User login
Lasers expunge mucosal tattoos
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
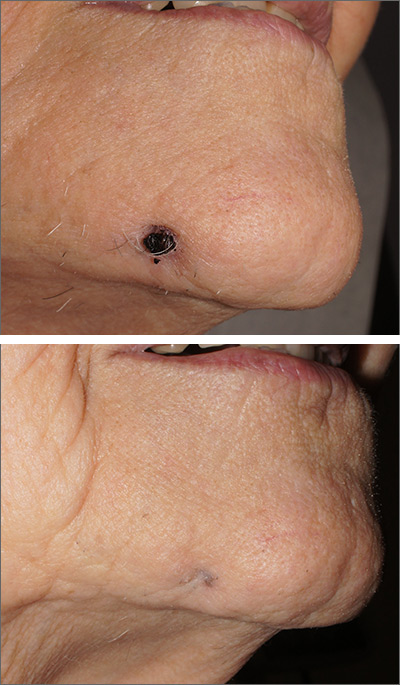
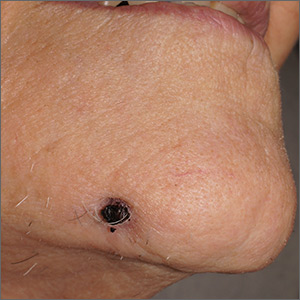
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
FROM LASERS IN SURGERY AND MEDICINE
Be ready for patient questions on sunscreen safety, SPF choice
ORLANDO – Dermatologists should be well versed in addressing common concerns that patients, family members, and the media have about photoprotection, Adam Friedman, MD, advised at the ODAC Dermatology, Aesthetic, & Surgical Conference.
“Know the controversies. Be armed and ready when these patients come to your office with questions,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview at the meeting, where he presented on issues related to photoprotection.
Sunscreen SPFs above 50 don’t technically provide a “meaningful” increase in ultraviolet protection, given that this value relates to filtering about 98% of UVB, but they still can provide some benefit, which has to do with real-world human error, Dr. Friedman said.
“Most people don’t use sunscreens the right way,” meaning they don’t apply enough to achieve the SPF listed, he added in the interview. “A higher SPF is meaningful, because if they apply less [sunscreen], they actually still are in that safety window,” with the higher SPF sunscreen. (The American Academy of Dermatology recommends an SPF of 30 or higher.) Several studies have shown that a SPF of 70 or 100 is superior to 50, likely because of this “dilutional” effect.
Patients may have concerns about the effects of sunscreen on vitamin D production, the environment, and hair loss, and whether they have endocrine disrupting effects, added Dr. Friedman, who is also the medical director of the meeting.
Inhibition of cutaneous vitamin D synthesis after using sunscreen can vary, based on whether a person has properly applied sunscreen, the season, latitude, and an individual’s age and obesity level. Patients with low vitamin D levels can use a vitamin D supplement to achieve sufficient levels, and patients concerned about the impact of sunscreen and vitamin D can be advised to take 600 IU of vitamin D3 a day, according to Dr. Friedman. Some studies have suggested that UVB exposure and risk of certain cancers are inversely correlated, implicating cutaneous vitamin D synthesis (J Clin Transl Endocrinol. 2014 Oct 5;1[4]:179-86). But correlation does not equal causation, he pointed out.
Other concerns stem from the potential for oxybenzone, a UVA/UVB filter in more than 70% of sunscreens, to act as an endocrine disruptor in people and whether it is potentially damaging the environment. The data driving these concerns “stem from the bench, not the real world,” Dr. Friedman said. While topical application of oxybenzone can result in systemic absorption, and even though it’s been detected in waters that are heavily populated or where people go on vacation, there is no evidence demonstrating toxicity to humans or the coral reefs. “At least the information we have to date says they don’t,” he added.
In a randomized clinical trial recently published in JAMA, Food and Drug Administration investigators found that systemic skin absorption with geometric mean plasma concentrations greater than 0.5 ng/mL with six active ingredients in sunscreen that were tested, including oxybenzone (JAMA. 2020;323[3]:256-7). The study was part of an FDA proposed rule requesting additional information on sunscreen ingredients; the plasma concentrations exceeded the level at which further safety studies could potentially be waived.
The study, Dr. Friedman said, “only demonstrated the ability to detect these UV filters at very small concentrations in the blood. They have yet to show any meaningful biologic correlation to these findings.”
For those patients who prefer not to use chemical filters, Dr. Friedman suggests recommending mineral-based sunscreens, of which he said micro- and nanoparticulate formulations offer the best cosmesis by sitting more evenly on the skin, being more amenable to thinner and less-lipophilic vehicles, and limiting visible light scattering (thereby limiting the unsightly white appearance) – while maintaining UV scattering efficacy. However, controversy has emerged as there are past studies that posit the theoretical danger of nanoparticles in sunscreens, given their potential to penetrate the skin and enter cells.
But continually emerging evidence has shown that commercially available nanosunscreens are safe, with no toxicity even at the cellular level when applied to the skin in sunscreen or in cosmetics. “All evidence to date suggests they do not do this,” Dr. Friedman said, noting that, in Europe, the European Commission’s Scientific Committee on Consumer Safety has stated that nanoparticles below a concentration of 25% in sunscreens is safe, “just don’t put them in aerosolized forms.”
Lastly, while some recent studies have detected titanium dioxide on the hair shafts of patients with and without frontal fibrosing alopecia, Dr. Friedman noted more evidence is needed before recommending that these patients avoid using sunscreen (Br J Dermatol. 2019 Jul;181[1]:216-7). “Correlation does not mean causation, and the current dogma is that there’s no connection between these two,” he commented.
Dr. Friedman reported consulting and advisory board relationships with numerous companies; he also reported speaking for Regeneron, Abbvie, and Dermira, and receiving grants with Pfizer and DF Pharma.
ORLANDO – Dermatologists should be well versed in addressing common concerns that patients, family members, and the media have about photoprotection, Adam Friedman, MD, advised at the ODAC Dermatology, Aesthetic, & Surgical Conference.
“Know the controversies. Be armed and ready when these patients come to your office with questions,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview at the meeting, where he presented on issues related to photoprotection.
Sunscreen SPFs above 50 don’t technically provide a “meaningful” increase in ultraviolet protection, given that this value relates to filtering about 98% of UVB, but they still can provide some benefit, which has to do with real-world human error, Dr. Friedman said.
“Most people don’t use sunscreens the right way,” meaning they don’t apply enough to achieve the SPF listed, he added in the interview. “A higher SPF is meaningful, because if they apply less [sunscreen], they actually still are in that safety window,” with the higher SPF sunscreen. (The American Academy of Dermatology recommends an SPF of 30 or higher.) Several studies have shown that a SPF of 70 or 100 is superior to 50, likely because of this “dilutional” effect.
Patients may have concerns about the effects of sunscreen on vitamin D production, the environment, and hair loss, and whether they have endocrine disrupting effects, added Dr. Friedman, who is also the medical director of the meeting.
Inhibition of cutaneous vitamin D synthesis after using sunscreen can vary, based on whether a person has properly applied sunscreen, the season, latitude, and an individual’s age and obesity level. Patients with low vitamin D levels can use a vitamin D supplement to achieve sufficient levels, and patients concerned about the impact of sunscreen and vitamin D can be advised to take 600 IU of vitamin D3 a day, according to Dr. Friedman. Some studies have suggested that UVB exposure and risk of certain cancers are inversely correlated, implicating cutaneous vitamin D synthesis (J Clin Transl Endocrinol. 2014 Oct 5;1[4]:179-86). But correlation does not equal causation, he pointed out.
Other concerns stem from the potential for oxybenzone, a UVA/UVB filter in more than 70% of sunscreens, to act as an endocrine disruptor in people and whether it is potentially damaging the environment. The data driving these concerns “stem from the bench, not the real world,” Dr. Friedman said. While topical application of oxybenzone can result in systemic absorption, and even though it’s been detected in waters that are heavily populated or where people go on vacation, there is no evidence demonstrating toxicity to humans or the coral reefs. “At least the information we have to date says they don’t,” he added.
In a randomized clinical trial recently published in JAMA, Food and Drug Administration investigators found that systemic skin absorption with geometric mean plasma concentrations greater than 0.5 ng/mL with six active ingredients in sunscreen that were tested, including oxybenzone (JAMA. 2020;323[3]:256-7). The study was part of an FDA proposed rule requesting additional information on sunscreen ingredients; the plasma concentrations exceeded the level at which further safety studies could potentially be waived.
The study, Dr. Friedman said, “only demonstrated the ability to detect these UV filters at very small concentrations in the blood. They have yet to show any meaningful biologic correlation to these findings.”
For those patients who prefer not to use chemical filters, Dr. Friedman suggests recommending mineral-based sunscreens, of which he said micro- and nanoparticulate formulations offer the best cosmesis by sitting more evenly on the skin, being more amenable to thinner and less-lipophilic vehicles, and limiting visible light scattering (thereby limiting the unsightly white appearance) – while maintaining UV scattering efficacy. However, controversy has emerged as there are past studies that posit the theoretical danger of nanoparticles in sunscreens, given their potential to penetrate the skin and enter cells.
But continually emerging evidence has shown that commercially available nanosunscreens are safe, with no toxicity even at the cellular level when applied to the skin in sunscreen or in cosmetics. “All evidence to date suggests they do not do this,” Dr. Friedman said, noting that, in Europe, the European Commission’s Scientific Committee on Consumer Safety has stated that nanoparticles below a concentration of 25% in sunscreens is safe, “just don’t put them in aerosolized forms.”
Lastly, while some recent studies have detected titanium dioxide on the hair shafts of patients with and without frontal fibrosing alopecia, Dr. Friedman noted more evidence is needed before recommending that these patients avoid using sunscreen (Br J Dermatol. 2019 Jul;181[1]:216-7). “Correlation does not mean causation, and the current dogma is that there’s no connection between these two,” he commented.
Dr. Friedman reported consulting and advisory board relationships with numerous companies; he also reported speaking for Regeneron, Abbvie, and Dermira, and receiving grants with Pfizer and DF Pharma.
ORLANDO – Dermatologists should be well versed in addressing common concerns that patients, family members, and the media have about photoprotection, Adam Friedman, MD, advised at the ODAC Dermatology, Aesthetic, & Surgical Conference.
“Know the controversies. Be armed and ready when these patients come to your office with questions,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview at the meeting, where he presented on issues related to photoprotection.
Sunscreen SPFs above 50 don’t technically provide a “meaningful” increase in ultraviolet protection, given that this value relates to filtering about 98% of UVB, but they still can provide some benefit, which has to do with real-world human error, Dr. Friedman said.
“Most people don’t use sunscreens the right way,” meaning they don’t apply enough to achieve the SPF listed, he added in the interview. “A higher SPF is meaningful, because if they apply less [sunscreen], they actually still are in that safety window,” with the higher SPF sunscreen. (The American Academy of Dermatology recommends an SPF of 30 or higher.) Several studies have shown that a SPF of 70 or 100 is superior to 50, likely because of this “dilutional” effect.
Patients may have concerns about the effects of sunscreen on vitamin D production, the environment, and hair loss, and whether they have endocrine disrupting effects, added Dr. Friedman, who is also the medical director of the meeting.
Inhibition of cutaneous vitamin D synthesis after using sunscreen can vary, based on whether a person has properly applied sunscreen, the season, latitude, and an individual’s age and obesity level. Patients with low vitamin D levels can use a vitamin D supplement to achieve sufficient levels, and patients concerned about the impact of sunscreen and vitamin D can be advised to take 600 IU of vitamin D3 a day, according to Dr. Friedman. Some studies have suggested that UVB exposure and risk of certain cancers are inversely correlated, implicating cutaneous vitamin D synthesis (J Clin Transl Endocrinol. 2014 Oct 5;1[4]:179-86). But correlation does not equal causation, he pointed out.
Other concerns stem from the potential for oxybenzone, a UVA/UVB filter in more than 70% of sunscreens, to act as an endocrine disruptor in people and whether it is potentially damaging the environment. The data driving these concerns “stem from the bench, not the real world,” Dr. Friedman said. While topical application of oxybenzone can result in systemic absorption, and even though it’s been detected in waters that are heavily populated or where people go on vacation, there is no evidence demonstrating toxicity to humans or the coral reefs. “At least the information we have to date says they don’t,” he added.
In a randomized clinical trial recently published in JAMA, Food and Drug Administration investigators found that systemic skin absorption with geometric mean plasma concentrations greater than 0.5 ng/mL with six active ingredients in sunscreen that were tested, including oxybenzone (JAMA. 2020;323[3]:256-7). The study was part of an FDA proposed rule requesting additional information on sunscreen ingredients; the plasma concentrations exceeded the level at which further safety studies could potentially be waived.
The study, Dr. Friedman said, “only demonstrated the ability to detect these UV filters at very small concentrations in the blood. They have yet to show any meaningful biologic correlation to these findings.”
For those patients who prefer not to use chemical filters, Dr. Friedman suggests recommending mineral-based sunscreens, of which he said micro- and nanoparticulate formulations offer the best cosmesis by sitting more evenly on the skin, being more amenable to thinner and less-lipophilic vehicles, and limiting visible light scattering (thereby limiting the unsightly white appearance) – while maintaining UV scattering efficacy. However, controversy has emerged as there are past studies that posit the theoretical danger of nanoparticles in sunscreens, given their potential to penetrate the skin and enter cells.
But continually emerging evidence has shown that commercially available nanosunscreens are safe, with no toxicity even at the cellular level when applied to the skin in sunscreen or in cosmetics. “All evidence to date suggests they do not do this,” Dr. Friedman said, noting that, in Europe, the European Commission’s Scientific Committee on Consumer Safety has stated that nanoparticles below a concentration of 25% in sunscreens is safe, “just don’t put them in aerosolized forms.”
Lastly, while some recent studies have detected titanium dioxide on the hair shafts of patients with and without frontal fibrosing alopecia, Dr. Friedman noted more evidence is needed before recommending that these patients avoid using sunscreen (Br J Dermatol. 2019 Jul;181[1]:216-7). “Correlation does not mean causation, and the current dogma is that there’s no connection between these two,” he commented.
Dr. Friedman reported consulting and advisory board relationships with numerous companies; he also reported speaking for Regeneron, Abbvie, and Dermira, and receiving grants with Pfizer and DF Pharma.
EXPERT ANALYSIS FROM ODAC 2020
High-dose chemo offers survival benefit only for highest-risk breast cancer
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
FROM JAMA ONCOLOGY
Key clinical point: High-dose chemotherapy offers a long-term breast cancer survival advantage only for women with very-high-risk disease.
Major finding: The absolute 20-year overall survival benefit for women with 10 or more involved lymph nodes was 14.6%.
Study details: Long-term, follow-up study of 885 women under age 56 years with stage III breast cancer treated with adjuvant high- or conventional-dose chemotherapy.
Disclosures: The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr. Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
Source: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
Understanding postpartum psychosis: From course to treatment
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Silent ischemia isn’t what it used to be
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Docs weigh pulling out of MIPS over paltry payments
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Risk of Suicide in the Year After an ED Visit
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
CDC: Risk in U.S. from 2019-nCoV remains low
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
Draining papule on chin
A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.
A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE