User login
Cannabis use in pregnancy and lactation: A changing landscape
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
CDC: Five confirmed 2019-nCoV cases in the U.S.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
FFR use nearly halved 1-year mortality risk in ischemic heart disease
Use of fractional flow reserve significantly improved 1-year mortality rates in adults with stable ischemic heart disease, according to a review of 17,989 patients.
Although fractional flow reserve (FFR) has demonstrated value in guiding coronary revascularization, its impact on outcomes in patients with stable ischemic heart disease has not been well studied in a large population, wrote Rushi V. Parikh, MD, of the University of California, Los Angeles, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers analyzed data from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program for adults who underwent coronary angiography between January 2009 and September 2017. The study included patients with angiographically intermediate disease, defined as 40%-69% diameter stenosis on visual inspection.
The rate of FFR use increased from 14.8% to 18.5% during the study period for all patients with intermediate lesions, and from 44% to 75% for those who had percutaneous coronary intervention, the researchers wrote.
Overall, based on hazard models, 1-year mortality was significantly lower in patients who underwent FFR, compared with those who had angiography only (2.8% vs. 5.9%; P less than 0.001). In addition, FFR use in revascularization was associated with a 43% reduced 1-year mortality risk, compared with angiography only.
The findings were limited by several factors, including the observational nature of the study, inability to distinguish between cardiovascular and noncardiovascular mortality, lack of data on the technical performance of the FFR, and a relatively short follow-up period, the researchers noted.
However, the results were strengthened by the large sample size, and support the use of FFR-guided revascularization in patients with angiographically intermediate stenosis, they wrote.
“Future registry-based studies accounting for all physiologic modalities are warranted to accurately quantify the landscape of coronary physiology-guided revascularization,” they added.
The study was supported in part by the Rocky Mountain Regional VA Medical Center in Aurora, Colo. Lead author Dr. Parikh had no financial conflicts to disclose.
SOURCE: Parikh RV et al. J Am Coll Cardiol. 2020 Feb 4;75:409-19.
Although the study suggests that the use of fractional flow reserve (FFR) has increased, it remains underused despite evidence and recommendations, wrote Julien Adjedj, MD, and Benoit Guillon, MD, in an accompanying editorial (J Am Coll Cardiol. 2020 Feb 4;75:420-1).
“Of course, time, cost, and need for hyperemia are often perceived as stumbling blocks. Yet, the real question is whether the cardiology community – not only interventional cardiologists – has truly adopted FFR (i.e., using it routinely and treating according to the results),” they wrote.
The editorialists noted that, in this study, typical predictors of FFR included younger age, multivessel or left main disease, previous history of percutaneous coronary intervention, no heart failure, and higher left ventricular ejection fraction.
“However, neither the absence of documented ischemia nor the presence of symptoms influenced the use of FFR. Significant site-level variation in FFR was observed,” they wrote. “This important finding suggests that the main reason for FFR underutilization in the contemporary era is operator belief regarding the utility of coronary physiology, and that revised reimbursement policies and additional education/training may not have a meaningful impact on FFR adoption.”
The editorialists emphasized that, although FFR use has increased, the findings of a significant decrease in mortality support additional use of FFR “and good reasons to do so.”
Dr. Adjedj is affiliated with the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, and had no financial conflicts to disclose. Dr. Guillon is affiliated with the University Hospital Jean Minjoz in Besançon, France, and disclosed a grant from Sanofi and participation in a conference for Abbott.
Although the study suggests that the use of fractional flow reserve (FFR) has increased, it remains underused despite evidence and recommendations, wrote Julien Adjedj, MD, and Benoit Guillon, MD, in an accompanying editorial (J Am Coll Cardiol. 2020 Feb 4;75:420-1).
“Of course, time, cost, and need for hyperemia are often perceived as stumbling blocks. Yet, the real question is whether the cardiology community – not only interventional cardiologists – has truly adopted FFR (i.e., using it routinely and treating according to the results),” they wrote.
The editorialists noted that, in this study, typical predictors of FFR included younger age, multivessel or left main disease, previous history of percutaneous coronary intervention, no heart failure, and higher left ventricular ejection fraction.
“However, neither the absence of documented ischemia nor the presence of symptoms influenced the use of FFR. Significant site-level variation in FFR was observed,” they wrote. “This important finding suggests that the main reason for FFR underutilization in the contemporary era is operator belief regarding the utility of coronary physiology, and that revised reimbursement policies and additional education/training may not have a meaningful impact on FFR adoption.”
The editorialists emphasized that, although FFR use has increased, the findings of a significant decrease in mortality support additional use of FFR “and good reasons to do so.”
Dr. Adjedj is affiliated with the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, and had no financial conflicts to disclose. Dr. Guillon is affiliated with the University Hospital Jean Minjoz in Besançon, France, and disclosed a grant from Sanofi and participation in a conference for Abbott.
Although the study suggests that the use of fractional flow reserve (FFR) has increased, it remains underused despite evidence and recommendations, wrote Julien Adjedj, MD, and Benoit Guillon, MD, in an accompanying editorial (J Am Coll Cardiol. 2020 Feb 4;75:420-1).
“Of course, time, cost, and need for hyperemia are often perceived as stumbling blocks. Yet, the real question is whether the cardiology community – not only interventional cardiologists – has truly adopted FFR (i.e., using it routinely and treating according to the results),” they wrote.
The editorialists noted that, in this study, typical predictors of FFR included younger age, multivessel or left main disease, previous history of percutaneous coronary intervention, no heart failure, and higher left ventricular ejection fraction.
“However, neither the absence of documented ischemia nor the presence of symptoms influenced the use of FFR. Significant site-level variation in FFR was observed,” they wrote. “This important finding suggests that the main reason for FFR underutilization in the contemporary era is operator belief regarding the utility of coronary physiology, and that revised reimbursement policies and additional education/training may not have a meaningful impact on FFR adoption.”
The editorialists emphasized that, although FFR use has increased, the findings of a significant decrease in mortality support additional use of FFR “and good reasons to do so.”
Dr. Adjedj is affiliated with the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, and had no financial conflicts to disclose. Dr. Guillon is affiliated with the University Hospital Jean Minjoz in Besançon, France, and disclosed a grant from Sanofi and participation in a conference for Abbott.
Use of fractional flow reserve significantly improved 1-year mortality rates in adults with stable ischemic heart disease, according to a review of 17,989 patients.
Although fractional flow reserve (FFR) has demonstrated value in guiding coronary revascularization, its impact on outcomes in patients with stable ischemic heart disease has not been well studied in a large population, wrote Rushi V. Parikh, MD, of the University of California, Los Angeles, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers analyzed data from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program for adults who underwent coronary angiography between January 2009 and September 2017. The study included patients with angiographically intermediate disease, defined as 40%-69% diameter stenosis on visual inspection.
The rate of FFR use increased from 14.8% to 18.5% during the study period for all patients with intermediate lesions, and from 44% to 75% for those who had percutaneous coronary intervention, the researchers wrote.
Overall, based on hazard models, 1-year mortality was significantly lower in patients who underwent FFR, compared with those who had angiography only (2.8% vs. 5.9%; P less than 0.001). In addition, FFR use in revascularization was associated with a 43% reduced 1-year mortality risk, compared with angiography only.
The findings were limited by several factors, including the observational nature of the study, inability to distinguish between cardiovascular and noncardiovascular mortality, lack of data on the technical performance of the FFR, and a relatively short follow-up period, the researchers noted.
However, the results were strengthened by the large sample size, and support the use of FFR-guided revascularization in patients with angiographically intermediate stenosis, they wrote.
“Future registry-based studies accounting for all physiologic modalities are warranted to accurately quantify the landscape of coronary physiology-guided revascularization,” they added.
The study was supported in part by the Rocky Mountain Regional VA Medical Center in Aurora, Colo. Lead author Dr. Parikh had no financial conflicts to disclose.
SOURCE: Parikh RV et al. J Am Coll Cardiol. 2020 Feb 4;75:409-19.
Use of fractional flow reserve significantly improved 1-year mortality rates in adults with stable ischemic heart disease, according to a review of 17,989 patients.
Although fractional flow reserve (FFR) has demonstrated value in guiding coronary revascularization, its impact on outcomes in patients with stable ischemic heart disease has not been well studied in a large population, wrote Rushi V. Parikh, MD, of the University of California, Los Angeles, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers analyzed data from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program for adults who underwent coronary angiography between January 2009 and September 2017. The study included patients with angiographically intermediate disease, defined as 40%-69% diameter stenosis on visual inspection.
The rate of FFR use increased from 14.8% to 18.5% during the study period for all patients with intermediate lesions, and from 44% to 75% for those who had percutaneous coronary intervention, the researchers wrote.
Overall, based on hazard models, 1-year mortality was significantly lower in patients who underwent FFR, compared with those who had angiography only (2.8% vs. 5.9%; P less than 0.001). In addition, FFR use in revascularization was associated with a 43% reduced 1-year mortality risk, compared with angiography only.
The findings were limited by several factors, including the observational nature of the study, inability to distinguish between cardiovascular and noncardiovascular mortality, lack of data on the technical performance of the FFR, and a relatively short follow-up period, the researchers noted.
However, the results were strengthened by the large sample size, and support the use of FFR-guided revascularization in patients with angiographically intermediate stenosis, they wrote.
“Future registry-based studies accounting for all physiologic modalities are warranted to accurately quantify the landscape of coronary physiology-guided revascularization,” they added.
The study was supported in part by the Rocky Mountain Regional VA Medical Center in Aurora, Colo. Lead author Dr. Parikh had no financial conflicts to disclose.
SOURCE: Parikh RV et al. J Am Coll Cardiol. 2020 Feb 4;75:409-19.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Use of fractional flow reserve to assist in revascularization significantly reduced the 1-year mortality risk in patients with stable ischemic heart disease.
Major finding:
Study details: The data come from a review of 17,989 patients who underwent coronary angiography during 2009-2017.
Disclosures: The study was supported in part by the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora, Colo. Lead author Dr. Parikh had no financial conflicts to disclose.
Source: Parikh RV et al. J Am Coll Cardiol. 2020 Feb 4;75:409-19.
Vigilance safely keeps AFib patients off anticoagulants post ablation
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM THE AF SYMPOSIUM 2020
FDA approves fidaxomicin for treatment of C. difficile–associated diarrhea
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
Zika virus: Birth defects rose fourfold in U.S. hardest-hit areas
according to the Centers for Disease Control and Prevention.
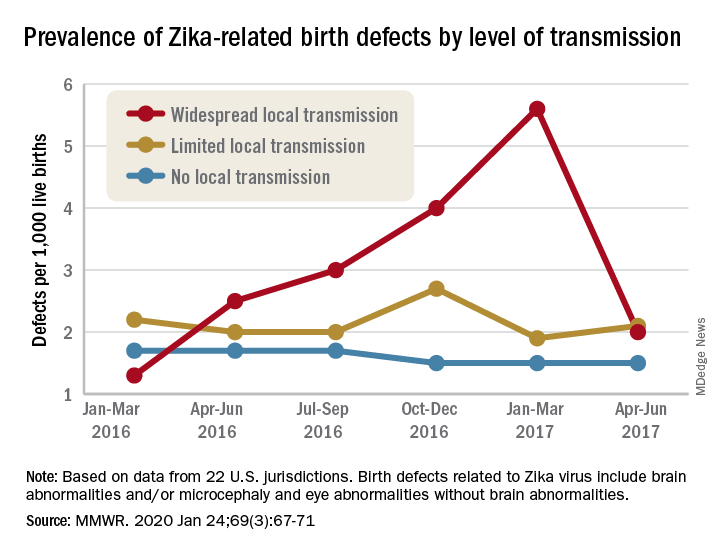
That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
FROM MMWR
Doctor wins $4.75 million award in defamation suit against hospital
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Cisplatin-gemcitabine is highly active in BRCA/PALB2+ pancreatic cancer
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Barbers have role in encouraging diabetes screening in black men
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Barbershops could offer a way to encourage diabetes screening among black men.
Major finding: HbA1c testing in barbershops identified a significant number of individuals with undiagnosed diabetes.
Study details: Study involving 895 black men attending eight barbershops in Brooklyn.
Disclosures: The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
Source: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Sociodemographic disadvantage confers poorer survival in young adults with CRC
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM














