User login
Lenvatinib/pembrolizumab has good activity in advanced RCC, other solid tumors
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Get familiar with evidence on these supplements
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
New frontier: Transgender men yield eggs, babies, even after testosterone
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
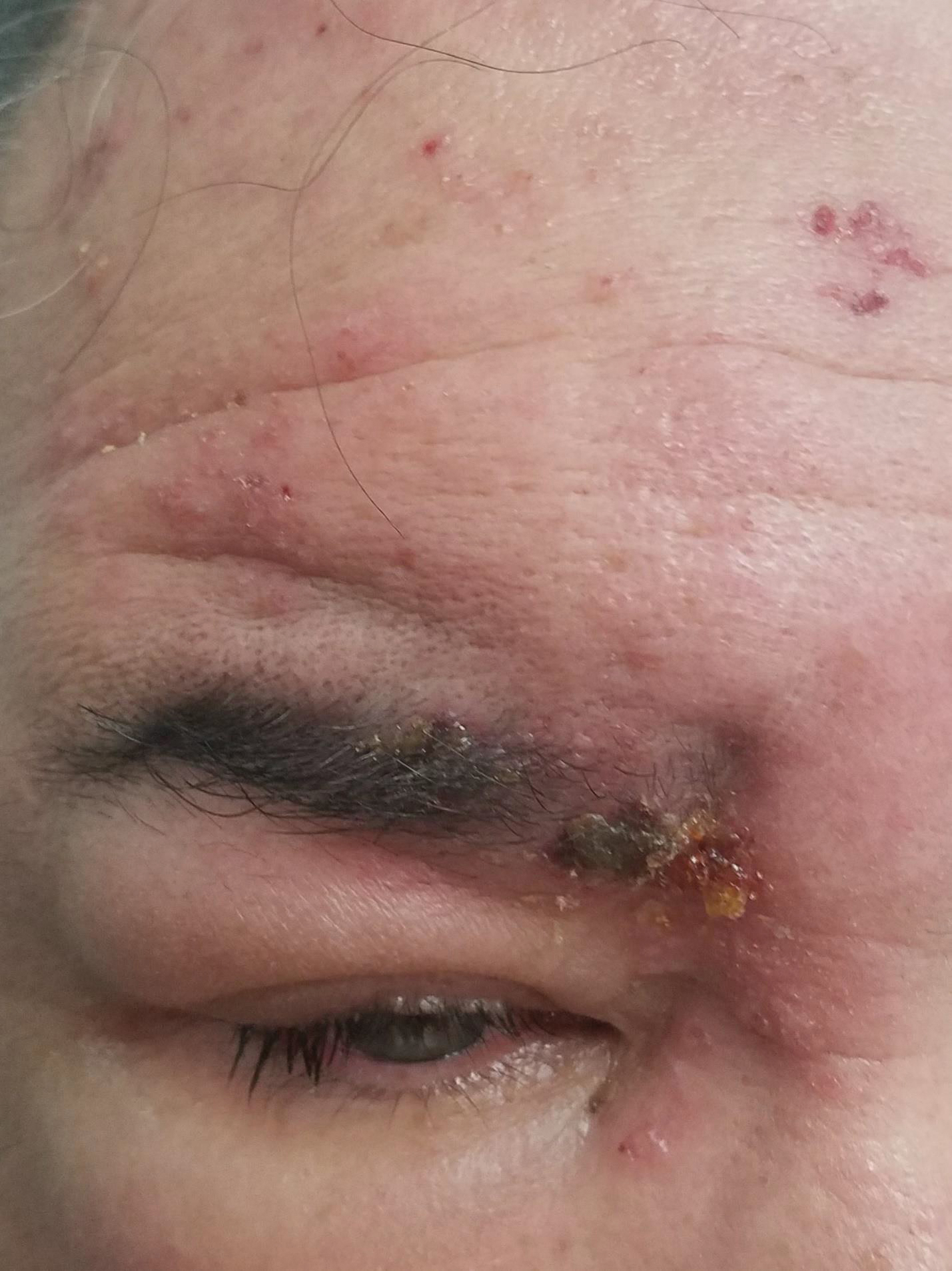
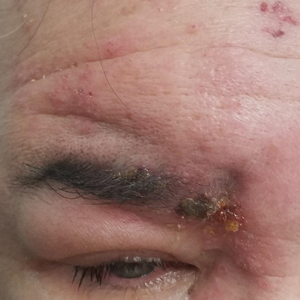
Periorbital Swelling and Rash Following Trauma
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
A 56-year-old man presented to an urgent care clinic with right periorbital swelling. He reported hitting his head on the door to a storage unit 2 days prior but did not lose consciousness. The swelling presented 2 days later. He reported mild headache and swelling around the right eye that coincided with an uncomfortable rash on the face and scalp. He also reported visual disruption due to the swelling but denied any eye pain, discharge from the eye, or painful eye movements. He had no lesions on the lips or inside the mouth. He denied any history of skin conditions. He further denied fever, joint pain, or any other systemic symptoms. His chronic medical conditions included diabetes mellitus, hypertension, and hyperlipidemia that were stable on metformin, carvedilol, amlodipine, enalapril, and simvastatin, which he had taken for several years. He had not started any new medications, and there were no recent changes in the dosing of his medications.
For pediatric use of supplements, rely on resources, evidence
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Zoledronate promotes postdenosumab bone retention
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
WHO declares public health emergency for novel coronavirus
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
February 2020: Question 2
Q2. Correct Answer: C
Rationale
Carvedilol is a nonselective beta-blocker with vasodilating properties that is used to decrease portal pressure and prevent first variceal hemorrhage. It has more robust effect on the reduction of portal pressure than nadolol or propranolol. A safe and effective dose is 12.5 mg/day. Doses higher than 12.5 mg a day are associated with increased side effects and hypotension in patients with impaired liver function caused alpha1 antagonist action and excessive first pass metabolism.
References
1. Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009 Sep;50(3):825-33.
2. Carvedilol (coreg) package insert Philadelphia. SmithKline Beecham Pharmaceuticals. May 1997.
ginews@gastro.org
Q2. Correct Answer: C
Rationale
Carvedilol is a nonselective beta-blocker with vasodilating properties that is used to decrease portal pressure and prevent first variceal hemorrhage. It has more robust effect on the reduction of portal pressure than nadolol or propranolol. A safe and effective dose is 12.5 mg/day. Doses higher than 12.5 mg a day are associated with increased side effects and hypotension in patients with impaired liver function caused alpha1 antagonist action and excessive first pass metabolism.
References
1. Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009 Sep;50(3):825-33.
2. Carvedilol (coreg) package insert Philadelphia. SmithKline Beecham Pharmaceuticals. May 1997.
ginews@gastro.org
Q2. Correct Answer: C
Rationale
Carvedilol is a nonselective beta-blocker with vasodilating properties that is used to decrease portal pressure and prevent first variceal hemorrhage. It has more robust effect on the reduction of portal pressure than nadolol or propranolol. A safe and effective dose is 12.5 mg/day. Doses higher than 12.5 mg a day are associated with increased side effects and hypotension in patients with impaired liver function caused alpha1 antagonist action and excessive first pass metabolism.
References
1. Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009 Sep;50(3):825-33.
2. Carvedilol (coreg) package insert Philadelphia. SmithKline Beecham Pharmaceuticals. May 1997.
ginews@gastro.org
Q2.
February 2020: Question 1
Q1. Correct Answer: B
Rationale
The leading cause of death in patients with NASH is cardiovascular disease. Death from liver-related causes is much more common in NASH than in the general population, but is not the leading cause of death. Cancer-related death is among the top three causes of death in patients with NASH, but is not the most common.
References
1. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21.
2. Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Disease. Hepatology 2018;67:328-57.
Q1. Correct Answer: B
Rationale
The leading cause of death in patients with NASH is cardiovascular disease. Death from liver-related causes is much more common in NASH than in the general population, but is not the leading cause of death. Cancer-related death is among the top three causes of death in patients with NASH, but is not the most common.
References
1. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21.
2. Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Disease. Hepatology 2018;67:328-57.
Q1. Correct Answer: B
Rationale
The leading cause of death in patients with NASH is cardiovascular disease. Death from liver-related causes is much more common in NASH than in the general population, but is not the leading cause of death. Cancer-related death is among the top three causes of death in patients with NASH, but is not the most common.
References
1. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21.
2. Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Disease. Hepatology 2018;67:328-57.
You recently diagnosed a 66-year-old man with cirrhosis due to nonalcoholic steatohepatitis. The patient presents to your clinic now inquiring about his long-term prognosis.
2019 Novel Coronavirus: Frequently asked questions for clinicians
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.