective equipment (PPE) is available, professional societies say.
Noting that some states have already lifted restrictions imposed to guard against COVID-19, the American Gastroenterological Association (AGA) and the Digestive Health Physicians Association (DHPA) on April 27 announced guidelines for resuming procedures delayed by the pandemic.
“Gastroenterologists are looking for some framework, however fluid it might be, to guide them in the next 2 to 4 weeks,” Paul Berggreen, MD, secretary of the DHPA, told Medscape Medical News.
The AGA and DHPA guidelines envision a return to elective procedures in areas where COVID-19 cases have been declining for at least 2 weeks and where they are permitted by government directives.
Decisions hinge on the availability of testing, Berggreen said. The guidelines recommend polymerase chain reaction (PCR) tests for COVID-19 infections prior to elective endoscopy. When these tests are not available, a daily temperature log for 10 days prior to the procedure may substitute, they say.
However, if no PCR test is done, the guidelines call on all procedure room personnel to use N95 masks or the equivalent. If these masks aren’t available, “consider delaying resumption of endoscopic procedures,” the guidelines say. The procedure should also be postponed or moved to an inpatient setting in the event of a positive test, according to the guidelines.
Only if the patient has a negative test result should the procedure go forward with the use of standard surgical masks rather than N95 masks or the equivalent, the guidelines say.
The mask recommendations differ slightly from a decision tree put forward during an American College of Gastroenterology (ACG) webinar on April 27, ACG President Mark Pochapin, MD, told Medscape Medical News.
The ACG decision tree takes into consideration local prevalence of COVID-19. “In a low prevalence area if you have a negative test you can wear a regular surgical mask if the patient wears a surgical mask,” Pochapin said.
The ACG decision tree also envisions the possibility of endoscopy with surgical masks along with face shields in areas with low prevalence of COVID-19, even in the absence of testing, if a patient doesn’t have symptoms.
In contrast, the ACG decision tree calls for N95 or elastomeric masks in areas of high prevalence even with a negative test and a negative symptom screen. And it calls for a hospital procedure with an N95 or elastomeric mask in patients with either a positive symptom screen or a positive test.
In addition to masks, the AGA and DHPA guidelines recommend use of other PPE, such as water-resistant gowns, shoe covers, scrubs, double-gloving, and surgical head coverings.
They recommend daily screening of endoscopy center staff with temperature checks and surveys of COVID-19 symptoms and exposure.
Moreover, they call for social distancing of patients, visitors, and staff, and high-level disinfection of endoscopes. They recommend against endotracheal intubation of patients undergoing elective upper endoscopy.
The number of states allowing elective procedures is changing by the day, Berggreen said. In Arizona, where he practices, Gov. Doug Ducey removed all restrictions on elective procedures starting May 1. But other states, where caseloads have been higher, including New York and Massachusetts, have yet to follow suit.
“We are still in kind of a holding pattern,” Richard Hodin, MD, AGAF, chief of gastrointestinal surgery at Massachusetts General Hospital in Boston, told Medscape Medical News. Currently his facility is only doing procedures when a patient’s life is in danger.
The availability of testing also ranges widely from one practice to another. New York University, where Pochapin is director of gastroenterology, is able to do its own tests. Berggreen’s practice, Arizona Digestive Health in Phoenix, has assigned staff to swab patients in its parking lot and then send the samples to a lab by courier for analysis.
Berggreen said his practice has been essentially closed for the past month. In May, he expects his team will do about 30% of its normal volume. They will not start with purely elective procedures, such as following up on a polyp, but with semi-urgent cases, said Berggreen.
“Elective procedures can still be delayed a little bit longer,” he said. “But we’re trying to take care of our patients that are not purely elective: somebody with abdominal pain that you think is very likely a stomach ulcer, somebody with rectal bleeding or persistent diarrhea that›s really impacting their life and you›re thinking this could be an inflammatory condition of the colon.»
Berggreen said he is reassured by a recent survey of 968 healthcare workers in Northern Italy who conducted gastrointestinal endoscopy there during the COVID-19 outbreak. Only 4.3% of respondents tested positive for COVID-19, and 85.7% of these infections occurred before the introduction of PPE and measures to reduce cases. Results were similarly encouraging for patients.
Providing more endoscopy will relieve many patients, Berggreen said. “We all understand the need to limit the spread of the coronavirus but we also have patients who are going to start to have more struggles and potentially worse outcomes by sitting on a condition that requires endoscopy to diagnose and appropriately manage.”
Neither Berggreen, Pochapin, nor Hodin reported any relevant financial interests. The authors of the survey did not report a source of funding or any relevant financial interests.
This article first appeared on Medscape.com.
Altering gut microbiome may reduce tumor-promoting effects of smoking
Altering the gut microbiome may reduce the tumor-promoting effects of cigarette smoking, based on a preclinical study.
Mice treated with microbiome-depleting antibiotics or genetically modified to lack an adaptive immune response did not show increased rates of cancer growth when exposed to cigarette smoke, reported lead author Prateek Sharma, MBBS, of the University of Miami, and colleagues.
Although previous research has shown that the gut microbiome plays a role in the progression of cancer and that smoking alters the gut microbiome, the collective effects of these changes have not been studied, the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“There is information that smoking changes the gut microbiome ... but the impact of this change is not known,” Dr. Sharma said in a virtual press conference.
To learn more, the investigators first performed an experiment using wild-type mice. All mice were injected with a cancer cell line from the pancreas, colon, or bladder. Mice were then sorted into four groups, in which they were given microbiome-depleting antibiotics and exposed to smoke, given antibiotics alone, exposed to smoke alone, or left untreated and unexposed to serve as controls. Tumor size was then measured over the course of 2 months.
The experiment revealed that mice exposed to smoke but not treated with antibiotics had increased rates of tumor growth regardless of cancer type. But in mice treated with antibiotics, the tumor-promoting effects of smoke exposure were completely lost; the mice had rates of tumor growth even lower than controls.
This experiment was repeated with mice genetically engineered to lack an adaptive immune response. Regardless of smoke or antibiotic exposure, all mice had comparable rates of tumor growth.
Dr. Sharma offered a summary of the findings and their possible implications for human medicine.
“Cigarette smoking changes the gut microbiome, and this changed gut microbiome interacts with the immune system to affect cancer progression,” he said. “If we can target this changed microbiome with modulation strategies like antibiotics, probiotics, or administration of good bacteria, we can alter this process. And if the same results are found in human studies, it could go a long way to affect cancer outcomes in smokers.”
In addition to human studies, Dr. Sharma said that future research should aim to uncover the underlying mechanisms involved in this process, including the types of bacteria that play a role.
When asked if the study might lessen concerns about the negative impacts of smoking among cancer patients, Dr. Sharma suggested that, even if the findings do translate to humans, smoking would still carry significant health risks.
“Even if gut microbiome modulation strategies do work in these patients, it may help a little, but it’s not going to bring it down to the level of nonsmokers, so it’s no way an excuse to not fear or continue [smoking],” he said.
The study was funded by the Florida Department of Health. The investigators reported no conflicts of interest.
Altering the gut microbiome may reduce the tumor-promoting effects of cigarette smoking, based on a preclinical study.
Mice treated with microbiome-depleting antibiotics or genetically modified to lack an adaptive immune response did not show increased rates of cancer growth when exposed to cigarette smoke, reported lead author Prateek Sharma, MBBS, of the University of Miami, and colleagues.
Although previous research has shown that the gut microbiome plays a role in the progression of cancer and that smoking alters the gut microbiome, the collective effects of these changes have not been studied, the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“There is information that smoking changes the gut microbiome ... but the impact of this change is not known,” Dr. Sharma said in a virtual press conference.
To learn more, the investigators first performed an experiment using wild-type mice. All mice were injected with a cancer cell line from the pancreas, colon, or bladder. Mice were then sorted into four groups, in which they were given microbiome-depleting antibiotics and exposed to smoke, given antibiotics alone, exposed to smoke alone, or left untreated and unexposed to serve as controls. Tumor size was then measured over the course of 2 months.
The experiment revealed that mice exposed to smoke but not treated with antibiotics had increased rates of tumor growth regardless of cancer type. But in mice treated with antibiotics, the tumor-promoting effects of smoke exposure were completely lost; the mice had rates of tumor growth even lower than controls.
This experiment was repeated with mice genetically engineered to lack an adaptive immune response. Regardless of smoke or antibiotic exposure, all mice had comparable rates of tumor growth.
Dr. Sharma offered a summary of the findings and their possible implications for human medicine.
“Cigarette smoking changes the gut microbiome, and this changed gut microbiome interacts with the immune system to affect cancer progression,” he said. “If we can target this changed microbiome with modulation strategies like antibiotics, probiotics, or administration of good bacteria, we can alter this process. And if the same results are found in human studies, it could go a long way to affect cancer outcomes in smokers.”
In addition to human studies, Dr. Sharma said that future research should aim to uncover the underlying mechanisms involved in this process, including the types of bacteria that play a role.
When asked if the study might lessen concerns about the negative impacts of smoking among cancer patients, Dr. Sharma suggested that, even if the findings do translate to humans, smoking would still carry significant health risks.
“Even if gut microbiome modulation strategies do work in these patients, it may help a little, but it’s not going to bring it down to the level of nonsmokers, so it’s no way an excuse to not fear or continue [smoking],” he said.
The study was funded by the Florida Department of Health. The investigators reported no conflicts of interest.
Altering the gut microbiome may reduce the tumor-promoting effects of cigarette smoking, based on a preclinical study.
Mice treated with microbiome-depleting antibiotics or genetically modified to lack an adaptive immune response did not show increased rates of cancer growth when exposed to cigarette smoke, reported lead author Prateek Sharma, MBBS, of the University of Miami, and colleagues.
Although previous research has shown that the gut microbiome plays a role in the progression of cancer and that smoking alters the gut microbiome, the collective effects of these changes have not been studied, the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“There is information that smoking changes the gut microbiome ... but the impact of this change is not known,” Dr. Sharma said in a virtual press conference.
To learn more, the investigators first performed an experiment using wild-type mice. All mice were injected with a cancer cell line from the pancreas, colon, or bladder. Mice were then sorted into four groups, in which they were given microbiome-depleting antibiotics and exposed to smoke, given antibiotics alone, exposed to smoke alone, or left untreated and unexposed to serve as controls. Tumor size was then measured over the course of 2 months.
The experiment revealed that mice exposed to smoke but not treated with antibiotics had increased rates of tumor growth regardless of cancer type. But in mice treated with antibiotics, the tumor-promoting effects of smoke exposure were completely lost; the mice had rates of tumor growth even lower than controls.
This experiment was repeated with mice genetically engineered to lack an adaptive immune response. Regardless of smoke or antibiotic exposure, all mice had comparable rates of tumor growth.
Dr. Sharma offered a summary of the findings and their possible implications for human medicine.
“Cigarette smoking changes the gut microbiome, and this changed gut microbiome interacts with the immune system to affect cancer progression,” he said. “If we can target this changed microbiome with modulation strategies like antibiotics, probiotics, or administration of good bacteria, we can alter this process. And if the same results are found in human studies, it could go a long way to affect cancer outcomes in smokers.”
In addition to human studies, Dr. Sharma said that future research should aim to uncover the underlying mechanisms involved in this process, including the types of bacteria that play a role.
When asked if the study might lessen concerns about the negative impacts of smoking among cancer patients, Dr. Sharma suggested that, even if the findings do translate to humans, smoking would still carry significant health risks.
“Even if gut microbiome modulation strategies do work in these patients, it may help a little, but it’s not going to bring it down to the level of nonsmokers, so it’s no way an excuse to not fear or continue [smoking],” he said.
The study was funded by the Florida Department of Health. The investigators reported no conflicts of interest.
FROM DDW 2020
Diastolic dysfunction is a common risk factor for cognitive decline
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Reporting for duty at the front lines of COVID-19: From your editor in chief
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
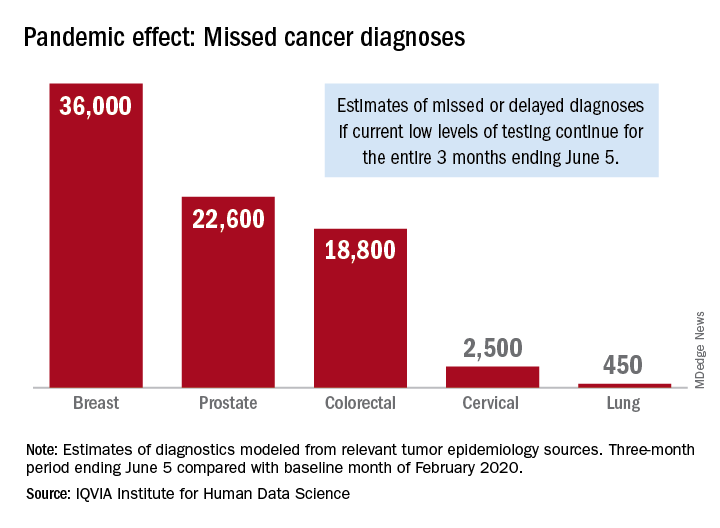
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
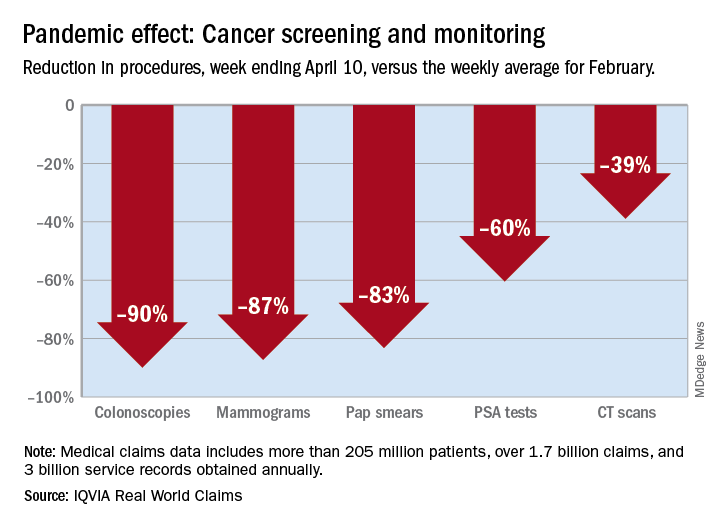
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
FDA tightens requirements for COVID-19 antibody tests
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
Multiple atopic dermatitis therapies completed or close to completing phase 3 studies
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.








