User login
Shared medical appointments educate and encourage MS patients
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
WEST PALM BEACH, FLA. – according to a presentation at the Americas Committee for Treatment and Research in Multiple Sclerosis.
“At first, this may sound to patients like an awkward concept – they may say, ‘Why would I want to have a medical appointment with other people?’ ” Mary R. Rensel, MD, who is the director of the program at the Mellen Center for Multiple Sclerosis, Cleveland Clinic Foundation, said. “But once they get there, it’s wonderful to see what happens – patients start to encourage each other and share resources, and it’s enjoyable for the patients and providers alike,” she said.
The main objective of the shared appointments concept was to increase education regarding comorbidity prevention and management of MS, however, importantly, if patients wish to discuss any issues privately, they are accommodated. In addition, family members, children, and caregivers are all welcome to attend. “Caregivers need support as well, so their participation is welcome,” Dr. Rensel said.
A significant benefit of the program is the extended time with providers – an hour and a half – which is a substantially longer period than patients and providers typically spend together, Dr. Rensel noted. “Medical visits are often so rushed, but this gives us much more time together, to learn more and talk about things like brain health,” she said.
With guidance from a multidisciplinary team including nurses, wellness providers, psychologists, and other experts, there are currently seven meeting themes that are rotated through the year, focusing on a variety of subjects. One, for instance, includes education from a nutritionist, and the center includes a kitchen for the group to learn about and try recipes. Other sessions include chair yoga, art therapy, guided imagery, and exercise physiology.
The Cleveland Clinic is a leader in the concept of SMA and offers it to as many as 360 disease states. With the pilot program now underway for more than 3 years, Dr. Rensel and her team conducted a study to investigate its effects.
For the study, the authors collected clinical data on 50 patients who had attended at least one session between January 2016 and June 2019. Among the patients, 94% were female, 80% had relapsing-remitting MS, and mean age was 50. Patients had a mean Determined Disease Steps (PDSS) score of 3.1 plus or minus 2.4 and the average 25-foot walk and nine-hole peg test (dominant hand) times were 9.4 plus or minus 7.8 seconds and 25.8 plus or minus 9.1 seconds, respectively.
The most common comorbidity was depression/anxiety, occurring in 44% of patients, however after participation in the shared medical appointment program, their mean Patient Health Questionnaire-9 scores, with higher scores indicative of worse depression, decreased from pretreatment scores of 7.3 plus or minus 5.5 to posttreatment scores of 5.1 plus or minus 5.6 (P = .001).
Notably, the program appears to have had a positive effect on patients’ use of health care services – while there was a significant decrease in the mean number of emergency room visits (n = 13 to n = 2; P = .0005), the results showed a favorable increase in mean number of follow-up visits with attendees’ primary care providers (n = 19 to n = 41; P = 3.47), physical therapists (n = 15 to n = 27; P = .004), or psychologists (n = 6 to n = 19; P = .003).
“The study was to evaluate the effect of the program after even just one appointment, and we found it really seemed to increase the use of more appropriate care, with less ER utilization and more visits to primary care,” Dr. Rensel said. The study even showed a small but significant reduction in pre- and postoutcome body mass index (BMI, 30.2 plus or minus 7.3 vs. 28.8 plus or minus 7.1; P = .03).
A critical metric that was not measured in the study – the effect of social interaction and camaraderie in a condition that can, for many, feel socially isolating – is clearly profound, Dr. Rensel said.
Amar Dhand, MD, associate professor of neurology at Brigham and Women’s Hospital, Harvard University, Boston, agreed that the peer support in such medical group settings can be highly valuable.
“Shared medical appointments offer an opportunity for peer-to-peer engagement, support, and education,” he said in an interview. “For many patients, this is a chance to bond with persons who are coexperiencing similar problems, allowing new social connections to emerge.”
Dr. Dhand, who spoke on the issue of the importance of social networks at the meeting, noted that, although there are numerous benefits with shared medical appointments, not all patients may respond well.
“Health care settings are one place to stimulate community among peers. This is one important ingredient of addressing social isolation,” he said. “However, there remain challenges such as sustainability of such relationships, paradoxical depression when persons see others with more severe disease, and infrastructure to support such programs.”
The findings from the study, however, do suggest favorable responses, he noted.
“I think, mechanistically, improved psychosocial outcomes are the most pertinent to the intervention,” Dr. Dhand said. “The health care utilization may be attributed to other factors and will need to be assessed in a case control design.”
Key benefits of the shared medical appointment concept
A recent article from Cleveland Clinic researchers reviewing the concept of shared medical appointments summarizes that the programs offer benefits based on nine key principles:
- Group exposure in shared medical appointments combats isolation, which in turn helps to remove doubts about one’s ability to manage illness.
- Patients learn about disease self-management vicariously by witnessing others’ illness experiences.
- Patients feel inspired by seeing others who are coping well.
- Group dynamics lead patients and providers to developing more equitable relationships.
- Providers feel increased appreciation and rapport toward colleagues leading to increased efficiency.
- Providers learn from the patients how better to meet their patients’ needs.
- Adequate time allotment of the SMA leads patients to feel supported.
- Patients receive professional expertise from the provider in combination with firsthand information from peers, resulting in more robust health knowledge.
- Patients have the opportunity to see how the physicians interact with fellow patients, which allows them to get to know the physician and better determine their level of trust.
The take-home message from the shared medical appointments concept is that “it may hit a quadruple aim,” Dr. Rensel said. “Access, cost, outcomes, and provider satisfaction.”
The Shared Medical Appointments program received a grant from Genzyme. Dr. Rensel reported consulting or advisory board relationships with Serono, Biogen, Teva, Genzyme, Novartis, and the National Multiple Sclerosis Society. Dr. Dhand had no disclosures to report.
REPORTING FROM ACTRIMS FORUM 2020
Is Transfer Always the Best Choice?
Some veterans who present to smaller facilities, such as rural hospitals, are transferred to larger facilities for diagnostic or therapeutic procedures. But that access also can mean hardship for rural veterans by taking them far from family and adding costs. Moreover, complex care coordination can cause “triage mismatch” when the patients are at their most vulnerable: “over-triage”—transferring patients unlikely to benefit and “under-triage”—failing to transfer those likely to benefit.
Researchers from VA Iowa City Healthcare System and University of Iowa conducted a study to find out what proportion of VHA transfers were potentially avoidable. Their study included all veterans treated in any of 120 VHA emergency departments (EDs) and transferred to a VHA acute care hospital between January 2012 and December 2014.
Potentially avoidable transfers (PATs) were defined as transfers in which the patient was either discharged from the referral ED or admitted to the referral hospital for < 24 hours, without having an invasive procedure. The researchers chose that definition to identify patients whose transfer might have been avoided if real-time specialty telemedicine were available at the index hospital. (They caution that the definition was not intended to suggest that all PATs were inappropriate.)
Over 3 years, 18,852 patients were transferred. Of the total patients transferred, 36% were transferred from 1 VHA ED to another VHA facility. Of the VHA transfers, 8,639 (46%) were transferred to another VHA ED; the rest were transferred to another VHA facility inpatient unit. The median transfer distance was 81.5 miles. Rural residents were transferred 3 times as often as urban residents.
The good news is that PATs are rare. Only 0.8% of VHA ED visits resulted in transfer, and of those, only one-fourth were deemed potentially avoidable. And while rural veterans were more likely to be transferred, PATs were less prevalent among those transfers (20.8% vs 23.9% for urban veterans).
More than half of VHA transfers were for patients diagnosed with mental health, cardiac, and digestive conditions. The top ICD-9 diagnosis related to VHA ED transfer was suicidal ideation. The diagnostic procedures associated with most PATs were mental health (11% potentially avoidable) and cardiac (21% potentially avoidable).
Their research turned up some unexpected data: For example, smaller EDs did not have a higher prevalence of PATs, suggesting that ED size was not associated with transfer appropriateness. And the proportion of PATs was higher in hospitals with > 50% board-certified emergency physicians.
The researchers say their findings highlight important differences between the VHA health care and civilian health care systems, emphasizing that the resources available within the VHA health system “might be unique” and underlining the need for VHA-specific solutions to health care delivery challenges.
The overall purpose of this study, the researchers say, was to identify areas where novel delivery of specialty care might reduce the need for some VHA transfers. Their analysis provides data for developing targeted intervention, such as ED-based telemedicine or “targeted remote care.”
Patients with mental health conditions—who made up more than one-third of all VHA-to-VHA interfacility transfers, higher than that reported in civilian hospitals—represent a “rich target population” for telehealth, the researchers suggest. They also note that because mental health providers are in critical shortage in most of the US, real-time telemedicine providing psychiatric resources could be an important and timely service.
Nearly half of medical directors of VHA EDs who responded to the VHA Healthcare Analysis and Information Group survey cited the transfer process as “overly burdensome,” and > 65% said administrative processes contribute to delay in transfer. Finding new ways to keep patients local could benefit providers as well.
Some veterans who present to smaller facilities, such as rural hospitals, are transferred to larger facilities for diagnostic or therapeutic procedures. But that access also can mean hardship for rural veterans by taking them far from family and adding costs. Moreover, complex care coordination can cause “triage mismatch” when the patients are at their most vulnerable: “over-triage”—transferring patients unlikely to benefit and “under-triage”—failing to transfer those likely to benefit.
Researchers from VA Iowa City Healthcare System and University of Iowa conducted a study to find out what proportion of VHA transfers were potentially avoidable. Their study included all veterans treated in any of 120 VHA emergency departments (EDs) and transferred to a VHA acute care hospital between January 2012 and December 2014.
Potentially avoidable transfers (PATs) were defined as transfers in which the patient was either discharged from the referral ED or admitted to the referral hospital for < 24 hours, without having an invasive procedure. The researchers chose that definition to identify patients whose transfer might have been avoided if real-time specialty telemedicine were available at the index hospital. (They caution that the definition was not intended to suggest that all PATs were inappropriate.)
Over 3 years, 18,852 patients were transferred. Of the total patients transferred, 36% were transferred from 1 VHA ED to another VHA facility. Of the VHA transfers, 8,639 (46%) were transferred to another VHA ED; the rest were transferred to another VHA facility inpatient unit. The median transfer distance was 81.5 miles. Rural residents were transferred 3 times as often as urban residents.
The good news is that PATs are rare. Only 0.8% of VHA ED visits resulted in transfer, and of those, only one-fourth were deemed potentially avoidable. And while rural veterans were more likely to be transferred, PATs were less prevalent among those transfers (20.8% vs 23.9% for urban veterans).
More than half of VHA transfers were for patients diagnosed with mental health, cardiac, and digestive conditions. The top ICD-9 diagnosis related to VHA ED transfer was suicidal ideation. The diagnostic procedures associated with most PATs were mental health (11% potentially avoidable) and cardiac (21% potentially avoidable).
Their research turned up some unexpected data: For example, smaller EDs did not have a higher prevalence of PATs, suggesting that ED size was not associated with transfer appropriateness. And the proportion of PATs was higher in hospitals with > 50% board-certified emergency physicians.
The researchers say their findings highlight important differences between the VHA health care and civilian health care systems, emphasizing that the resources available within the VHA health system “might be unique” and underlining the need for VHA-specific solutions to health care delivery challenges.
The overall purpose of this study, the researchers say, was to identify areas where novel delivery of specialty care might reduce the need for some VHA transfers. Their analysis provides data for developing targeted intervention, such as ED-based telemedicine or “targeted remote care.”
Patients with mental health conditions—who made up more than one-third of all VHA-to-VHA interfacility transfers, higher than that reported in civilian hospitals—represent a “rich target population” for telehealth, the researchers suggest. They also note that because mental health providers are in critical shortage in most of the US, real-time telemedicine providing psychiatric resources could be an important and timely service.
Nearly half of medical directors of VHA EDs who responded to the VHA Healthcare Analysis and Information Group survey cited the transfer process as “overly burdensome,” and > 65% said administrative processes contribute to delay in transfer. Finding new ways to keep patients local could benefit providers as well.
Some veterans who present to smaller facilities, such as rural hospitals, are transferred to larger facilities for diagnostic or therapeutic procedures. But that access also can mean hardship for rural veterans by taking them far from family and adding costs. Moreover, complex care coordination can cause “triage mismatch” when the patients are at their most vulnerable: “over-triage”—transferring patients unlikely to benefit and “under-triage”—failing to transfer those likely to benefit.
Researchers from VA Iowa City Healthcare System and University of Iowa conducted a study to find out what proportion of VHA transfers were potentially avoidable. Their study included all veterans treated in any of 120 VHA emergency departments (EDs) and transferred to a VHA acute care hospital between January 2012 and December 2014.
Potentially avoidable transfers (PATs) were defined as transfers in which the patient was either discharged from the referral ED or admitted to the referral hospital for < 24 hours, without having an invasive procedure. The researchers chose that definition to identify patients whose transfer might have been avoided if real-time specialty telemedicine were available at the index hospital. (They caution that the definition was not intended to suggest that all PATs were inappropriate.)
Over 3 years, 18,852 patients were transferred. Of the total patients transferred, 36% were transferred from 1 VHA ED to another VHA facility. Of the VHA transfers, 8,639 (46%) were transferred to another VHA ED; the rest were transferred to another VHA facility inpatient unit. The median transfer distance was 81.5 miles. Rural residents were transferred 3 times as often as urban residents.
The good news is that PATs are rare. Only 0.8% of VHA ED visits resulted in transfer, and of those, only one-fourth were deemed potentially avoidable. And while rural veterans were more likely to be transferred, PATs were less prevalent among those transfers (20.8% vs 23.9% for urban veterans).
More than half of VHA transfers were for patients diagnosed with mental health, cardiac, and digestive conditions. The top ICD-9 diagnosis related to VHA ED transfer was suicidal ideation. The diagnostic procedures associated with most PATs were mental health (11% potentially avoidable) and cardiac (21% potentially avoidable).
Their research turned up some unexpected data: For example, smaller EDs did not have a higher prevalence of PATs, suggesting that ED size was not associated with transfer appropriateness. And the proportion of PATs was higher in hospitals with > 50% board-certified emergency physicians.
The researchers say their findings highlight important differences between the VHA health care and civilian health care systems, emphasizing that the resources available within the VHA health system “might be unique” and underlining the need for VHA-specific solutions to health care delivery challenges.
The overall purpose of this study, the researchers say, was to identify areas where novel delivery of specialty care might reduce the need for some VHA transfers. Their analysis provides data for developing targeted intervention, such as ED-based telemedicine or “targeted remote care.”
Patients with mental health conditions—who made up more than one-third of all VHA-to-VHA interfacility transfers, higher than that reported in civilian hospitals—represent a “rich target population” for telehealth, the researchers suggest. They also note that because mental health providers are in critical shortage in most of the US, real-time telemedicine providing psychiatric resources could be an important and timely service.
Nearly half of medical directors of VHA EDs who responded to the VHA Healthcare Analysis and Information Group survey cited the transfer process as “overly burdensome,” and > 65% said administrative processes contribute to delay in transfer. Finding new ways to keep patients local could benefit providers as well.
New study suggests milk could increase breast cancer risk
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Angiosarcoma Imitating a Morpheaform Basal Cell Carcinoma
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
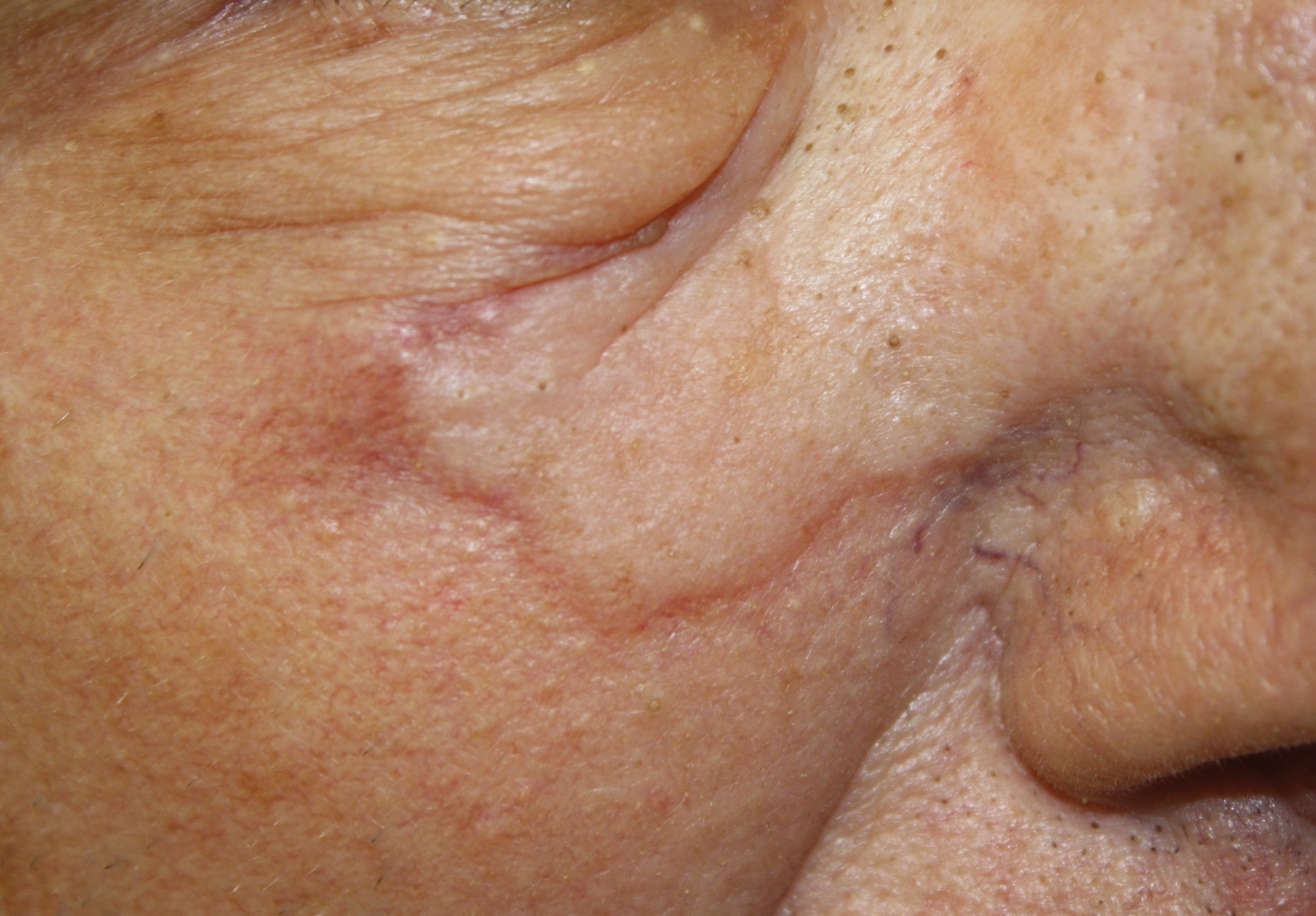
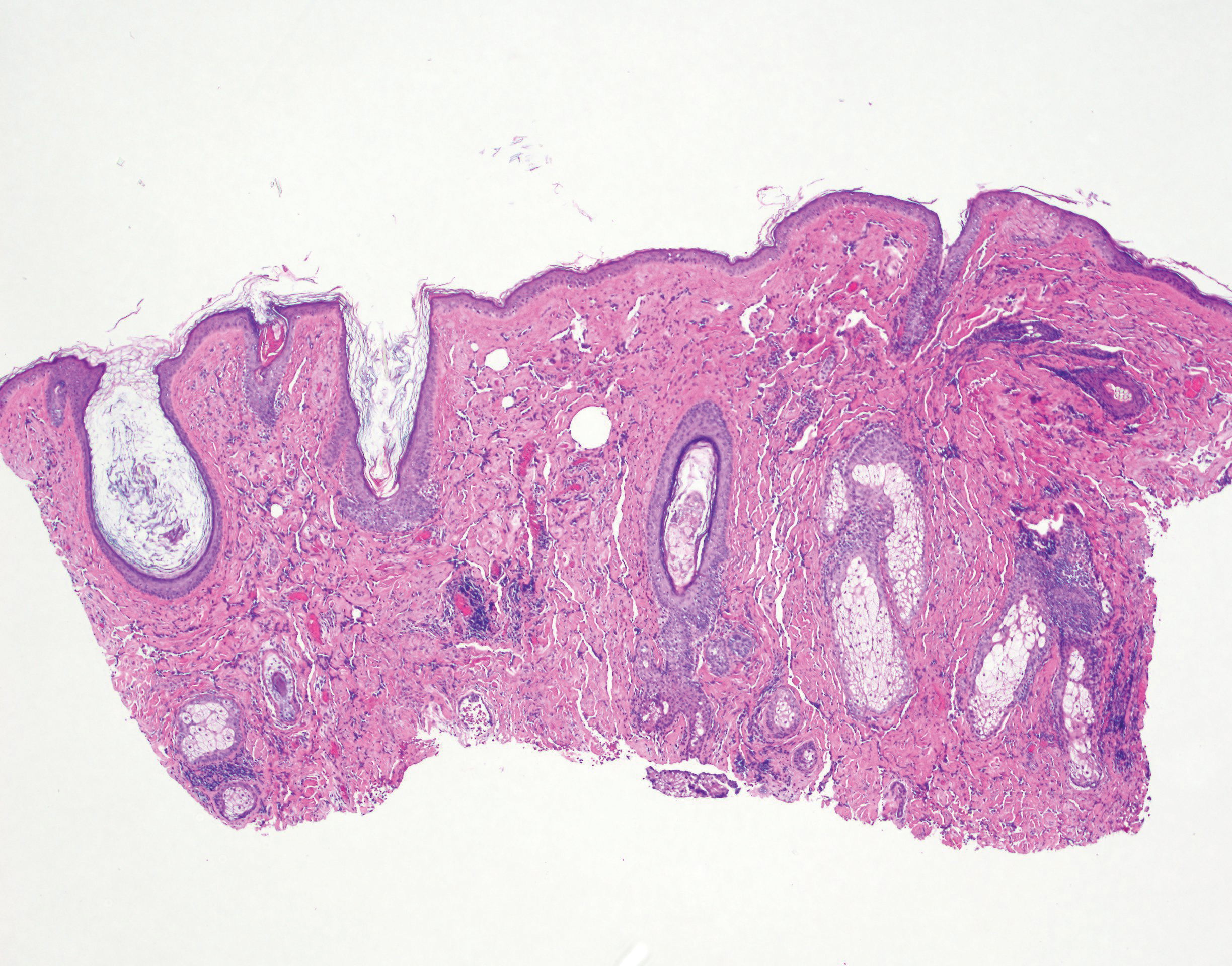
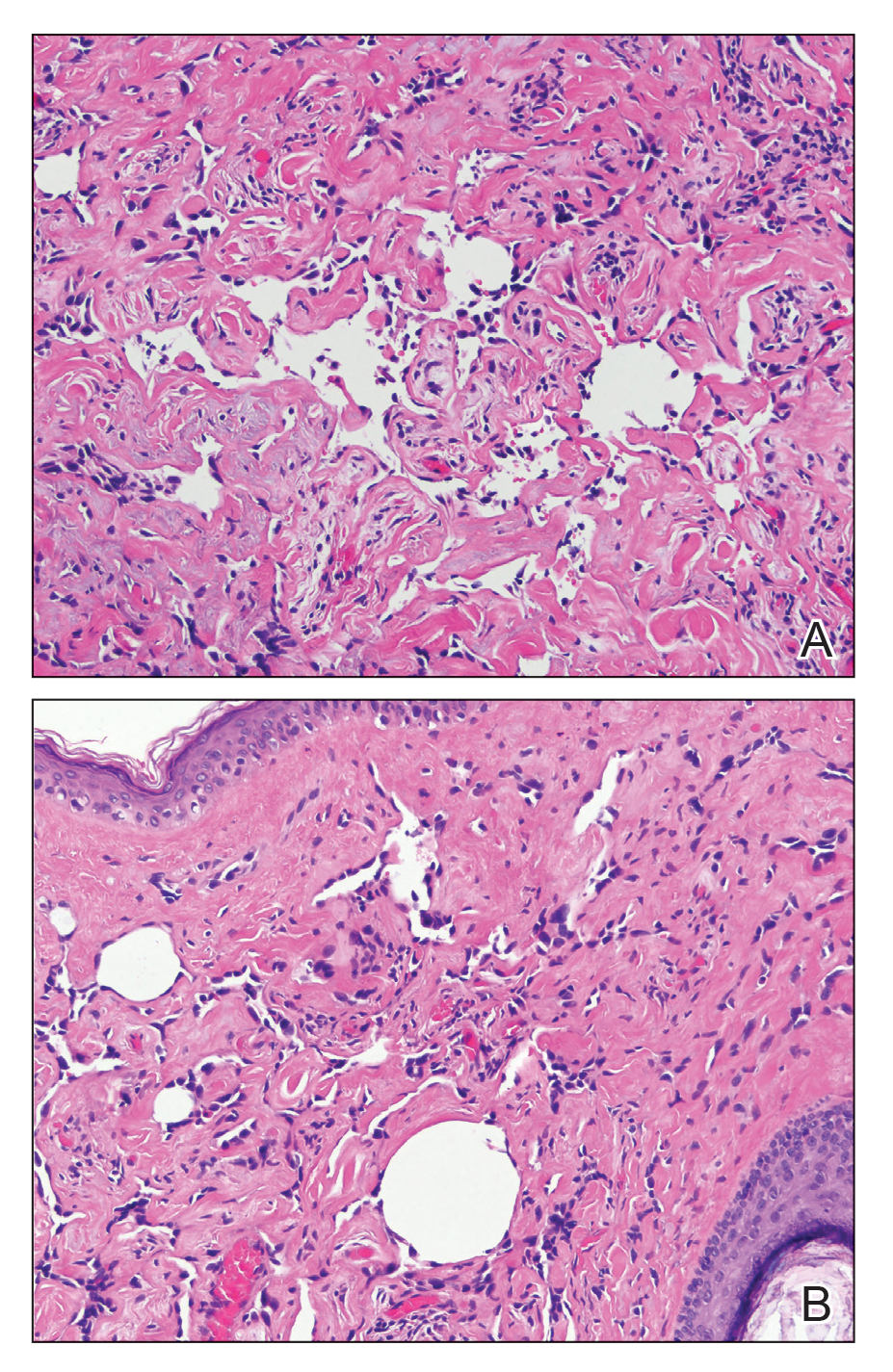
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
Practice Points
- Angiosarcoma is an aggressive vascular tumor with a poor prognosis.
- Angiosarcomas can arise in the setting of chronic lymphedema or prior radiation therapy or can arise spontaneously.
- Classically, angiosarcoma presents as a violaceous patch or plaque but occasionally can exhibit atypical clinical features. Angiosarcomas should be considered on the differential for any changing plaque on the head or neck.
Sharp climb in weight gain after breast cancer diagnosis
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
‘Promising’ responses with preoperative immunotherapy in oral cavity cancer
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
Can this patient get IV contrast?
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
Mortality sevenfold higher post TAVR with severe kidney injury
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
REPORTING FROM CRT 2020
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
HLA-B27 status predicts radiographic phenotype of axSpA
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
FROM ARTHRITIS CARE & RESEARCH