User login
FMT may improve outcomes without clearing multidrug-resistant organisms
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
FROM DDW 2020
Researchers identify a cause of L-DOPA–induced dyskinesia in Parkinson’s disease
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
FROM Science Advances
Telemedicine: A primer for today’s ObGyn
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
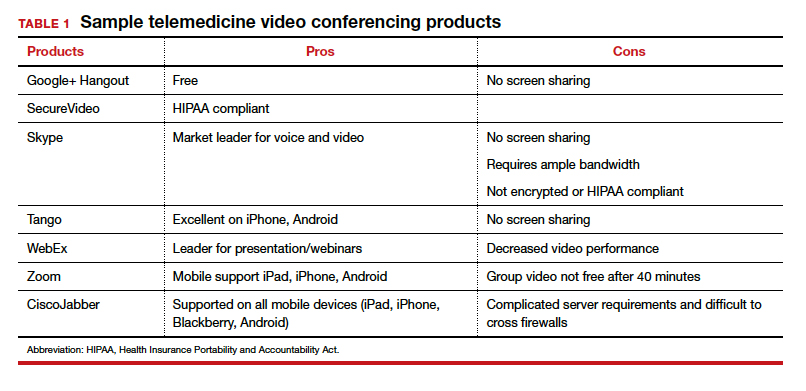
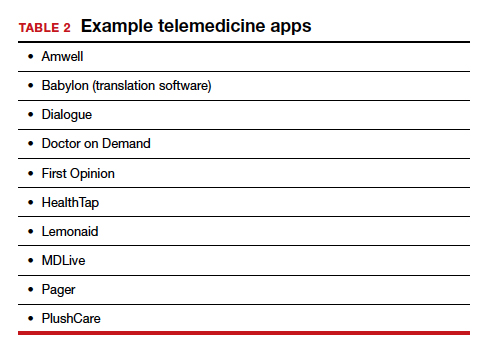
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.
Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
COVID-19 apps for the ObGyn health care provider

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.
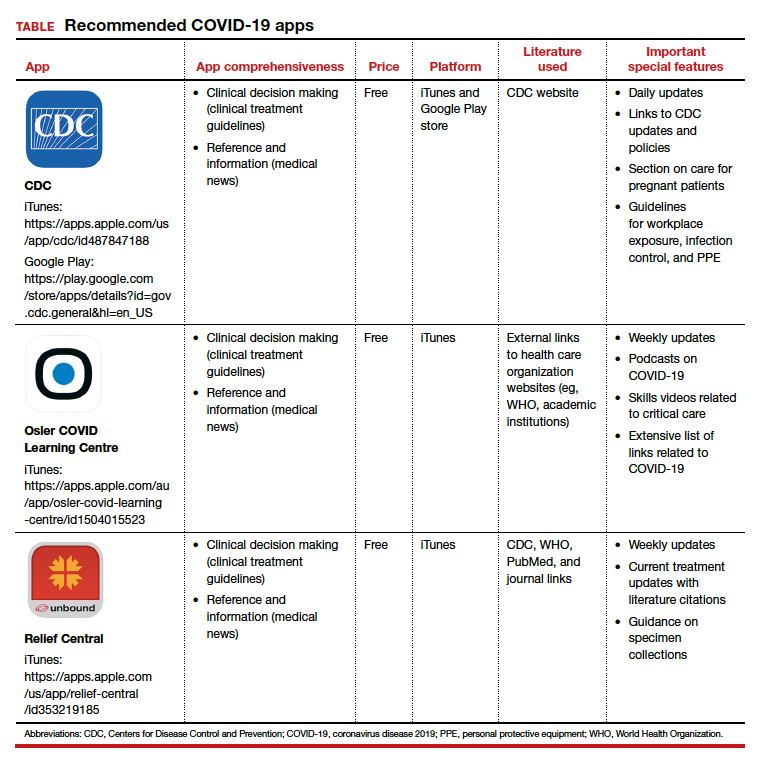
Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.

Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.

Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Pandemic effect: All other health care visits can wait
according to survey conducted at the end of April.
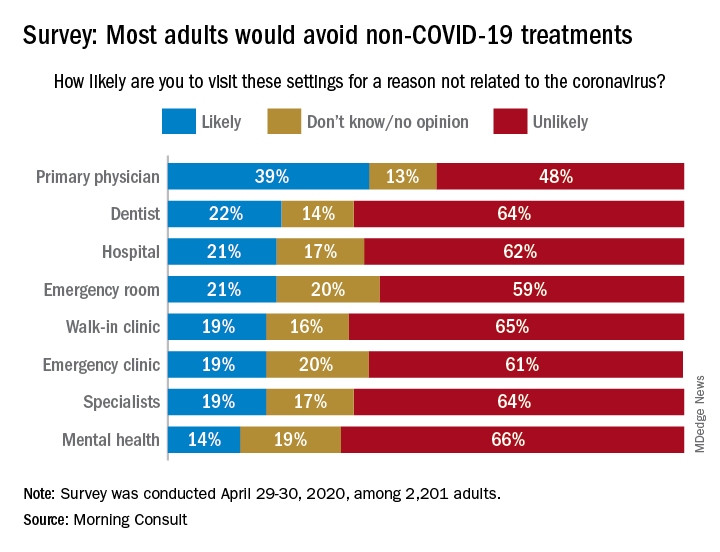
When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
COVID-19: We are in a war, without the most effective weapons to fight a novel viral pathogen
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
Tip Sheet: Teledermatology 101
FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
Cell and gene research raise hopes for recessive dystrophic EB treatments
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
REPORTING FROM EB 2020
Initial high-efficacy MS therapy tied to less disability later
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.









