User login
Sunitinib for mRCC: Real-world experience differs somewhat
First-line sunitinib therapy for metastatic renal cell carcinoma (mRCC) has somewhat differing effectiveness and safety when used in real-world practice, compared with clinical trials, suggests the OSSMAR multicenter retrospective cohort study.
Investigators led by Marwan Ghosn, MD, Hotel-Dieu de France University Hospital and Saint Joseph University, Beirut, Lebanon, analyzed outcomes among 289 patients with mRCC started on sunitinib (Sutent), an oral multitargeted tyrosine kinase inhibitor, between 2006 and 2016 at 10 centers in Africa and the Middle East region.
The patients had a median age at diagnosis of 58.7 years, and 85.8% had clear cell histology. Two-thirds had metastases at diagnosis, and 15.2% and 31.4% had favorable- and poor-risk disease, respectively, according to expanded Memorial Sloan Kettering Cancer Center criteria. Overall, 52.2% had undergone partial or radical nephrectomy, and nearly all were receiving sunitinib as first-line therapy.
Study results, reported in the Journal of Global Oncology, showed that the mean total sunitinib starting dose was 48.1 mg, with most patients (87.6%) started on a dose of 50 mg. On average, the drug was given for 9.6 months.
The overall response rate was 20.8%, and responses lasted for a median of of 8.2 months. With a median follow-up of 7.8 months, patients had a median time to progression of 5.7 months and a median overall survival of 7.8 months. The 12- and 24-month rates of overall survival were 34.3% and 11.4%, respectively.
Although 60.9% of patients experienced adverse events, only 8.0% experienced serious adverse events. The main adverse events were gastrointestinal and hematologic, mirroring those seen previously in trials. About a third of the whole study cohort (28.7%) discontinued sunitinib therapy.
“OSSMAR is the first study in the Middle East involving several Arab countries and evaluating the use of real-time sunitinib in the treatment of mRCC. As a result, this study is of primary importance because it allows for a better assessment of the actual effectiveness and practical adverse events of sunitinib in the population in our region,” Dr. Ghosn and coinvestigators maintained.
Compared with patients in clinical trials, OSSMAR patients had poorer overall survival, possibly due to factors such as a relatively lower nephrectomy rate, real-world influences such as comorbidities, and losses to follow-up, the investigators proposed.
At the same time, the OSSMAR cohort also had a lower rate of adverse events, although this difference likely reflects less rigorous assessment of toxicity in real-world practice, they noted.
Dr. Ghosn disclosed that he has relationships with numerous pharmaceutical companies. The study did not receive any specific funding.
SOURCE: Ghosn M et al. J Glob Oncol. 2019 Oct 5. doi: 10.1200/JGO.18.00238.
First-line sunitinib therapy for metastatic renal cell carcinoma (mRCC) has somewhat differing effectiveness and safety when used in real-world practice, compared with clinical trials, suggests the OSSMAR multicenter retrospective cohort study.
Investigators led by Marwan Ghosn, MD, Hotel-Dieu de France University Hospital and Saint Joseph University, Beirut, Lebanon, analyzed outcomes among 289 patients with mRCC started on sunitinib (Sutent), an oral multitargeted tyrosine kinase inhibitor, between 2006 and 2016 at 10 centers in Africa and the Middle East region.
The patients had a median age at diagnosis of 58.7 years, and 85.8% had clear cell histology. Two-thirds had metastases at diagnosis, and 15.2% and 31.4% had favorable- and poor-risk disease, respectively, according to expanded Memorial Sloan Kettering Cancer Center criteria. Overall, 52.2% had undergone partial or radical nephrectomy, and nearly all were receiving sunitinib as first-line therapy.
Study results, reported in the Journal of Global Oncology, showed that the mean total sunitinib starting dose was 48.1 mg, with most patients (87.6%) started on a dose of 50 mg. On average, the drug was given for 9.6 months.
The overall response rate was 20.8%, and responses lasted for a median of of 8.2 months. With a median follow-up of 7.8 months, patients had a median time to progression of 5.7 months and a median overall survival of 7.8 months. The 12- and 24-month rates of overall survival were 34.3% and 11.4%, respectively.
Although 60.9% of patients experienced adverse events, only 8.0% experienced serious adverse events. The main adverse events were gastrointestinal and hematologic, mirroring those seen previously in trials. About a third of the whole study cohort (28.7%) discontinued sunitinib therapy.
“OSSMAR is the first study in the Middle East involving several Arab countries and evaluating the use of real-time sunitinib in the treatment of mRCC. As a result, this study is of primary importance because it allows for a better assessment of the actual effectiveness and practical adverse events of sunitinib in the population in our region,” Dr. Ghosn and coinvestigators maintained.
Compared with patients in clinical trials, OSSMAR patients had poorer overall survival, possibly due to factors such as a relatively lower nephrectomy rate, real-world influences such as comorbidities, and losses to follow-up, the investigators proposed.
At the same time, the OSSMAR cohort also had a lower rate of adverse events, although this difference likely reflects less rigorous assessment of toxicity in real-world practice, they noted.
Dr. Ghosn disclosed that he has relationships with numerous pharmaceutical companies. The study did not receive any specific funding.
SOURCE: Ghosn M et al. J Glob Oncol. 2019 Oct 5. doi: 10.1200/JGO.18.00238.
First-line sunitinib therapy for metastatic renal cell carcinoma (mRCC) has somewhat differing effectiveness and safety when used in real-world practice, compared with clinical trials, suggests the OSSMAR multicenter retrospective cohort study.
Investigators led by Marwan Ghosn, MD, Hotel-Dieu de France University Hospital and Saint Joseph University, Beirut, Lebanon, analyzed outcomes among 289 patients with mRCC started on sunitinib (Sutent), an oral multitargeted tyrosine kinase inhibitor, between 2006 and 2016 at 10 centers in Africa and the Middle East region.
The patients had a median age at diagnosis of 58.7 years, and 85.8% had clear cell histology. Two-thirds had metastases at diagnosis, and 15.2% and 31.4% had favorable- and poor-risk disease, respectively, according to expanded Memorial Sloan Kettering Cancer Center criteria. Overall, 52.2% had undergone partial or radical nephrectomy, and nearly all were receiving sunitinib as first-line therapy.
Study results, reported in the Journal of Global Oncology, showed that the mean total sunitinib starting dose was 48.1 mg, with most patients (87.6%) started on a dose of 50 mg. On average, the drug was given for 9.6 months.
The overall response rate was 20.8%, and responses lasted for a median of of 8.2 months. With a median follow-up of 7.8 months, patients had a median time to progression of 5.7 months and a median overall survival of 7.8 months. The 12- and 24-month rates of overall survival were 34.3% and 11.4%, respectively.
Although 60.9% of patients experienced adverse events, only 8.0% experienced serious adverse events. The main adverse events were gastrointestinal and hematologic, mirroring those seen previously in trials. About a third of the whole study cohort (28.7%) discontinued sunitinib therapy.
“OSSMAR is the first study in the Middle East involving several Arab countries and evaluating the use of real-time sunitinib in the treatment of mRCC. As a result, this study is of primary importance because it allows for a better assessment of the actual effectiveness and practical adverse events of sunitinib in the population in our region,” Dr. Ghosn and coinvestigators maintained.
Compared with patients in clinical trials, OSSMAR patients had poorer overall survival, possibly due to factors such as a relatively lower nephrectomy rate, real-world influences such as comorbidities, and losses to follow-up, the investigators proposed.
At the same time, the OSSMAR cohort also had a lower rate of adverse events, although this difference likely reflects less rigorous assessment of toxicity in real-world practice, they noted.
Dr. Ghosn disclosed that he has relationships with numerous pharmaceutical companies. The study did not receive any specific funding.
SOURCE: Ghosn M et al. J Glob Oncol. 2019 Oct 5. doi: 10.1200/JGO.18.00238.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Supplemental oxygen: More isn’t always better
ILLUSTRATIVE CASE
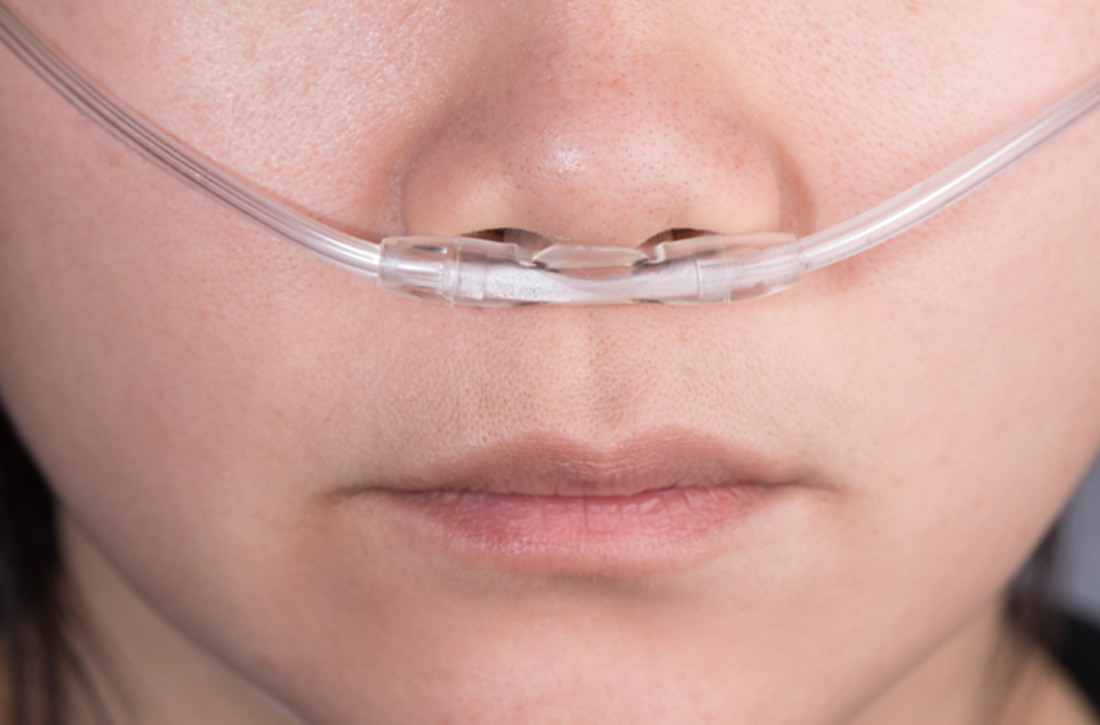
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
ILLUSTRATIVE CASE
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
PRACTICE CHANGER
Do not use liberal oxygen therapy (SpO2 > 96%) in acutely ill adults, as it is associated with increased all-cause mortality.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 25 randomized controlled trials.
Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
45-year-old woman • fever and chills • diffuse abdominal pain • shortness of breath • Dx?
THE CASE
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; lauren_cowen@urmc.rochester.edu
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
THE CASE
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; lauren_cowen@urmc.rochester.edu
THE CASE
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; lauren_cowen@urmc.rochester.edu
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
Head & neck cancers: What you’ll see, how to proceed
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
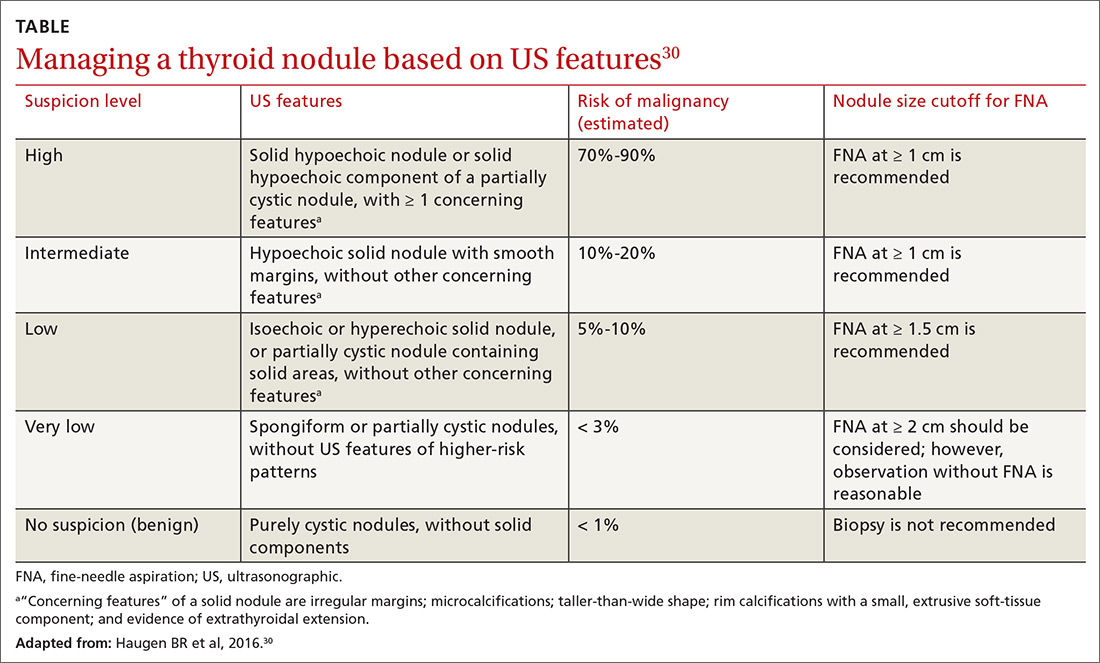
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
PRACTICE RECOMMENDATIONS
› Do not treat a neck mass with antibiotics unless it has features consistent with infection. C
› Order laryngoscopy for all patients with hoarseness that does not resolve after 3 months—or sooner, if malignancy is suspected. C
› Order ultrasonography-guided fine-needle aspiration for diagnostic evaluation of salivary gland masses. B
› Manage a thyroid nodule based on its sonographic features, including size, consistency, and the presence of concerning features. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Better overall survival with nivolumab vs. chemo for advanced ESCC
BARCELONA – Nivolumab was associated with improved overall survival and a favorable safety profile, compared with chemotherapy, in patients with previously treated advanced esophageal squamous cell carcinoma (ESCC) in the open-label phase 3 ATTRACTION-3 study.
The overall survival (OS) benefit was observed regardless of tumor programmed death-ligand 1 (PD-L1) expression, Byoung Chul Cho, MD, reported at the European Society for Medical Oncology Congress.
The findings were reported online simultaneously in The Lancet Oncology.
Median OS at a minimum follow-up of 17.6 months was 10.9 vs. 8.4 months in 210 patients randomized to receive treatment with the PD-1 inhibitor nivolumab and 209 who received chemotherapy, respectively (hazard ratio, 0.77), said Dr. Cho of Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, South Korea.
“Notably, there was a 13% and 10% improvement in overall survival rates at 12 months (47% vs. 34%) and 18 months (31% vs. 21%), respectively,” he said, also noting that the HRs for death favored nivolumab vs. chemotherapy across multiple prespecified subgroups, including those based on tumor PD-L1 expression (HRs, 0.69 and 0.84 for PD-L1 of 1% or greater and less than 1%, respectively).
No meaningful difference was seen in progression-free survival between the nivolumab and chemotherapy groups (12% vs. 7%; HR, 1.08), or in objective response rates (19% vs. 22%), he said.
“However, responses were substantially more durable with nivolumab, compared to chemotherapy; duration of response was 6.9 months with nivolumab vs. 3.9 months in the chemotherapy arm,” he said. “Notably, 21% of patients in the nivolumab arm were still in response, compared to only 6% in the chemotherapy arm.”
Patients enrolled in the open label study had unresectable advanced or recurrent ESCC refractory or intolerant to one prior fluoropyrimidine/platinum-based therapy. They were randomized 1:1 to receive 240 mg of nivolumab every 2 weeks or investigators’ choice of paclitaxel or docetaxel.
Fewer treatment-related adverse events (TRAEs) were reported with nivolumab, Dr. Cho said.
Any grade TRAEs occurred in 66% vs. 95% of patients in the groups, respectively, and grade 3-4 TRAEs occurred in 18% vs. 63%. The majority of select TRAEs – defined as those with potential immunologic etiology, including endocrine, gastrointestinal, hepatic, pulmonary, renal, and skin effects – were grade 1 or 2, and the only difference between the nivolumab and chemotherapy groups with respect to those was in endocrine effects, which affected 11% vs. less than 1% of patients, respectively.
Grade 3/4 select TRAEs occurred in less than 2% of patients, Dr. Cho noted.
An exploratory analysis further showed significant overall improvement in health-related quality of life with nivolumab through week 42 on treatment, he added.
The findings are of note, because metastatic esophageal cancer has a 5-year relative survival rate of less than 8%, and ESCC accounts for about 90% of cases worldwide, he said, adding that current second-line chemotherapy options for ESCC offer poor long-term survival and are associated with toxicity.
Nivolumab, which showed promising antitumor activity and manageable toxicity for advanced ESCC in patients who were refractory to or intolerant of standard chemotherapies in the phase 2 ATTRACTION-1 study, is the first immune checkpoint inhibitor to demonstrate a statistically significant, clinically meaningful improvement in OS vs. chemotherapy in this setting, he said.
The findings of this final analysis of ATTRACTION-3, which shows a 23% reduction in the risk of death, a 2.5-month improvement in median OS, benefit across PD-L1 subgroups, and a favorable safety profile, suggest that nivolumab represents a new standard second-line treatment option for patients with advanced ESCC, he concluded.
ATTRACTION-3 was funded by Ono Pharmaceutical Co., in collaboration with Bristol-Myers Squibb. Dr. Cho reported relationships with Bristol-Myers Squibb, Ono Pharmaceutical, and others. He also reported stock ownership and/or patents with TheraCanVac and Champions Oncology.
SOURCE: Cho B et al. ESMO 2019, Abstract LBA11.
BARCELONA – Nivolumab was associated with improved overall survival and a favorable safety profile, compared with chemotherapy, in patients with previously treated advanced esophageal squamous cell carcinoma (ESCC) in the open-label phase 3 ATTRACTION-3 study.
The overall survival (OS) benefit was observed regardless of tumor programmed death-ligand 1 (PD-L1) expression, Byoung Chul Cho, MD, reported at the European Society for Medical Oncology Congress.
The findings were reported online simultaneously in The Lancet Oncology.
Median OS at a minimum follow-up of 17.6 months was 10.9 vs. 8.4 months in 210 patients randomized to receive treatment with the PD-1 inhibitor nivolumab and 209 who received chemotherapy, respectively (hazard ratio, 0.77), said Dr. Cho of Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, South Korea.
“Notably, there was a 13% and 10% improvement in overall survival rates at 12 months (47% vs. 34%) and 18 months (31% vs. 21%), respectively,” he said, also noting that the HRs for death favored nivolumab vs. chemotherapy across multiple prespecified subgroups, including those based on tumor PD-L1 expression (HRs, 0.69 and 0.84 for PD-L1 of 1% or greater and less than 1%, respectively).
No meaningful difference was seen in progression-free survival between the nivolumab and chemotherapy groups (12% vs. 7%; HR, 1.08), or in objective response rates (19% vs. 22%), he said.
“However, responses were substantially more durable with nivolumab, compared to chemotherapy; duration of response was 6.9 months with nivolumab vs. 3.9 months in the chemotherapy arm,” he said. “Notably, 21% of patients in the nivolumab arm were still in response, compared to only 6% in the chemotherapy arm.”
Patients enrolled in the open label study had unresectable advanced or recurrent ESCC refractory or intolerant to one prior fluoropyrimidine/platinum-based therapy. They were randomized 1:1 to receive 240 mg of nivolumab every 2 weeks or investigators’ choice of paclitaxel or docetaxel.
Fewer treatment-related adverse events (TRAEs) were reported with nivolumab, Dr. Cho said.
Any grade TRAEs occurred in 66% vs. 95% of patients in the groups, respectively, and grade 3-4 TRAEs occurred in 18% vs. 63%. The majority of select TRAEs – defined as those with potential immunologic etiology, including endocrine, gastrointestinal, hepatic, pulmonary, renal, and skin effects – were grade 1 or 2, and the only difference between the nivolumab and chemotherapy groups with respect to those was in endocrine effects, which affected 11% vs. less than 1% of patients, respectively.
Grade 3/4 select TRAEs occurred in less than 2% of patients, Dr. Cho noted.
An exploratory analysis further showed significant overall improvement in health-related quality of life with nivolumab through week 42 on treatment, he added.
The findings are of note, because metastatic esophageal cancer has a 5-year relative survival rate of less than 8%, and ESCC accounts for about 90% of cases worldwide, he said, adding that current second-line chemotherapy options for ESCC offer poor long-term survival and are associated with toxicity.
Nivolumab, which showed promising antitumor activity and manageable toxicity for advanced ESCC in patients who were refractory to or intolerant of standard chemotherapies in the phase 2 ATTRACTION-1 study, is the first immune checkpoint inhibitor to demonstrate a statistically significant, clinically meaningful improvement in OS vs. chemotherapy in this setting, he said.
The findings of this final analysis of ATTRACTION-3, which shows a 23% reduction in the risk of death, a 2.5-month improvement in median OS, benefit across PD-L1 subgroups, and a favorable safety profile, suggest that nivolumab represents a new standard second-line treatment option for patients with advanced ESCC, he concluded.
ATTRACTION-3 was funded by Ono Pharmaceutical Co., in collaboration with Bristol-Myers Squibb. Dr. Cho reported relationships with Bristol-Myers Squibb, Ono Pharmaceutical, and others. He also reported stock ownership and/or patents with TheraCanVac and Champions Oncology.
SOURCE: Cho B et al. ESMO 2019, Abstract LBA11.
BARCELONA – Nivolumab was associated with improved overall survival and a favorable safety profile, compared with chemotherapy, in patients with previously treated advanced esophageal squamous cell carcinoma (ESCC) in the open-label phase 3 ATTRACTION-3 study.
The overall survival (OS) benefit was observed regardless of tumor programmed death-ligand 1 (PD-L1) expression, Byoung Chul Cho, MD, reported at the European Society for Medical Oncology Congress.
The findings were reported online simultaneously in The Lancet Oncology.
Median OS at a minimum follow-up of 17.6 months was 10.9 vs. 8.4 months in 210 patients randomized to receive treatment with the PD-1 inhibitor nivolumab and 209 who received chemotherapy, respectively (hazard ratio, 0.77), said Dr. Cho of Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, South Korea.
“Notably, there was a 13% and 10% improvement in overall survival rates at 12 months (47% vs. 34%) and 18 months (31% vs. 21%), respectively,” he said, also noting that the HRs for death favored nivolumab vs. chemotherapy across multiple prespecified subgroups, including those based on tumor PD-L1 expression (HRs, 0.69 and 0.84 for PD-L1 of 1% or greater and less than 1%, respectively).
No meaningful difference was seen in progression-free survival between the nivolumab and chemotherapy groups (12% vs. 7%; HR, 1.08), or in objective response rates (19% vs. 22%), he said.
“However, responses were substantially more durable with nivolumab, compared to chemotherapy; duration of response was 6.9 months with nivolumab vs. 3.9 months in the chemotherapy arm,” he said. “Notably, 21% of patients in the nivolumab arm were still in response, compared to only 6% in the chemotherapy arm.”
Patients enrolled in the open label study had unresectable advanced or recurrent ESCC refractory or intolerant to one prior fluoropyrimidine/platinum-based therapy. They were randomized 1:1 to receive 240 mg of nivolumab every 2 weeks or investigators’ choice of paclitaxel or docetaxel.
Fewer treatment-related adverse events (TRAEs) were reported with nivolumab, Dr. Cho said.
Any grade TRAEs occurred in 66% vs. 95% of patients in the groups, respectively, and grade 3-4 TRAEs occurred in 18% vs. 63%. The majority of select TRAEs – defined as those with potential immunologic etiology, including endocrine, gastrointestinal, hepatic, pulmonary, renal, and skin effects – were grade 1 or 2, and the only difference between the nivolumab and chemotherapy groups with respect to those was in endocrine effects, which affected 11% vs. less than 1% of patients, respectively.
Grade 3/4 select TRAEs occurred in less than 2% of patients, Dr. Cho noted.
An exploratory analysis further showed significant overall improvement in health-related quality of life with nivolumab through week 42 on treatment, he added.
The findings are of note, because metastatic esophageal cancer has a 5-year relative survival rate of less than 8%, and ESCC accounts for about 90% of cases worldwide, he said, adding that current second-line chemotherapy options for ESCC offer poor long-term survival and are associated with toxicity.
Nivolumab, which showed promising antitumor activity and manageable toxicity for advanced ESCC in patients who were refractory to or intolerant of standard chemotherapies in the phase 2 ATTRACTION-1 study, is the first immune checkpoint inhibitor to demonstrate a statistically significant, clinically meaningful improvement in OS vs. chemotherapy in this setting, he said.
The findings of this final analysis of ATTRACTION-3, which shows a 23% reduction in the risk of death, a 2.5-month improvement in median OS, benefit across PD-L1 subgroups, and a favorable safety profile, suggest that nivolumab represents a new standard second-line treatment option for patients with advanced ESCC, he concluded.
ATTRACTION-3 was funded by Ono Pharmaceutical Co., in collaboration with Bristol-Myers Squibb. Dr. Cho reported relationships with Bristol-Myers Squibb, Ono Pharmaceutical, and others. He also reported stock ownership and/or patents with TheraCanVac and Champions Oncology.
SOURCE: Cho B et al. ESMO 2019, Abstract LBA11.
REPORTING FROM ESMO 2019
Key clinical point: Nivolumab was associated with improved OS vs. chemotherapy, in previously treated advanced ESCC.
Major finding: Median OS was 10.9 vs. 8.4 months with nivolumab vs. chemotherapy, respectively (hazard ratio, 0.77).
Study details: A randomized, open-label, phase 3 study of 419 patients.
Disclosures: ATTRACTION-3 was funded by Ono Pharmaceutical Co., in collaboration with Bristol-Myers Squibb. Dr. Cho reported relationships with Bristol-Myers Squibb, Ono Pharmaceutical, and others. He reported stock ownership and/or patents with TheraCanVac and Champions Oncology.
Source: Cho B et al. ESMO 2019, Abstract LBA11.
Court strikes down Trump’s conscience rule
A federal court has struck down a Trump administration rule that would have allowed clinicians to refuse to provide medical care to patients for religious or moral reasons.
In a Nov. 6 decision, the U.S. District Court for the Southern District of New York vacated President Trump’s rule in its entirety, concluding that the rule had no justification and that its provisions were arbitrary and capricious. In his 147-page opinion, District Judge Paul Engelmayer wrote that the U.S. Department of Health & Human Services did not have the authority to enact such an expansive rule and that the measure conflicts with the Administrative Procedure Act, Title VII of the Civil Rights Act, and the Emergency Medical Treatment & Labor Act, among other laws.
“Had the court found only narrow parts of the rule infirm, a remedy tailoring the vacatur to only the problematic provision might well have been viable,” Judge Engelmayer wrote. “The [Administrative Procedure Act] violations that the court has found, however, are numerous, fundamental, and far reaching ... In these circumstances, a decision to leave standing isolated shards of the rule that have not been found specifically infirm would ignore the big picture: that the rulemaking exercise here was sufficiently shot through with glaring legal defects as to not justify a search for survivors [and] leaving stray nonsubstantive provisions intact would not serve a useful purpose.”
At press time, the Trump administration had not indicated whether they plan to file an appeal.
Clare Coleman, president & CEO for the National Family Planning & Reproductive Health Association, a plaintiff in the case, said the organization was heartened by the ruling and that the judge’s decision protects health care for millions of Americans.
“The court safeguarded the public’s health by striking down the Trump administration’s health care refusal rule,” Ms. Coleman said in a statement. “This unlawful rule is an outright attack on the health and wellness of millions of people across the country, and the court heard clear and compelling arguments about the harm communities face when our health care system is distorted to the point in which a patient’s health care needs are not paramount.”
The conscience rule, finalized in May 2019 by HHS, would have allowed clinicians to refuse care to patients if they deemed that care was in conflict with their religious or moral beliefs. The provisions principally – although not exclusively – addressed objections to abortion, sterilization, and assisted suicide, as well as counseling and referrals associated with these services.
According to HHS, the final rule fulfills President Trump’s promise to promote and protect rights of conscience and religious liberty. “This rule ensures that health care entities and professionals won’t be bullied out of the health care field because they decline to participate in actions that violate their conscience, including the taking of human life,” Roger Severino, director of the Office for Civil Rights, said in a statement. “Protecting conscience and religious freedom not only fosters greater diversity in health care, it’s the law.”
The judge’s order invalidating the rule consolidated three legal challenges against HHS over the rule. Plaintiffs included more than 15 states, Planned Parenthood Federation of America, and the National Family Planning & Reproductive Health Association, among others. The plaintiffs argued that the rule, scheduled to take effect on Nov. 22, would have threatened the ability of clinicians to provide essential, potentially life-saving medical care and would have exacerbated health disparities.
Stephanie Taub, senior counsel at the First Liberty Institute, an organization that represents religious freedom cases, said the court’s decision leaves health care professionals across America vulnerable to being forced “to perform, facilitate, or refer for procedures that violate their conscience.”
“The Trump administration’s HHS protections would ensure that health care professionals are free to work consistent with their religious beliefs while providing the best care to their patients,” Ms. Taub said in a statement.
The court’s decision comes less than a week after another district judge temporarily blocked an order by President Trump that would make having health insurance, or the ability to pay for medical care, a requirement for immigrants seeking U.S. visas. In that case, the judge said there are serious questions about whether President Trump’s immigration rule was arbitrary and capricious and, therefore, a violation of the Administrative Procedure Act. The order is on hold while the case continues through the courts.
A federal court has struck down a Trump administration rule that would have allowed clinicians to refuse to provide medical care to patients for religious or moral reasons.
In a Nov. 6 decision, the U.S. District Court for the Southern District of New York vacated President Trump’s rule in its entirety, concluding that the rule had no justification and that its provisions were arbitrary and capricious. In his 147-page opinion, District Judge Paul Engelmayer wrote that the U.S. Department of Health & Human Services did not have the authority to enact such an expansive rule and that the measure conflicts with the Administrative Procedure Act, Title VII of the Civil Rights Act, and the Emergency Medical Treatment & Labor Act, among other laws.
“Had the court found only narrow parts of the rule infirm, a remedy tailoring the vacatur to only the problematic provision might well have been viable,” Judge Engelmayer wrote. “The [Administrative Procedure Act] violations that the court has found, however, are numerous, fundamental, and far reaching ... In these circumstances, a decision to leave standing isolated shards of the rule that have not been found specifically infirm would ignore the big picture: that the rulemaking exercise here was sufficiently shot through with glaring legal defects as to not justify a search for survivors [and] leaving stray nonsubstantive provisions intact would not serve a useful purpose.”
At press time, the Trump administration had not indicated whether they plan to file an appeal.
Clare Coleman, president & CEO for the National Family Planning & Reproductive Health Association, a plaintiff in the case, said the organization was heartened by the ruling and that the judge’s decision protects health care for millions of Americans.
“The court safeguarded the public’s health by striking down the Trump administration’s health care refusal rule,” Ms. Coleman said in a statement. “This unlawful rule is an outright attack on the health and wellness of millions of people across the country, and the court heard clear and compelling arguments about the harm communities face when our health care system is distorted to the point in which a patient’s health care needs are not paramount.”
The conscience rule, finalized in May 2019 by HHS, would have allowed clinicians to refuse care to patients if they deemed that care was in conflict with their religious or moral beliefs. The provisions principally – although not exclusively – addressed objections to abortion, sterilization, and assisted suicide, as well as counseling and referrals associated with these services.
According to HHS, the final rule fulfills President Trump’s promise to promote and protect rights of conscience and religious liberty. “This rule ensures that health care entities and professionals won’t be bullied out of the health care field because they decline to participate in actions that violate their conscience, including the taking of human life,” Roger Severino, director of the Office for Civil Rights, said in a statement. “Protecting conscience and religious freedom not only fosters greater diversity in health care, it’s the law.”
The judge’s order invalidating the rule consolidated three legal challenges against HHS over the rule. Plaintiffs included more than 15 states, Planned Parenthood Federation of America, and the National Family Planning & Reproductive Health Association, among others. The plaintiffs argued that the rule, scheduled to take effect on Nov. 22, would have threatened the ability of clinicians to provide essential, potentially life-saving medical care and would have exacerbated health disparities.
Stephanie Taub, senior counsel at the First Liberty Institute, an organization that represents religious freedom cases, said the court’s decision leaves health care professionals across America vulnerable to being forced “to perform, facilitate, or refer for procedures that violate their conscience.”
“The Trump administration’s HHS protections would ensure that health care professionals are free to work consistent with their religious beliefs while providing the best care to their patients,” Ms. Taub said in a statement.
The court’s decision comes less than a week after another district judge temporarily blocked an order by President Trump that would make having health insurance, or the ability to pay for medical care, a requirement for immigrants seeking U.S. visas. In that case, the judge said there are serious questions about whether President Trump’s immigration rule was arbitrary and capricious and, therefore, a violation of the Administrative Procedure Act. The order is on hold while the case continues through the courts.
A federal court has struck down a Trump administration rule that would have allowed clinicians to refuse to provide medical care to patients for religious or moral reasons.
In a Nov. 6 decision, the U.S. District Court for the Southern District of New York vacated President Trump’s rule in its entirety, concluding that the rule had no justification and that its provisions were arbitrary and capricious. In his 147-page opinion, District Judge Paul Engelmayer wrote that the U.S. Department of Health & Human Services did not have the authority to enact such an expansive rule and that the measure conflicts with the Administrative Procedure Act, Title VII of the Civil Rights Act, and the Emergency Medical Treatment & Labor Act, among other laws.
“Had the court found only narrow parts of the rule infirm, a remedy tailoring the vacatur to only the problematic provision might well have been viable,” Judge Engelmayer wrote. “The [Administrative Procedure Act] violations that the court has found, however, are numerous, fundamental, and far reaching ... In these circumstances, a decision to leave standing isolated shards of the rule that have not been found specifically infirm would ignore the big picture: that the rulemaking exercise here was sufficiently shot through with glaring legal defects as to not justify a search for survivors [and] leaving stray nonsubstantive provisions intact would not serve a useful purpose.”
At press time, the Trump administration had not indicated whether they plan to file an appeal.
Clare Coleman, president & CEO for the National Family Planning & Reproductive Health Association, a plaintiff in the case, said the organization was heartened by the ruling and that the judge’s decision protects health care for millions of Americans.
“The court safeguarded the public’s health by striking down the Trump administration’s health care refusal rule,” Ms. Coleman said in a statement. “This unlawful rule is an outright attack on the health and wellness of millions of people across the country, and the court heard clear and compelling arguments about the harm communities face when our health care system is distorted to the point in which a patient’s health care needs are not paramount.”
The conscience rule, finalized in May 2019 by HHS, would have allowed clinicians to refuse care to patients if they deemed that care was in conflict with their religious or moral beliefs. The provisions principally – although not exclusively – addressed objections to abortion, sterilization, and assisted suicide, as well as counseling and referrals associated with these services.
According to HHS, the final rule fulfills President Trump’s promise to promote and protect rights of conscience and religious liberty. “This rule ensures that health care entities and professionals won’t be bullied out of the health care field because they decline to participate in actions that violate their conscience, including the taking of human life,” Roger Severino, director of the Office for Civil Rights, said in a statement. “Protecting conscience and religious freedom not only fosters greater diversity in health care, it’s the law.”
The judge’s order invalidating the rule consolidated three legal challenges against HHS over the rule. Plaintiffs included more than 15 states, Planned Parenthood Federation of America, and the National Family Planning & Reproductive Health Association, among others. The plaintiffs argued that the rule, scheduled to take effect on Nov. 22, would have threatened the ability of clinicians to provide essential, potentially life-saving medical care and would have exacerbated health disparities.
Stephanie Taub, senior counsel at the First Liberty Institute, an organization that represents religious freedom cases, said the court’s decision leaves health care professionals across America vulnerable to being forced “to perform, facilitate, or refer for procedures that violate their conscience.”
“The Trump administration’s HHS protections would ensure that health care professionals are free to work consistent with their religious beliefs while providing the best care to their patients,” Ms. Taub said in a statement.
The court’s decision comes less than a week after another district judge temporarily blocked an order by President Trump that would make having health insurance, or the ability to pay for medical care, a requirement for immigrants seeking U.S. visas. In that case, the judge said there are serious questions about whether President Trump’s immigration rule was arbitrary and capricious and, therefore, a violation of the Administrative Procedure Act. The order is on hold while the case continues through the courts.
Chronic blistering rash on hands
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
Worsening nausea, vomiting, and dizziness • 20-pound weight loss in 2 months • mild hearing loss • reoccurring episodes of falls • Dx?
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.
Best timing for measuring orthostatic vital signs?
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
PRACTICE CHANGER
Measure orthostatic vital signs within 1 minute of standing to most accurately correlate dizziness with long-term adverse outcomes. 1
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, prospective cohort study with patient-oriented outcomes and good follow-up.
Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316-1323.
Suicide screening: How to recognize and treat at-risk adults
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; meredith.williamson@tamu.edu.
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; meredith.williamson@tamu.edu.
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; meredith.williamson@tamu.edu.
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.