User login
GAO Finds DoD Can Do More to Recruit and Retain Physicians and Dentists
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
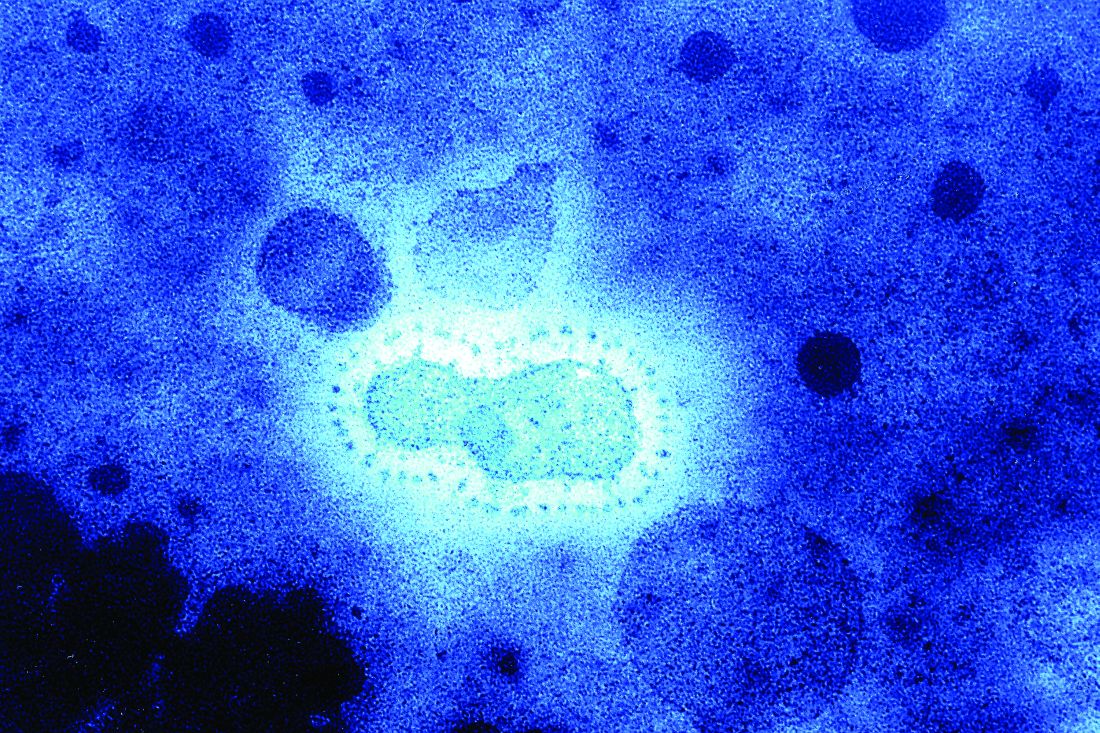
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
FDA: Cybersecurity vulnerabilities identified in GE Healthcare monitoring devices
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
Neurologic disease doesn’t discriminate against anyone
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
New Vascepa indication opens up treatment to millions; “Most significant event since statins”
The newly approved U.S. indication for icosapent ethyl (Vascepa; Amarin) is broadly in line with the entry criteria for the REDUCE-IT trial and includes a large high-risk primary-prevention population, as well as those with established cardiovascular disease (CVD). The drug, thus, could well be used by millions of patients in the United States alone.
The high-dose, purified eicosapentaenoic acid product was approved last week by the Food and Drug Administration for cardiovascular risk reduction among adults already taking maximally tolerated statins with triglyceride levels of 150 mg/dL or higher who have either established CVD or diabetes and two or more additional risk factors for CVD.
The approval is based largely on the REDUCE-IT trial’s finding of a 25% reduction in risk for major adverse cardiovascular events versus placebo. The FDA stated that the approval is the first for an agent with this specific indication.
Noting that it recognizes the need for additional medical treatments for CVD, the FDA says the new approval “will give patients with elevated triglycerides and other important risk factors, including heart disease, stroke, and diabetes, an adjunctive treatment option that can help decrease their risk of cardiovascular events.”
The drug was unanimously recommended for approval by the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee last month. But while the committee all agreed on its use in patients with established CVD, which made up 70% of the REDUCE-IT population, they were divided on whether the indication should be extended to the high-risk primary-prevention population, who made up just 30% of patients in the study.
Nonetheless, the FDA has gone for a broad indication based on the whole REDUCE-IT population.
In a conference call following the approval, Steven Ketchum, PhD, chief scientific officer at Amarin, pointed out that the primary-prevention population stipulated in the new approval differed very slightly from the REDUCE-IT enrollment criteria.
The trial specified that patients with diabetes should be older than 50 with one other cardiovascular risk factor, whereas the approved population is for diabetes and two cardiovascular risk factors. But as these two risk factors are not specified, they could include age, cigarette smoking, hypertension or use of an antihypertensive agent, low HDL cholesterol, high C-reactive protein, body mass index above 25 kg/m2, renal dysfunction, retinopathy, albuminuria, or an ankle branchial index below 0.9, Dr. Ketchum said.
“So while the label asks for two other risk factors, one of these could be age; so we believe the label is actually slightly broader than the REDUCE-IT inclusion criteria, and doctors have been left with significant leeway to decide which risk factors to consider on top of diabetes.”
Deepak Bhatt, MD, the lead investigator of REDUCE-IT, described the Vascepa approval as “the most significant event in the field of cardiovascular prevention since the introduction of statins nearly 3 decades ago.”
He commended the FDA on “a very evidence-based, prescriber-friendly, and most importantly, patient-friendly label,” which he said was in line with guidelines from multiple professional societies that have already incorporated the REDUCE-IT findings for secondary prevention and diabetic primary prevention.
Dr. Bhatt, who is a professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, Boston, said the label essentially matches the REDUCE-IT population.
“The entry criteria for REDUCE-IT was fasting triglycerides greater than or equal to 150 mg/dL, with a 10% variance allowed (giving a minimum triglyceride value of 135 mg/dL). In actuality, we ended up with about 10% of the population with triglycerides between 100 and 150 mg/dL, and they had a similar degree of benefit as those with higher levels,” he reported.
“In the label, the 150 mg/dL does not specify fasting, and in fact many practices have moved away from fasting lipid measurements for the sake of patient comfort,” Dr. Bhatt added. “On average, nonfasting levels are about 50 mg/dL higher, so the label essentially mirrors those we studied, with the FDA applying good common sense and not being overly dogmatic about the exact wording of the trial inclusion criteria.”
No price change foreseen
Vascepa is already on the market for patients with very high triglyceride levels, and the company says it is not increasing the current price of about $300 a month, which is “relatively low, compared to other new breakthrough drugs.” However, it says it expects sales to grow from vastly increased volume based on the new indication.
Dr. Bhatt noted that REDUCE-IT cost-effectiveness data presented at the recent American Heart Association scientific sessions found the drug to be cost saving in the majority of cases. “That is something that is quite rare in cost-effectiveness research,” he said.
“Now, the key challenge is to identify and treat appropriate patients,” Dr. Bhatt noted. He says this task will largely fall on cardiologists, endocrinologists, and primary care physicians, though stroke neurologists, nephrologists, and vascular medicine specialists will also have patients for whom the data are relevant.
“I believe the drug will ultimately be widely prescribed, initially by subspecialists, but by primary care physicians also. It is overall very well tolerated, safe, and easy to use,” he said. “Much like statin prescription started in subspecialty practices but then became quite common in primary care, I envision the same happening with icosapent ethyl.”
Lipid expert Roger Blumenthal, MD, who was not involved in the REDUCE-IT trial, also welcomed the new approval for Vascepa.
“The indication is very appropriate; it is great to have another disease-modifying medication in our prevention toolkit,” Dr. Blumenthal, who is director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease in Baltimore, said in an interview.
Some still unsure
But not everyone is in full agreement with the broad indication granted.
One expert who has reservations is James de Lemos, MD, professor of medicine at the University of Texas, Dallas, who sat on the FDA advisory committee that assessed the drug last month.
“I would have preferred a narrower label for now, limited to the secondary prevention indication, because I felt that REDUCE-IT did not include sufficient numbers of patients to justify the high-risk primary-prevention indication. We need an adequately powered, randomized, controlled trial to establish the risk/benefit and cost/benefit in primary prevention, and with this broad label, I worry there will be little incentive for the company to pursue this,” Dr. de Lemos commented in an interview.
“This is a slippery slope, and we should not allow broad indications that extend to primary prevention for drugs that were studied in mixed secondary- and primary-prevention patients, with the results driven by the secondary-prevention subset. These two subgroups are fundamentally different populations in whom the pathophysiology and the background treatments are very different,” he added.
However, Dr. de Lemos acknowledged that he would use Vascepa for some high-risk primary-prevention patients in his practice – those with diabetes, high triglycerides, and multiple risk factors. “I just wish we had more data coming so that I could be more certain of the benefit in this group,” he said.
Dr. Bhatt disclosed sitting on advisory boards for Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio, and Regado Biosciences; conducting unfunded research in association with FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda; and receiving research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, and Forest Laboratories. Dr. De Lemos reported receiving grant and consulting income from Roche Diagnostics and Abbott Diagnostics; and consulting for Ortho Clinical Diagnostics. Dr. Blumenthal has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The newly approved U.S. indication for icosapent ethyl (Vascepa; Amarin) is broadly in line with the entry criteria for the REDUCE-IT trial and includes a large high-risk primary-prevention population, as well as those with established cardiovascular disease (CVD). The drug, thus, could well be used by millions of patients in the United States alone.
The high-dose, purified eicosapentaenoic acid product was approved last week by the Food and Drug Administration for cardiovascular risk reduction among adults already taking maximally tolerated statins with triglyceride levels of 150 mg/dL or higher who have either established CVD or diabetes and two or more additional risk factors for CVD.
The approval is based largely on the REDUCE-IT trial’s finding of a 25% reduction in risk for major adverse cardiovascular events versus placebo. The FDA stated that the approval is the first for an agent with this specific indication.
Noting that it recognizes the need for additional medical treatments for CVD, the FDA says the new approval “will give patients with elevated triglycerides and other important risk factors, including heart disease, stroke, and diabetes, an adjunctive treatment option that can help decrease their risk of cardiovascular events.”
The drug was unanimously recommended for approval by the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee last month. But while the committee all agreed on its use in patients with established CVD, which made up 70% of the REDUCE-IT population, they were divided on whether the indication should be extended to the high-risk primary-prevention population, who made up just 30% of patients in the study.
Nonetheless, the FDA has gone for a broad indication based on the whole REDUCE-IT population.
In a conference call following the approval, Steven Ketchum, PhD, chief scientific officer at Amarin, pointed out that the primary-prevention population stipulated in the new approval differed very slightly from the REDUCE-IT enrollment criteria.
The trial specified that patients with diabetes should be older than 50 with one other cardiovascular risk factor, whereas the approved population is for diabetes and two cardiovascular risk factors. But as these two risk factors are not specified, they could include age, cigarette smoking, hypertension or use of an antihypertensive agent, low HDL cholesterol, high C-reactive protein, body mass index above 25 kg/m2, renal dysfunction, retinopathy, albuminuria, or an ankle branchial index below 0.9, Dr. Ketchum said.
“So while the label asks for two other risk factors, one of these could be age; so we believe the label is actually slightly broader than the REDUCE-IT inclusion criteria, and doctors have been left with significant leeway to decide which risk factors to consider on top of diabetes.”
Deepak Bhatt, MD, the lead investigator of REDUCE-IT, described the Vascepa approval as “the most significant event in the field of cardiovascular prevention since the introduction of statins nearly 3 decades ago.”
He commended the FDA on “a very evidence-based, prescriber-friendly, and most importantly, patient-friendly label,” which he said was in line with guidelines from multiple professional societies that have already incorporated the REDUCE-IT findings for secondary prevention and diabetic primary prevention.
Dr. Bhatt, who is a professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, Boston, said the label essentially matches the REDUCE-IT population.
“The entry criteria for REDUCE-IT was fasting triglycerides greater than or equal to 150 mg/dL, with a 10% variance allowed (giving a minimum triglyceride value of 135 mg/dL). In actuality, we ended up with about 10% of the population with triglycerides between 100 and 150 mg/dL, and they had a similar degree of benefit as those with higher levels,” he reported.
“In the label, the 150 mg/dL does not specify fasting, and in fact many practices have moved away from fasting lipid measurements for the sake of patient comfort,” Dr. Bhatt added. “On average, nonfasting levels are about 50 mg/dL higher, so the label essentially mirrors those we studied, with the FDA applying good common sense and not being overly dogmatic about the exact wording of the trial inclusion criteria.”
No price change foreseen
Vascepa is already on the market for patients with very high triglyceride levels, and the company says it is not increasing the current price of about $300 a month, which is “relatively low, compared to other new breakthrough drugs.” However, it says it expects sales to grow from vastly increased volume based on the new indication.
Dr. Bhatt noted that REDUCE-IT cost-effectiveness data presented at the recent American Heart Association scientific sessions found the drug to be cost saving in the majority of cases. “That is something that is quite rare in cost-effectiveness research,” he said.
“Now, the key challenge is to identify and treat appropriate patients,” Dr. Bhatt noted. He says this task will largely fall on cardiologists, endocrinologists, and primary care physicians, though stroke neurologists, nephrologists, and vascular medicine specialists will also have patients for whom the data are relevant.
“I believe the drug will ultimately be widely prescribed, initially by subspecialists, but by primary care physicians also. It is overall very well tolerated, safe, and easy to use,” he said. “Much like statin prescription started in subspecialty practices but then became quite common in primary care, I envision the same happening with icosapent ethyl.”
Lipid expert Roger Blumenthal, MD, who was not involved in the REDUCE-IT trial, also welcomed the new approval for Vascepa.
“The indication is very appropriate; it is great to have another disease-modifying medication in our prevention toolkit,” Dr. Blumenthal, who is director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease in Baltimore, said in an interview.
Some still unsure
But not everyone is in full agreement with the broad indication granted.
One expert who has reservations is James de Lemos, MD, professor of medicine at the University of Texas, Dallas, who sat on the FDA advisory committee that assessed the drug last month.
“I would have preferred a narrower label for now, limited to the secondary prevention indication, because I felt that REDUCE-IT did not include sufficient numbers of patients to justify the high-risk primary-prevention indication. We need an adequately powered, randomized, controlled trial to establish the risk/benefit and cost/benefit in primary prevention, and with this broad label, I worry there will be little incentive for the company to pursue this,” Dr. de Lemos commented in an interview.
“This is a slippery slope, and we should not allow broad indications that extend to primary prevention for drugs that were studied in mixed secondary- and primary-prevention patients, with the results driven by the secondary-prevention subset. These two subgroups are fundamentally different populations in whom the pathophysiology and the background treatments are very different,” he added.
However, Dr. de Lemos acknowledged that he would use Vascepa for some high-risk primary-prevention patients in his practice – those with diabetes, high triglycerides, and multiple risk factors. “I just wish we had more data coming so that I could be more certain of the benefit in this group,” he said.
Dr. Bhatt disclosed sitting on advisory boards for Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio, and Regado Biosciences; conducting unfunded research in association with FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda; and receiving research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, and Forest Laboratories. Dr. De Lemos reported receiving grant and consulting income from Roche Diagnostics and Abbott Diagnostics; and consulting for Ortho Clinical Diagnostics. Dr. Blumenthal has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The newly approved U.S. indication for icosapent ethyl (Vascepa; Amarin) is broadly in line with the entry criteria for the REDUCE-IT trial and includes a large high-risk primary-prevention population, as well as those with established cardiovascular disease (CVD). The drug, thus, could well be used by millions of patients in the United States alone.
The high-dose, purified eicosapentaenoic acid product was approved last week by the Food and Drug Administration for cardiovascular risk reduction among adults already taking maximally tolerated statins with triglyceride levels of 150 mg/dL or higher who have either established CVD or diabetes and two or more additional risk factors for CVD.
The approval is based largely on the REDUCE-IT trial’s finding of a 25% reduction in risk for major adverse cardiovascular events versus placebo. The FDA stated that the approval is the first for an agent with this specific indication.
Noting that it recognizes the need for additional medical treatments for CVD, the FDA says the new approval “will give patients with elevated triglycerides and other important risk factors, including heart disease, stroke, and diabetes, an adjunctive treatment option that can help decrease their risk of cardiovascular events.”
The drug was unanimously recommended for approval by the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee last month. But while the committee all agreed on its use in patients with established CVD, which made up 70% of the REDUCE-IT population, they were divided on whether the indication should be extended to the high-risk primary-prevention population, who made up just 30% of patients in the study.
Nonetheless, the FDA has gone for a broad indication based on the whole REDUCE-IT population.
In a conference call following the approval, Steven Ketchum, PhD, chief scientific officer at Amarin, pointed out that the primary-prevention population stipulated in the new approval differed very slightly from the REDUCE-IT enrollment criteria.
The trial specified that patients with diabetes should be older than 50 with one other cardiovascular risk factor, whereas the approved population is for diabetes and two cardiovascular risk factors. But as these two risk factors are not specified, they could include age, cigarette smoking, hypertension or use of an antihypertensive agent, low HDL cholesterol, high C-reactive protein, body mass index above 25 kg/m2, renal dysfunction, retinopathy, albuminuria, or an ankle branchial index below 0.9, Dr. Ketchum said.
“So while the label asks for two other risk factors, one of these could be age; so we believe the label is actually slightly broader than the REDUCE-IT inclusion criteria, and doctors have been left with significant leeway to decide which risk factors to consider on top of diabetes.”
Deepak Bhatt, MD, the lead investigator of REDUCE-IT, described the Vascepa approval as “the most significant event in the field of cardiovascular prevention since the introduction of statins nearly 3 decades ago.”
He commended the FDA on “a very evidence-based, prescriber-friendly, and most importantly, patient-friendly label,” which he said was in line with guidelines from multiple professional societies that have already incorporated the REDUCE-IT findings for secondary prevention and diabetic primary prevention.
Dr. Bhatt, who is a professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, Boston, said the label essentially matches the REDUCE-IT population.
“The entry criteria for REDUCE-IT was fasting triglycerides greater than or equal to 150 mg/dL, with a 10% variance allowed (giving a minimum triglyceride value of 135 mg/dL). In actuality, we ended up with about 10% of the population with triglycerides between 100 and 150 mg/dL, and they had a similar degree of benefit as those with higher levels,” he reported.
“In the label, the 150 mg/dL does not specify fasting, and in fact many practices have moved away from fasting lipid measurements for the sake of patient comfort,” Dr. Bhatt added. “On average, nonfasting levels are about 50 mg/dL higher, so the label essentially mirrors those we studied, with the FDA applying good common sense and not being overly dogmatic about the exact wording of the trial inclusion criteria.”
No price change foreseen
Vascepa is already on the market for patients with very high triglyceride levels, and the company says it is not increasing the current price of about $300 a month, which is “relatively low, compared to other new breakthrough drugs.” However, it says it expects sales to grow from vastly increased volume based on the new indication.
Dr. Bhatt noted that REDUCE-IT cost-effectiveness data presented at the recent American Heart Association scientific sessions found the drug to be cost saving in the majority of cases. “That is something that is quite rare in cost-effectiveness research,” he said.
“Now, the key challenge is to identify and treat appropriate patients,” Dr. Bhatt noted. He says this task will largely fall on cardiologists, endocrinologists, and primary care physicians, though stroke neurologists, nephrologists, and vascular medicine specialists will also have patients for whom the data are relevant.
“I believe the drug will ultimately be widely prescribed, initially by subspecialists, but by primary care physicians also. It is overall very well tolerated, safe, and easy to use,” he said. “Much like statin prescription started in subspecialty practices but then became quite common in primary care, I envision the same happening with icosapent ethyl.”
Lipid expert Roger Blumenthal, MD, who was not involved in the REDUCE-IT trial, also welcomed the new approval for Vascepa.
“The indication is very appropriate; it is great to have another disease-modifying medication in our prevention toolkit,” Dr. Blumenthal, who is director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease in Baltimore, said in an interview.
Some still unsure
But not everyone is in full agreement with the broad indication granted.
One expert who has reservations is James de Lemos, MD, professor of medicine at the University of Texas, Dallas, who sat on the FDA advisory committee that assessed the drug last month.
“I would have preferred a narrower label for now, limited to the secondary prevention indication, because I felt that REDUCE-IT did not include sufficient numbers of patients to justify the high-risk primary-prevention indication. We need an adequately powered, randomized, controlled trial to establish the risk/benefit and cost/benefit in primary prevention, and with this broad label, I worry there will be little incentive for the company to pursue this,” Dr. de Lemos commented in an interview.
“This is a slippery slope, and we should not allow broad indications that extend to primary prevention for drugs that were studied in mixed secondary- and primary-prevention patients, with the results driven by the secondary-prevention subset. These two subgroups are fundamentally different populations in whom the pathophysiology and the background treatments are very different,” he added.
However, Dr. de Lemos acknowledged that he would use Vascepa for some high-risk primary-prevention patients in his practice – those with diabetes, high triglycerides, and multiple risk factors. “I just wish we had more data coming so that I could be more certain of the benefit in this group,” he said.
Dr. Bhatt disclosed sitting on advisory boards for Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio, and Regado Biosciences; conducting unfunded research in association with FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda; and receiving research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, and Forest Laboratories. Dr. De Lemos reported receiving grant and consulting income from Roche Diagnostics and Abbott Diagnostics; and consulting for Ortho Clinical Diagnostics. Dr. Blumenthal has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Blood test may advance diagnosis of GI cancers
SAN FRANCISCO – A new assay based on methylation patterns in plasma cell-free DNA holds promise for improving detection of diverse gastrointestinal (GI) cancers when they are still curable, findings of the Circulating Cell-free Genome Atlas (CCGA) study suggest.
Among 447 patients with GI cancers of varied types and stages, the multicancer assay had sensitivity exceeding 80% for all stages (exceeding 70% for stages I to III) and specificity exceeding 99%, investigators reported at the 2020 GI Cancers Symposium. In addition, the assay correctly determined the tissue of origin about 90% of the time.
“To pursue early detection at a population scale while minimizing harm and cost, several features are important, including a low false-positive rate, a high detection rate, the ability to localize the site of origin of the malignancy, and limiting overdiagnosis,” noted lead investigator Brian M. Wolpin, MD, MPH, director of the Gastrointestinal Cancer Center and director of the Hale Family Center for Pancreatic Cancer Research at Dana-Farber Cancer Institute in Boston. “This multicancer early detection test evaluating cell-free DNA methylation may be a useful test to detect GI cancers and may guide further evaluation and workup.”
About three-quarters of all cancer patients in the study had symptoms that ultimately led to their diagnosis, Dr. Wolpin acknowledged. But the investigators are conducting two large additional studies among asymptomatic populations to better assess the assay’s screening potential: the STRIVE study, which has enrolled nearly 100,000 women undergoing screening mammography, and the SUMMIT study, which is enrolling 50,000 men and women without a known cancer diagnosis, with enrichment of the sample for smokers.
Clinical potential
“The holy grail, of course, is to try to find any test – a blood test, a urine test, a breath test, a stool test – that will allow us to detect cancer at an earlier and more curable stage, and we have hints now that we are headed in the right direction,” session cochair George A. Fisher Jr., MD, PhD, said in an interview. “I don’t know that this is the technology that will do it, but I think it’s superior to the technology just looking at DNA mutations.”
It is a further merit that the assay also has good accuracy in ascertaining the cancer’s tissue of origin, as that should help streamline the diagnostic workup, he agreed.
The assay is already attractive for patients with suspicious lesions or symptoms, but further research will be needed to assess its performance in detecting asymptomatic cancer, and logistic issues would have to be addressed, according to Dr. Fisher of Stanford (Calif.) University.
“As an outright screening test, I don’t think we are there yet. We would have to identify the populations in whom to use the test and come up with some frequency of how often we use it,” he said. Consideration would need to be given to informed consent, especially as some patients may have a positive assay result but a cancer that can’t be found, generating psychological distress.
And cost will come into play. “The problem is, for most stage I cancers you can screen for, they are still very low in likelihood, so you have to screen a lot of people, which means a lot of expense,” he elaborated.
“It will be interesting in the research world to decide when such a test would become a reasonable standard of care, and how regulators will view that and how insurers will view that in terms of cost-efficacy analysis,” Dr. Fisher concluded.
Study details
The CCGA study has enrolled 15,254 participants with and without cancer at 142 U.S and Canadian sites. Participants provide blood samples (at diagnosis for those with cancer) and are followed up for 5 years.
To develop the assay, the investigators performed targeted methylation sequencing of cell-free DNA from plasma and trained an algorithm to use the methylation patterns to detect more than 20 cancer types and classify them based on the organ of origin.
Dr. Wolpin reported results for a CCGA substudy conducted among 2,185 patients with cancer and 2,131 individuals without cancer. About a fifth of the former group had cancers of the GI tract (colon or rectum in 174 patients, pancreas in 123, esophagus in 71, liver or bile duct in 40, stomach in 25, gallbladder in 14). The population was split into training and validation sets.
The assay had a specificity of 99.8% in the entire training set and 99.3% in the entire validation set, corresponding to false-positives in just 0.2% and 0.7% of the individuals without cancer, according to data reported at the symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
For GI cancers, assay sensitivity for stage I-IV disease was 82% in the training set and 81% in the validation set, and sensitivity for stage I-III disease was 73% and 71%, respectively. By specific nonmetastatic stage, sensitivity was about 50% for stage I disease, 75% for stage II disease, and 85% for stage III disease.
Fully 97% of the GI cancers having detectable cell-free DNA were assigned a tissue of origin by the assay. The predicted tissue of origin had an accuracy of 91% in the training set and 89% in the validation set, with similar values across GI cancer types.
Dr. Wolpin disclosed having a relationship with various pharmaceutical companies, including GRAIL, which funded the study. Dr. Fisher disclosed relationships with numerous pharmaceutical companies and that an immediate family member has stock in Seattle Genetics
SOURCE: Wolpin BM et al. 2020 GI Symposium, Abstract 283.
SAN FRANCISCO – A new assay based on methylation patterns in plasma cell-free DNA holds promise for improving detection of diverse gastrointestinal (GI) cancers when they are still curable, findings of the Circulating Cell-free Genome Atlas (CCGA) study suggest.
Among 447 patients with GI cancers of varied types and stages, the multicancer assay had sensitivity exceeding 80% for all stages (exceeding 70% for stages I to III) and specificity exceeding 99%, investigators reported at the 2020 GI Cancers Symposium. In addition, the assay correctly determined the tissue of origin about 90% of the time.
“To pursue early detection at a population scale while minimizing harm and cost, several features are important, including a low false-positive rate, a high detection rate, the ability to localize the site of origin of the malignancy, and limiting overdiagnosis,” noted lead investigator Brian M. Wolpin, MD, MPH, director of the Gastrointestinal Cancer Center and director of the Hale Family Center for Pancreatic Cancer Research at Dana-Farber Cancer Institute in Boston. “This multicancer early detection test evaluating cell-free DNA methylation may be a useful test to detect GI cancers and may guide further evaluation and workup.”
About three-quarters of all cancer patients in the study had symptoms that ultimately led to their diagnosis, Dr. Wolpin acknowledged. But the investigators are conducting two large additional studies among asymptomatic populations to better assess the assay’s screening potential: the STRIVE study, which has enrolled nearly 100,000 women undergoing screening mammography, and the SUMMIT study, which is enrolling 50,000 men and women without a known cancer diagnosis, with enrichment of the sample for smokers.
Clinical potential
“The holy grail, of course, is to try to find any test – a blood test, a urine test, a breath test, a stool test – that will allow us to detect cancer at an earlier and more curable stage, and we have hints now that we are headed in the right direction,” session cochair George A. Fisher Jr., MD, PhD, said in an interview. “I don’t know that this is the technology that will do it, but I think it’s superior to the technology just looking at DNA mutations.”
It is a further merit that the assay also has good accuracy in ascertaining the cancer’s tissue of origin, as that should help streamline the diagnostic workup, he agreed.
The assay is already attractive for patients with suspicious lesions or symptoms, but further research will be needed to assess its performance in detecting asymptomatic cancer, and logistic issues would have to be addressed, according to Dr. Fisher of Stanford (Calif.) University.
“As an outright screening test, I don’t think we are there yet. We would have to identify the populations in whom to use the test and come up with some frequency of how often we use it,” he said. Consideration would need to be given to informed consent, especially as some patients may have a positive assay result but a cancer that can’t be found, generating psychological distress.
And cost will come into play. “The problem is, for most stage I cancers you can screen for, they are still very low in likelihood, so you have to screen a lot of people, which means a lot of expense,” he elaborated.
“It will be interesting in the research world to decide when such a test would become a reasonable standard of care, and how regulators will view that and how insurers will view that in terms of cost-efficacy analysis,” Dr. Fisher concluded.
Study details
The CCGA study has enrolled 15,254 participants with and without cancer at 142 U.S and Canadian sites. Participants provide blood samples (at diagnosis for those with cancer) and are followed up for 5 years.
To develop the assay, the investigators performed targeted methylation sequencing of cell-free DNA from plasma and trained an algorithm to use the methylation patterns to detect more than 20 cancer types and classify them based on the organ of origin.
Dr. Wolpin reported results for a CCGA substudy conducted among 2,185 patients with cancer and 2,131 individuals without cancer. About a fifth of the former group had cancers of the GI tract (colon or rectum in 174 patients, pancreas in 123, esophagus in 71, liver or bile duct in 40, stomach in 25, gallbladder in 14). The population was split into training and validation sets.
The assay had a specificity of 99.8% in the entire training set and 99.3% in the entire validation set, corresponding to false-positives in just 0.2% and 0.7% of the individuals without cancer, according to data reported at the symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
For GI cancers, assay sensitivity for stage I-IV disease was 82% in the training set and 81% in the validation set, and sensitivity for stage I-III disease was 73% and 71%, respectively. By specific nonmetastatic stage, sensitivity was about 50% for stage I disease, 75% for stage II disease, and 85% for stage III disease.
Fully 97% of the GI cancers having detectable cell-free DNA were assigned a tissue of origin by the assay. The predicted tissue of origin had an accuracy of 91% in the training set and 89% in the validation set, with similar values across GI cancer types.
Dr. Wolpin disclosed having a relationship with various pharmaceutical companies, including GRAIL, which funded the study. Dr. Fisher disclosed relationships with numerous pharmaceutical companies and that an immediate family member has stock in Seattle Genetics
SOURCE: Wolpin BM et al. 2020 GI Symposium, Abstract 283.
SAN FRANCISCO – A new assay based on methylation patterns in plasma cell-free DNA holds promise for improving detection of diverse gastrointestinal (GI) cancers when they are still curable, findings of the Circulating Cell-free Genome Atlas (CCGA) study suggest.
Among 447 patients with GI cancers of varied types and stages, the multicancer assay had sensitivity exceeding 80% for all stages (exceeding 70% for stages I to III) and specificity exceeding 99%, investigators reported at the 2020 GI Cancers Symposium. In addition, the assay correctly determined the tissue of origin about 90% of the time.
“To pursue early detection at a population scale while minimizing harm and cost, several features are important, including a low false-positive rate, a high detection rate, the ability to localize the site of origin of the malignancy, and limiting overdiagnosis,” noted lead investigator Brian M. Wolpin, MD, MPH, director of the Gastrointestinal Cancer Center and director of the Hale Family Center for Pancreatic Cancer Research at Dana-Farber Cancer Institute in Boston. “This multicancer early detection test evaluating cell-free DNA methylation may be a useful test to detect GI cancers and may guide further evaluation and workup.”
About three-quarters of all cancer patients in the study had symptoms that ultimately led to their diagnosis, Dr. Wolpin acknowledged. But the investigators are conducting two large additional studies among asymptomatic populations to better assess the assay’s screening potential: the STRIVE study, which has enrolled nearly 100,000 women undergoing screening mammography, and the SUMMIT study, which is enrolling 50,000 men and women without a known cancer diagnosis, with enrichment of the sample for smokers.
Clinical potential
“The holy grail, of course, is to try to find any test – a blood test, a urine test, a breath test, a stool test – that will allow us to detect cancer at an earlier and more curable stage, and we have hints now that we are headed in the right direction,” session cochair George A. Fisher Jr., MD, PhD, said in an interview. “I don’t know that this is the technology that will do it, but I think it’s superior to the technology just looking at DNA mutations.”
It is a further merit that the assay also has good accuracy in ascertaining the cancer’s tissue of origin, as that should help streamline the diagnostic workup, he agreed.
The assay is already attractive for patients with suspicious lesions or symptoms, but further research will be needed to assess its performance in detecting asymptomatic cancer, and logistic issues would have to be addressed, according to Dr. Fisher of Stanford (Calif.) University.
“As an outright screening test, I don’t think we are there yet. We would have to identify the populations in whom to use the test and come up with some frequency of how often we use it,” he said. Consideration would need to be given to informed consent, especially as some patients may have a positive assay result but a cancer that can’t be found, generating psychological distress.
And cost will come into play. “The problem is, for most stage I cancers you can screen for, they are still very low in likelihood, so you have to screen a lot of people, which means a lot of expense,” he elaborated.
“It will be interesting in the research world to decide when such a test would become a reasonable standard of care, and how regulators will view that and how insurers will view that in terms of cost-efficacy analysis,” Dr. Fisher concluded.
Study details
The CCGA study has enrolled 15,254 participants with and without cancer at 142 U.S and Canadian sites. Participants provide blood samples (at diagnosis for those with cancer) and are followed up for 5 years.
To develop the assay, the investigators performed targeted methylation sequencing of cell-free DNA from plasma and trained an algorithm to use the methylation patterns to detect more than 20 cancer types and classify them based on the organ of origin.
Dr. Wolpin reported results for a CCGA substudy conducted among 2,185 patients with cancer and 2,131 individuals without cancer. About a fifth of the former group had cancers of the GI tract (colon or rectum in 174 patients, pancreas in 123, esophagus in 71, liver or bile duct in 40, stomach in 25, gallbladder in 14). The population was split into training and validation sets.
The assay had a specificity of 99.8% in the entire training set and 99.3% in the entire validation set, corresponding to false-positives in just 0.2% and 0.7% of the individuals without cancer, according to data reported at the symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
For GI cancers, assay sensitivity for stage I-IV disease was 82% in the training set and 81% in the validation set, and sensitivity for stage I-III disease was 73% and 71%, respectively. By specific nonmetastatic stage, sensitivity was about 50% for stage I disease, 75% for stage II disease, and 85% for stage III disease.
Fully 97% of the GI cancers having detectable cell-free DNA were assigned a tissue of origin by the assay. The predicted tissue of origin had an accuracy of 91% in the training set and 89% in the validation set, with similar values across GI cancer types.
Dr. Wolpin disclosed having a relationship with various pharmaceutical companies, including GRAIL, which funded the study. Dr. Fisher disclosed relationships with numerous pharmaceutical companies and that an immediate family member has stock in Seattle Genetics
SOURCE: Wolpin BM et al. 2020 GI Symposium, Abstract 283.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Surgeon General scolds docs for failing to help patients quit smoking
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
What will it take to lower the cost of insulin in the United States?
Mayo Clinic hematologist S. Vincent Rajkumar, MD, argues in a new commentary.
The High Cost of Insulin in the United States: An Urgent Call to Action was published in the January 2020 issue of the Mayo Clinic Proceedings by Dr. Rajkumar, professor of medicine at the Mayo Clinic, Rochester, Minn., who specializes in treating myeloma and, more recently, has become an expert in drug pricing.
He also presented the information in a YouTube video.
As has been widely reported and examined by Congress in the past few years, the cost of insulin in the United States has risen at a far higher rate than inflation. For example, the list price of a single vial of Humalog jumped from $21 in 1999 to $275 in 2019, a far higher price than anywhere else in the world.
Stories of one in four patients having to ration their insulin use because of cost, and of some dying, have fueled protests, leading to legislative efforts and to a few initiatives by some of the manufacturers to address the cost problem.
Collective advocacy
Much of the blame has been placed on the manufacturers for charging such high prices and on the pharmacy benefit managers (PBMs) – also known as “middlemen” – for incentivizing higher-priced products on formularies through rebates. Those are major factors, Dr. Rajkumar argues, but they are not the only ones.
“There is no one reason why this is happening, and no one solution. It’s very complicated. It’s multiple factors all playing together. The only way to tackle it is to really understand it 360,” he said in an interview.
This is true of drug prices overall in the United States, but insulin is a special case. The current analog formulations have not changed in more than 20 years, yet only in the past 5 years have a handful of biosimilar and generic versions started to appear from the same manufacturers as the branded products.
“Insulin is a window into what’s wrong with the pharma industry. ... It’s the best example of how the system is broken,” Dr. Rajkumar stressed.
Physicians can help ease the problem, he said, by becoming educated about drug prices, taking cost into account when prescribing, and routinely discussing prescription drug affordability with patients.
Resources such as www.goodrx.com and www.blinkhealth.com provide information about drug prices and pharmacies that offer drugs at the lowest prices.
“Doctors need to not be suspicious of biosimilars and generics,” he emphasized.
Physicians can also advocate for policies that will lower insulin prices, and their institutions can establish preferences for lower-cost biosimilars in formularies.
“Our individual and collective advocacy gives voice to the needs of our patients,” Dr. Rajkumar emphasized.
‘Everyone in the supply chain benefits’
In his commentary, Dr. Rajkumar lists six major reasons for the high cost of insulin:
1. People with type 1 diabetes are a “vulnerable population” who will die without insulin and are therefore willing to pay a high price to stay alive.
2. Just three manufacturers – Eli Lilly, Novo Nordisk, and Sanofi-Aventis – control nearly the entire insulin market in the United States, with no regulations to cap or control the prices they can charge.
3. The manufacturers continually file new patents for existing insulin products – 70 in the case of Lantus, for example – that provide additional years of monopoly protection from competition.
4. Although the Food and Drug Administration has been receptive to approving insulin biosimilars, it still requires manufacturers to go through a long and cumbersome process to obtain licensure, sometimes taking as long as 10 years.
5.PBMs, paid by insurance companies to negotiate prices with retail pharmacies and pharmaceutical companies (through rebates), stand to benefit from higher, not lower, list prices.
6. Pharmaceutical companies have vast lobbying power.
In regard to the fifth point, about PBMs, Dr. Rajkumar said, “It’s not just the PBMs – it’s the whole supply chain. It’s hard to put a finger on the source of the problem. There’s no transparency in any of the arrangements for you to know why only certain drugs are on a given formulary of an insurance company or PBM. But we do know that, in general, the whole supply chain benefits from the higher list price. Everyone.”
‘Authorized generic’ insulins
That is why Dr. Rajkumar is not convinced that Eli Lilly’s recent launch of half-priced “authorized generic” insulins – first the Lispro injection in March 2019, and then two combination pen products in January 2020 – or Novo Nordisk’s My$99Insulin program and “follow-on” authorized generic versions of Novolog and NovoLog Mix, launched Jan. 2, 2020, will have a huge impact.
“It’s common sense. If Apple made the same iPhone for two different prices, who would pay the full price? It gives you a window into asking what is wrong with the system that allows that? To pay for the higher-priced product, somebody is being paid,” he said.
Indeed, in December 2019, the offices of Sen. Elizabeth Warren (D-MA) and Sen. Richard Blumenthal (D-CT) issued a report from a survey of 400 pharmacies nationwide that found that 83% of the less expensive authorized generic Insulin Lispro was not in stock.
More than two-thirds of pharmacies reported they could not order the product.
“Eli Lilly has failed to take consequential steps – such as simply lowering the list price of Humalog, as it has in foreign markets – to provide lower-cost access to this important diabetes drug,” according to the senators’ report, which concludes by urging Eli Lilly to lower the list price of its insulin and calling for Congress to take steps to enact systemic change to reduce drug prices nationwide.
Asked for comment, Dani Barnhizer, Eli Lilly’s manager of global diabetes communications, said, “Like Senators Warren and Blumenthal, Lilly would like to see even broader use of Insulin Lispro injection because it’s a real solution that can lower copays for people living with diabetes.”
“But the Senators’ paper failed to identify the system challenges that have inhibited access to this lower list price product,” Ms. Barnhizer said. “Payers determine an individual’s premiums and copays, which are subsidized by rebates that pharmaceutical companies pay. And many payers prioritize these rebates to lower premiums instead of offering low copays for chronic medications such as insulin.
“It’s why only one in four people using Medicare Part D, and one in five with commercial plans, have coverage for Insulin Lispro injection. That will not change until payers prioritize providing consistent, affordable insulin copays,” she added.
However, Ms. Barnhizer also noted, “It is not unusual for pharmacies to not stock a medicine. Any pharmacy can place an order for Insulin Lispro injection, with delivery in 1-2 days. All major U.S. wholesalers are now distributing Insulin Lispro injection.”
She also said that people who earn 400% or less of the federal poverty level may be eligible for free insulin, and that Lilly can provide free insulin to anyone in emergency situations. (Novo Nordisk offers that as well.)
Asked why Lilly does not simply lower the price of all their insulins, Ms. Barnhizer responded: “Cutting the list prices would significantly disrupt access to branded insulins, which thousands of insured patients depend on. Launching lower-priced insulin options is a less disruptive approach to help reduce the amount people pay at the pharmacy for people who need the help.”
Government role needed
In addition to the recommendations for physicians and their institutions, Dr. Rajkumar listed several potential policy-level solutions:
1. At the state and federal level, regulations should protect against excessive drug launch prices through the same type of value-based pricing approaches used in other developed countries. “Capping the maximum price increases to the rate of inflation is needed and can happen only through state and/or federal legislation,” he wrote.
Although some may see this as antithetical to “free market” economics, Dr. Rajkumar points out that, in the case of many prescription drugs, including insulin, “we not only have an unregulated monopoly, but it’s a prolonged unregulated monopoly for a lifesaving product, not a luxury item.”
“When you grant monopoly protection and put a barrier on competition, that’s not a free market. People have always had regulations on monopolies. Unregulated monopoly is a recipe for high prices. You have to have some regulation that protects citizens from exploitation.”
And here, he believes, the United States could adopt price negotiations as practiced in Europe and other parts of the world, but which are currently forbidden in the United States.
“Other countries negotiate the price in exchange for monopoly protection. You don’t have to reinvent the wheel,” he said.
2. Reform of the regulatory and legal processes to ease the path for approval of generics and biosimilars to enter the market. This could include reciprocal approval so that biosimilars approved in Canada or the European Union could automatically be granted FDA approval in the United States.
3. Reform of the patent system to prevent overpatenting and patent abuse, by capping patent life to 7-10 years and forbidding use of additional patents as a way of prolonging market exclusivity.
To this point, Barnhizer pointed out that “none of the Lilly insulins are patent protected. Our most commonly used insulin, Humalog U-100, lost patent protection in 2014.”
4. A nongovernmental agency should oversee pricing and make recommendations to Medicare and insurers on the maximum price of new and existing drugs, including insulin. One body poised to do that work is the Institute for Clinical and Economic Review, which receives 77% of its funding from nonprofit foundations. “I have worked with them and they are the best there is,” Dr. Rajkumar said.
5. Any rebates paid by manufacturers to PBMs should be transparent to all stakeholders, including patients.
6. Nonprofit generic manufacturing should be established. The Mayo Clinic has recently partnered with Intermountain Healthcare and several other organizations in creating Civica Rx, a nonprofit generic company.
7. Measures and laws that provide access to insulin in emergency situations are needed, particularly for people with type 1 diabetes.
Recent proposed and enacted legislation has been aimed at some of these goals.
The Insulin Price Reduction Act, introduced in October 2019 and just endorsed by the American Diabetes Association, would reduce insulin costs by providing incentives for manufacturers to revert to the 2006 list price of all insulins.
The Affordable Insulin Approvals Now Act, introduced in July 2019, aims to speed up approvals of generic and biosimilar insulins.
And in May 2019, the state of Colorado passed a bill to cap patient copays for insulin at $100 a month, and similar legislation has been introduced in several other states.
Dr. Rajkumar says that an overall fix will require changes at the federal level.
But, he said, they do not need to happen all at once.
“There are a number of changes that need to happen, but we can do one legislation at a time. ... I’m optimistic that something will happen. This is a national conversation. Both sides of the aisle want to do something about it. People are indeed feeling the pinch, so maybe something will get done,” he said.
Dr. Rajkumar has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, argues in a new commentary.
The High Cost of Insulin in the United States: An Urgent Call to Action was published in the January 2020 issue of the Mayo Clinic Proceedings by Dr. Rajkumar, professor of medicine at the Mayo Clinic, Rochester, Minn., who specializes in treating myeloma and, more recently, has become an expert in drug pricing.
He also presented the information in a YouTube video.
As has been widely reported and examined by Congress in the past few years, the cost of insulin in the United States has risen at a far higher rate than inflation. For example, the list price of a single vial of Humalog jumped from $21 in 1999 to $275 in 2019, a far higher price than anywhere else in the world.
Stories of one in four patients having to ration their insulin use because of cost, and of some dying, have fueled protests, leading to legislative efforts and to a few initiatives by some of the manufacturers to address the cost problem.
Collective advocacy
Much of the blame has been placed on the manufacturers for charging such high prices and on the pharmacy benefit managers (PBMs) – also known as “middlemen” – for incentivizing higher-priced products on formularies through rebates. Those are major factors, Dr. Rajkumar argues, but they are not the only ones.
“There is no one reason why this is happening, and no one solution. It’s very complicated. It’s multiple factors all playing together. The only way to tackle it is to really understand it 360,” he said in an interview.
This is true of drug prices overall in the United States, but insulin is a special case. The current analog formulations have not changed in more than 20 years, yet only in the past 5 years have a handful of biosimilar and generic versions started to appear from the same manufacturers as the branded products.
“Insulin is a window into what’s wrong with the pharma industry. ... It’s the best example of how the system is broken,” Dr. Rajkumar stressed.
Physicians can help ease the problem, he said, by becoming educated about drug prices, taking cost into account when prescribing, and routinely discussing prescription drug affordability with patients.
Resources such as www.goodrx.com and www.blinkhealth.com provide information about drug prices and pharmacies that offer drugs at the lowest prices.
“Doctors need to not be suspicious of biosimilars and generics,” he emphasized.
Physicians can also advocate for policies that will lower insulin prices, and their institutions can establish preferences for lower-cost biosimilars in formularies.
“Our individual and collective advocacy gives voice to the needs of our patients,” Dr. Rajkumar emphasized.
‘Everyone in the supply chain benefits’
In his commentary, Dr. Rajkumar lists six major reasons for the high cost of insulin:
1. People with type 1 diabetes are a “vulnerable population” who will die without insulin and are therefore willing to pay a high price to stay alive.
2. Just three manufacturers – Eli Lilly, Novo Nordisk, and Sanofi-Aventis – control nearly the entire insulin market in the United States, with no regulations to cap or control the prices they can charge.
3. The manufacturers continually file new patents for existing insulin products – 70 in the case of Lantus, for example – that provide additional years of monopoly protection from competition.
4. Although the Food and Drug Administration has been receptive to approving insulin biosimilars, it still requires manufacturers to go through a long and cumbersome process to obtain licensure, sometimes taking as long as 10 years.
5.PBMs, paid by insurance companies to negotiate prices with retail pharmacies and pharmaceutical companies (through rebates), stand to benefit from higher, not lower, list prices.
6. Pharmaceutical companies have vast lobbying power.
In regard to the fifth point, about PBMs, Dr. Rajkumar said, “It’s not just the PBMs – it’s the whole supply chain. It’s hard to put a finger on the source of the problem. There’s no transparency in any of the arrangements for you to know why only certain drugs are on a given formulary of an insurance company or PBM. But we do know that, in general, the whole supply chain benefits from the higher list price. Everyone.”
‘Authorized generic’ insulins
That is why Dr. Rajkumar is not convinced that Eli Lilly’s recent launch of half-priced “authorized generic” insulins – first the Lispro injection in March 2019, and then two combination pen products in January 2020 – or Novo Nordisk’s My$99Insulin program and “follow-on” authorized generic versions of Novolog and NovoLog Mix, launched Jan. 2, 2020, will have a huge impact.
“It’s common sense. If Apple made the same iPhone for two different prices, who would pay the full price? It gives you a window into asking what is wrong with the system that allows that? To pay for the higher-priced product, somebody is being paid,” he said.
Indeed, in December 2019, the offices of Sen. Elizabeth Warren (D-MA) and Sen. Richard Blumenthal (D-CT) issued a report from a survey of 400 pharmacies nationwide that found that 83% of the less expensive authorized generic Insulin Lispro was not in stock.
More than two-thirds of pharmacies reported they could not order the product.
“Eli Lilly has failed to take consequential steps – such as simply lowering the list price of Humalog, as it has in foreign markets – to provide lower-cost access to this important diabetes drug,” according to the senators’ report, which concludes by urging Eli Lilly to lower the list price of its insulin and calling for Congress to take steps to enact systemic change to reduce drug prices nationwide.
Asked for comment, Dani Barnhizer, Eli Lilly’s manager of global diabetes communications, said, “Like Senators Warren and Blumenthal, Lilly would like to see even broader use of Insulin Lispro injection because it’s a real solution that can lower copays for people living with diabetes.”
“But the Senators’ paper failed to identify the system challenges that have inhibited access to this lower list price product,” Ms. Barnhizer said. “Payers determine an individual’s premiums and copays, which are subsidized by rebates that pharmaceutical companies pay. And many payers prioritize these rebates to lower premiums instead of offering low copays for chronic medications such as insulin.
“It’s why only one in four people using Medicare Part D, and one in five with commercial plans, have coverage for Insulin Lispro injection. That will not change until payers prioritize providing consistent, affordable insulin copays,” she added.
However, Ms. Barnhizer also noted, “It is not unusual for pharmacies to not stock a medicine. Any pharmacy can place an order for Insulin Lispro injection, with delivery in 1-2 days. All major U.S. wholesalers are now distributing Insulin Lispro injection.”
She also said that people who earn 400% or less of the federal poverty level may be eligible for free insulin, and that Lilly can provide free insulin to anyone in emergency situations. (Novo Nordisk offers that as well.)
Asked why Lilly does not simply lower the price of all their insulins, Ms. Barnhizer responded: “Cutting the list prices would significantly disrupt access to branded insulins, which thousands of insured patients depend on. Launching lower-priced insulin options is a less disruptive approach to help reduce the amount people pay at the pharmacy for people who need the help.”
Government role needed
In addition to the recommendations for physicians and their institutions, Dr. Rajkumar listed several potential policy-level solutions:
1. At the state and federal level, regulations should protect against excessive drug launch prices through the same type of value-based pricing approaches used in other developed countries. “Capping the maximum price increases to the rate of inflation is needed and can happen only through state and/or federal legislation,” he wrote.
Although some may see this as antithetical to “free market” economics, Dr. Rajkumar points out that, in the case of many prescription drugs, including insulin, “we not only have an unregulated monopoly, but it’s a prolonged unregulated monopoly for a lifesaving product, not a luxury item.”
“When you grant monopoly protection and put a barrier on competition, that’s not a free market. People have always had regulations on monopolies. Unregulated monopoly is a recipe for high prices. You have to have some regulation that protects citizens from exploitation.”
And here, he believes, the United States could adopt price negotiations as practiced in Europe and other parts of the world, but which are currently forbidden in the United States.
“Other countries negotiate the price in exchange for monopoly protection. You don’t have to reinvent the wheel,” he said.
2. Reform of the regulatory and legal processes to ease the path for approval of generics and biosimilars to enter the market. This could include reciprocal approval so that biosimilars approved in Canada or the European Union could automatically be granted FDA approval in the United States.
3. Reform of the patent system to prevent overpatenting and patent abuse, by capping patent life to 7-10 years and forbidding use of additional patents as a way of prolonging market exclusivity.
To this point, Barnhizer pointed out that “none of the Lilly insulins are patent protected. Our most commonly used insulin, Humalog U-100, lost patent protection in 2014.”
4. A nongovernmental agency should oversee pricing and make recommendations to Medicare and insurers on the maximum price of new and existing drugs, including insulin. One body poised to do that work is the Institute for Clinical and Economic Review, which receives 77% of its funding from nonprofit foundations. “I have worked with them and they are the best there is,” Dr. Rajkumar said.
5. Any rebates paid by manufacturers to PBMs should be transparent to all stakeholders, including patients.
6. Nonprofit generic manufacturing should be established. The Mayo Clinic has recently partnered with Intermountain Healthcare and several other organizations in creating Civica Rx, a nonprofit generic company.
7. Measures and laws that provide access to insulin in emergency situations are needed, particularly for people with type 1 diabetes.
Recent proposed and enacted legislation has been aimed at some of these goals.
The Insulin Price Reduction Act, introduced in October 2019 and just endorsed by the American Diabetes Association, would reduce insulin costs by providing incentives for manufacturers to revert to the 2006 list price of all insulins.
The Affordable Insulin Approvals Now Act, introduced in July 2019, aims to speed up approvals of generic and biosimilar insulins.
And in May 2019, the state of Colorado passed a bill to cap patient copays for insulin at $100 a month, and similar legislation has been introduced in several other states.
Dr. Rajkumar says that an overall fix will require changes at the federal level.
But, he said, they do not need to happen all at once.
“There are a number of changes that need to happen, but we can do one legislation at a time. ... I’m optimistic that something will happen. This is a national conversation. Both sides of the aisle want to do something about it. People are indeed feeling the pinch, so maybe something will get done,” he said.
Dr. Rajkumar has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, argues in a new commentary.
The High Cost of Insulin in the United States: An Urgent Call to Action was published in the January 2020 issue of the Mayo Clinic Proceedings by Dr. Rajkumar, professor of medicine at the Mayo Clinic, Rochester, Minn., who specializes in treating myeloma and, more recently, has become an expert in drug pricing.
He also presented the information in a YouTube video.
As has been widely reported and examined by Congress in the past few years, the cost of insulin in the United States has risen at a far higher rate than inflation. For example, the list price of a single vial of Humalog jumped from $21 in 1999 to $275 in 2019, a far higher price than anywhere else in the world.
Stories of one in four patients having to ration their insulin use because of cost, and of some dying, have fueled protests, leading to legislative efforts and to a few initiatives by some of the manufacturers to address the cost problem.
Collective advocacy
Much of the blame has been placed on the manufacturers for charging such high prices and on the pharmacy benefit managers (PBMs) – also known as “middlemen” – for incentivizing higher-priced products on formularies through rebates. Those are major factors, Dr. Rajkumar argues, but they are not the only ones.
“There is no one reason why this is happening, and no one solution. It’s very complicated. It’s multiple factors all playing together. The only way to tackle it is to really understand it 360,” he said in an interview.
This is true of drug prices overall in the United States, but insulin is a special case. The current analog formulations have not changed in more than 20 years, yet only in the past 5 years have a handful of biosimilar and generic versions started to appear from the same manufacturers as the branded products.
“Insulin is a window into what’s wrong with the pharma industry. ... It’s the best example of how the system is broken,” Dr. Rajkumar stressed.
Physicians can help ease the problem, he said, by becoming educated about drug prices, taking cost into account when prescribing, and routinely discussing prescription drug affordability with patients.
Resources such as www.goodrx.com and www.blinkhealth.com provide information about drug prices and pharmacies that offer drugs at the lowest prices.
“Doctors need to not be suspicious of biosimilars and generics,” he emphasized.
Physicians can also advocate for policies that will lower insulin prices, and their institutions can establish preferences for lower-cost biosimilars in formularies.
“Our individual and collective advocacy gives voice to the needs of our patients,” Dr. Rajkumar emphasized.
‘Everyone in the supply chain benefits’
In his commentary, Dr. Rajkumar lists six major reasons for the high cost of insulin:
1. People with type 1 diabetes are a “vulnerable population” who will die without insulin and are therefore willing to pay a high price to stay alive.
2. Just three manufacturers – Eli Lilly, Novo Nordisk, and Sanofi-Aventis – control nearly the entire insulin market in the United States, with no regulations to cap or control the prices they can charge.
3. The manufacturers continually file new patents for existing insulin products – 70 in the case of Lantus, for example – that provide additional years of monopoly protection from competition.
4. Although the Food and Drug Administration has been receptive to approving insulin biosimilars, it still requires manufacturers to go through a long and cumbersome process to obtain licensure, sometimes taking as long as 10 years.
5.PBMs, paid by insurance companies to negotiate prices with retail pharmacies and pharmaceutical companies (through rebates), stand to benefit from higher, not lower, list prices.
6. Pharmaceutical companies have vast lobbying power.
In regard to the fifth point, about PBMs, Dr. Rajkumar said, “It’s not just the PBMs – it’s the whole supply chain. It’s hard to put a finger on the source of the problem. There’s no transparency in any of the arrangements for you to know why only certain drugs are on a given formulary of an insurance company or PBM. But we do know that, in general, the whole supply chain benefits from the higher list price. Everyone.”
‘Authorized generic’ insulins
That is why Dr. Rajkumar is not convinced that Eli Lilly’s recent launch of half-priced “authorized generic” insulins – first the Lispro injection in March 2019, and then two combination pen products in January 2020 – or Novo Nordisk’s My$99Insulin program and “follow-on” authorized generic versions of Novolog and NovoLog Mix, launched Jan. 2, 2020, will have a huge impact.
“It’s common sense. If Apple made the same iPhone for two different prices, who would pay the full price? It gives you a window into asking what is wrong with the system that allows that? To pay for the higher-priced product, somebody is being paid,” he said.
Indeed, in December 2019, the offices of Sen. Elizabeth Warren (D-MA) and Sen. Richard Blumenthal (D-CT) issued a report from a survey of 400 pharmacies nationwide that found that 83% of the less expensive authorized generic Insulin Lispro was not in stock.
More than two-thirds of pharmacies reported they could not order the product.
“Eli Lilly has failed to take consequential steps – such as simply lowering the list price of Humalog, as it has in foreign markets – to provide lower-cost access to this important diabetes drug,” according to the senators’ report, which concludes by urging Eli Lilly to lower the list price of its insulin and calling for Congress to take steps to enact systemic change to reduce drug prices nationwide.
Asked for comment, Dani Barnhizer, Eli Lilly’s manager of global diabetes communications, said, “Like Senators Warren and Blumenthal, Lilly would like to see even broader use of Insulin Lispro injection because it’s a real solution that can lower copays for people living with diabetes.”
“But the Senators’ paper failed to identify the system challenges that have inhibited access to this lower list price product,” Ms. Barnhizer said. “Payers determine an individual’s premiums and copays, which are subsidized by rebates that pharmaceutical companies pay. And many payers prioritize these rebates to lower premiums instead of offering low copays for chronic medications such as insulin.
“It’s why only one in four people using Medicare Part D, and one in five with commercial plans, have coverage for Insulin Lispro injection. That will not change until payers prioritize providing consistent, affordable insulin copays,” she added.
However, Ms. Barnhizer also noted, “It is not unusual for pharmacies to not stock a medicine. Any pharmacy can place an order for Insulin Lispro injection, with delivery in 1-2 days. All major U.S. wholesalers are now distributing Insulin Lispro injection.”
She also said that people who earn 400% or less of the federal poverty level may be eligible for free insulin, and that Lilly can provide free insulin to anyone in emergency situations. (Novo Nordisk offers that as well.)
Asked why Lilly does not simply lower the price of all their insulins, Ms. Barnhizer responded: “Cutting the list prices would significantly disrupt access to branded insulins, which thousands of insured patients depend on. Launching lower-priced insulin options is a less disruptive approach to help reduce the amount people pay at the pharmacy for people who need the help.”
Government role needed
In addition to the recommendations for physicians and their institutions, Dr. Rajkumar listed several potential policy-level solutions:
1. At the state and federal level, regulations should protect against excessive drug launch prices through the same type of value-based pricing approaches used in other developed countries. “Capping the maximum price increases to the rate of inflation is needed and can happen only through state and/or federal legislation,” he wrote.
Although some may see this as antithetical to “free market” economics, Dr. Rajkumar points out that, in the case of many prescription drugs, including insulin, “we not only have an unregulated monopoly, but it’s a prolonged unregulated monopoly for a lifesaving product, not a luxury item.”
“When you grant monopoly protection and put a barrier on competition, that’s not a free market. People have always had regulations on monopolies. Unregulated monopoly is a recipe for high prices. You have to have some regulation that protects citizens from exploitation.”
And here, he believes, the United States could adopt price negotiations as practiced in Europe and other parts of the world, but which are currently forbidden in the United States.
“Other countries negotiate the price in exchange for monopoly protection. You don’t have to reinvent the wheel,” he said.
2. Reform of the regulatory and legal processes to ease the path for approval of generics and biosimilars to enter the market. This could include reciprocal approval so that biosimilars approved in Canada or the European Union could automatically be granted FDA approval in the United States.
3. Reform of the patent system to prevent overpatenting and patent abuse, by capping patent life to 7-10 years and forbidding use of additional patents as a way of prolonging market exclusivity.
To this point, Barnhizer pointed out that “none of the Lilly insulins are patent protected. Our most commonly used insulin, Humalog U-100, lost patent protection in 2014.”
4. A nongovernmental agency should oversee pricing and make recommendations to Medicare and insurers on the maximum price of new and existing drugs, including insulin. One body poised to do that work is the Institute for Clinical and Economic Review, which receives 77% of its funding from nonprofit foundations. “I have worked with them and they are the best there is,” Dr. Rajkumar said.
5. Any rebates paid by manufacturers to PBMs should be transparent to all stakeholders, including patients.
6. Nonprofit generic manufacturing should be established. The Mayo Clinic has recently partnered with Intermountain Healthcare and several other organizations in creating Civica Rx, a nonprofit generic company.
7. Measures and laws that provide access to insulin in emergency situations are needed, particularly for people with type 1 diabetes.
Recent proposed and enacted legislation has been aimed at some of these goals.
The Insulin Price Reduction Act, introduced in October 2019 and just endorsed by the American Diabetes Association, would reduce insulin costs by providing incentives for manufacturers to revert to the 2006 list price of all insulins.
The Affordable Insulin Approvals Now Act, introduced in July 2019, aims to speed up approvals of generic and biosimilar insulins.
And in May 2019, the state of Colorado passed a bill to cap patient copays for insulin at $100 a month, and similar legislation has been introduced in several other states.
Dr. Rajkumar says that an overall fix will require changes at the federal level.
But, he said, they do not need to happen all at once.
“There are a number of changes that need to happen, but we can do one legislation at a time. ... I’m optimistic that something will happen. This is a national conversation. Both sides of the aisle want to do something about it. People are indeed feeling the pinch, so maybe something will get done,” he said.
Dr. Rajkumar has reported no relevant financial relationships.
This article first appeared on Medscape.com.
FDA approves first treatment for advanced epithelioid sarcoma
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
Family-focused therapy linked to longer remissions in youth at risk for bipolar disorder
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
FROM JAMA PSYCHIATRY