User login
Heparin-Induced Bullous Hemorrhagic Dermatosis Confined to the Oral Mucosa
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
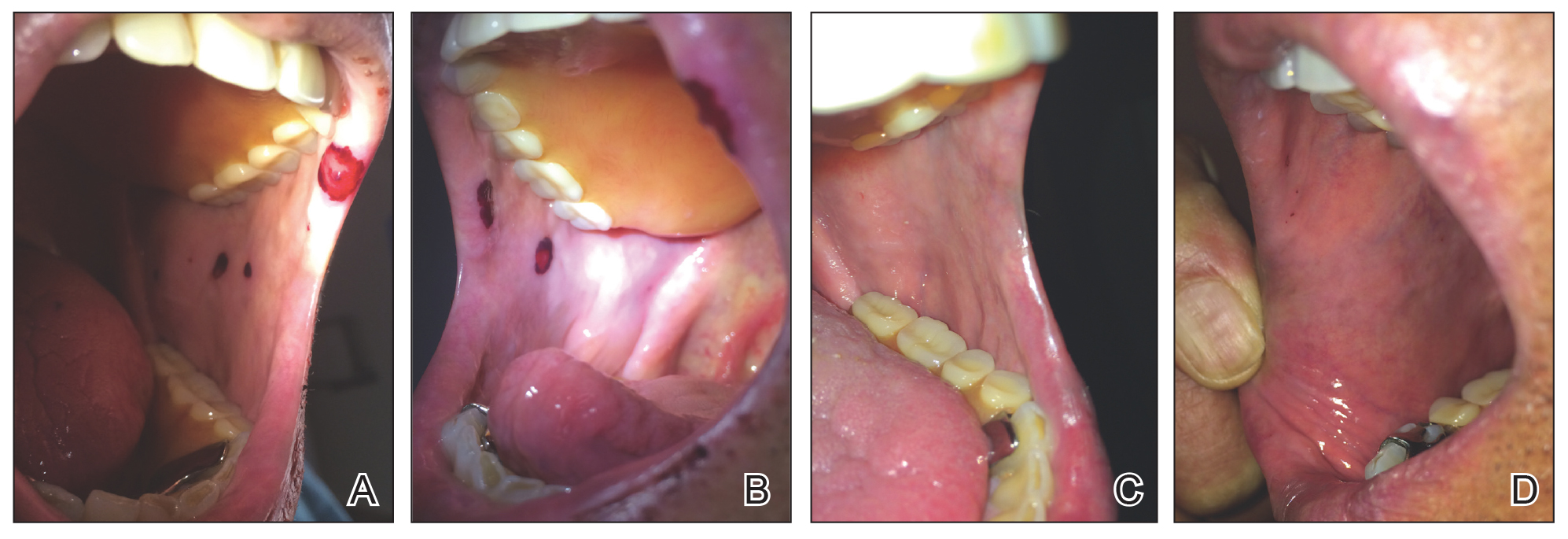
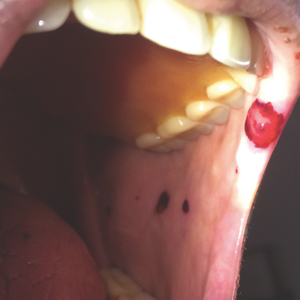
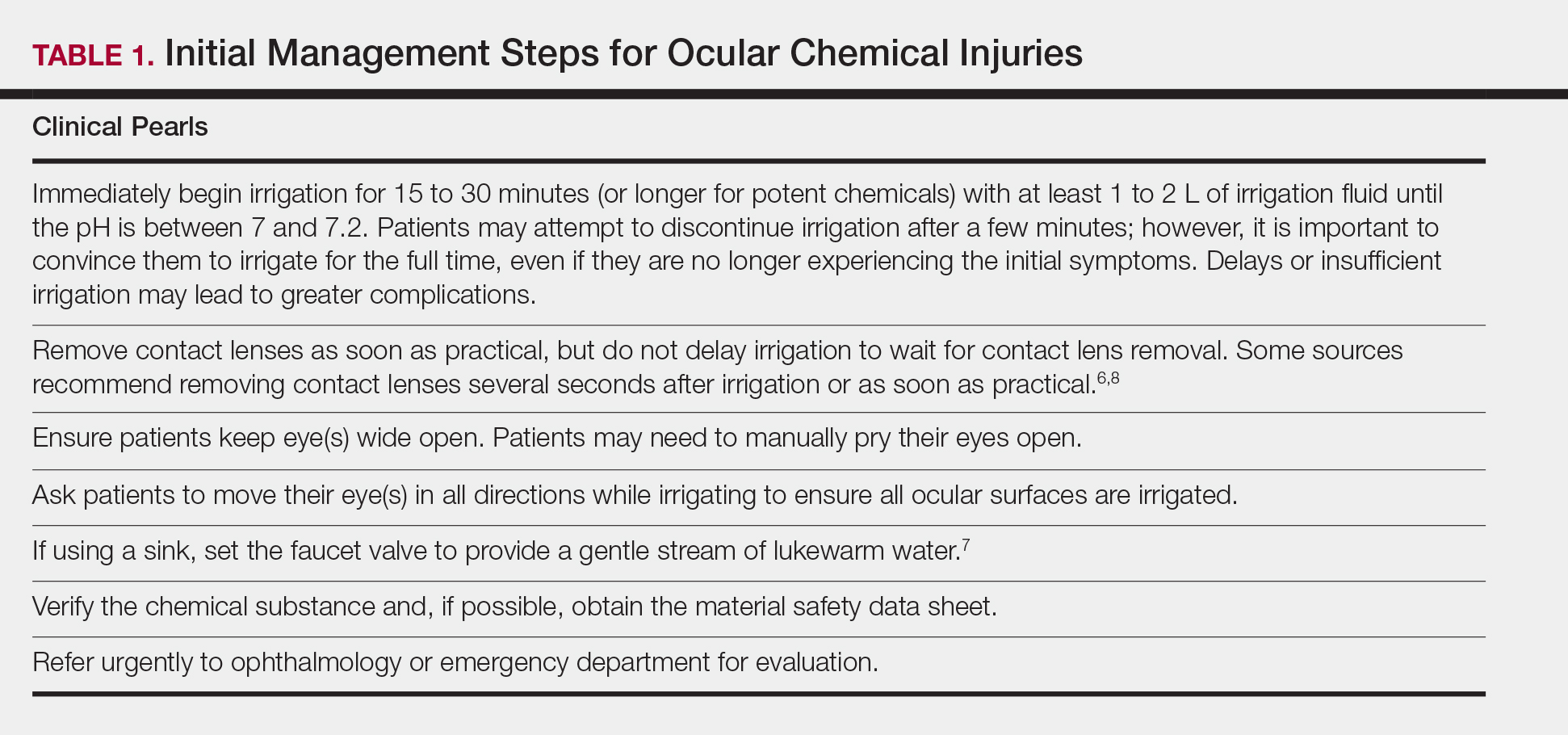
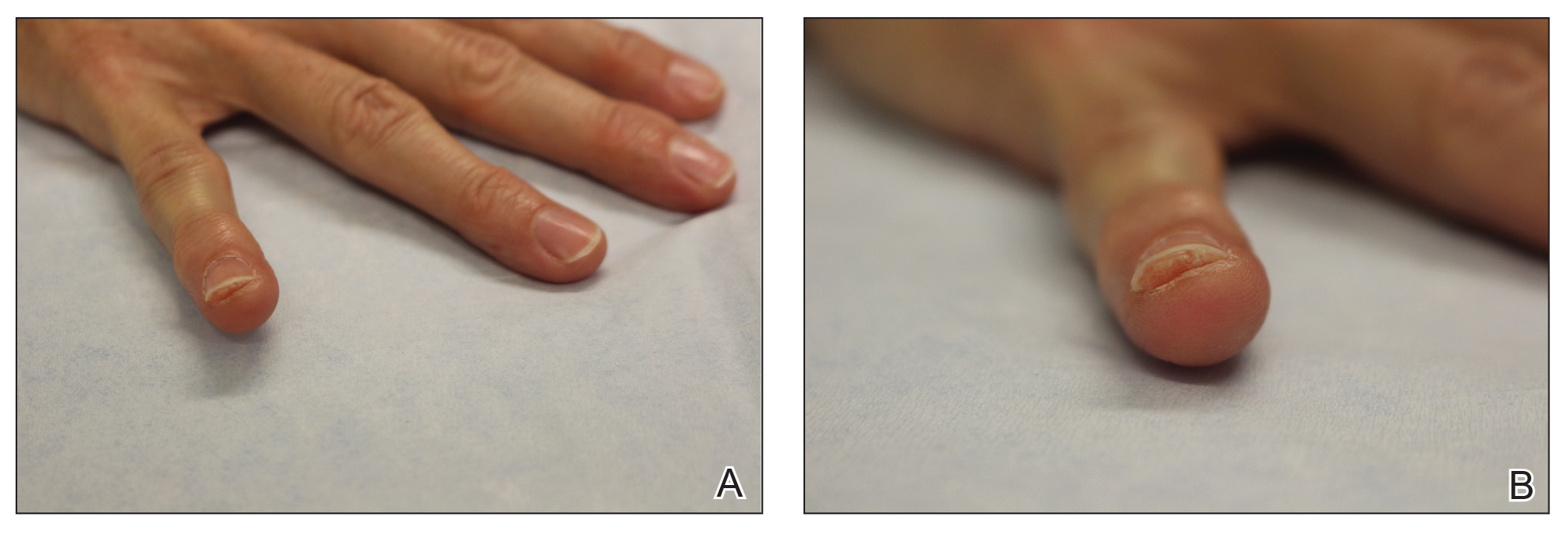
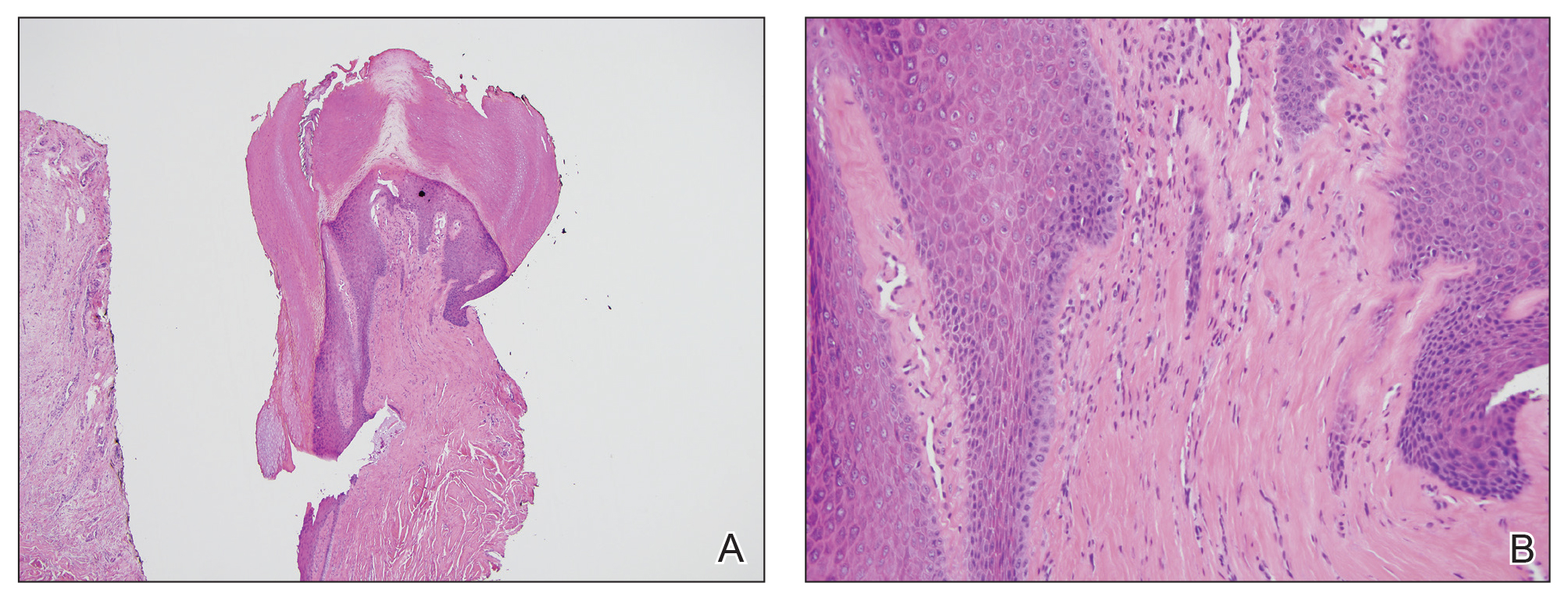
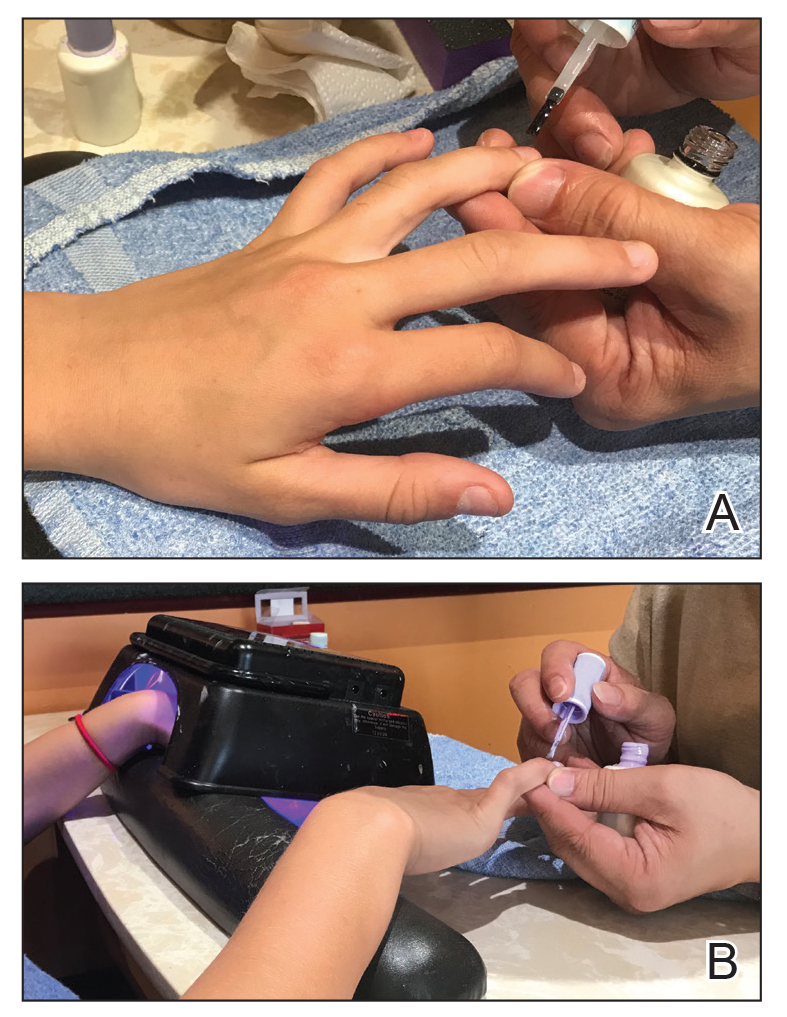
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Practice Points
- It is important for physicians to recognize the clinical appearance of cutaneous adverse reactions to heparin, including bullous hemorrhagic dermatosis.
- Heparin-induced bullous hemorrhagic dermatosis tends to self-resolve, even with continuation of unfractionated heparin.
Air-conditioned cognition, brain worm, and six-fingered success
(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

Erythema Gyratum Repens–like Eruption in Sézary Syndrome: Evidence for the Role of a Dermatophyte
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
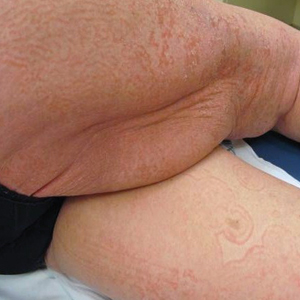
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
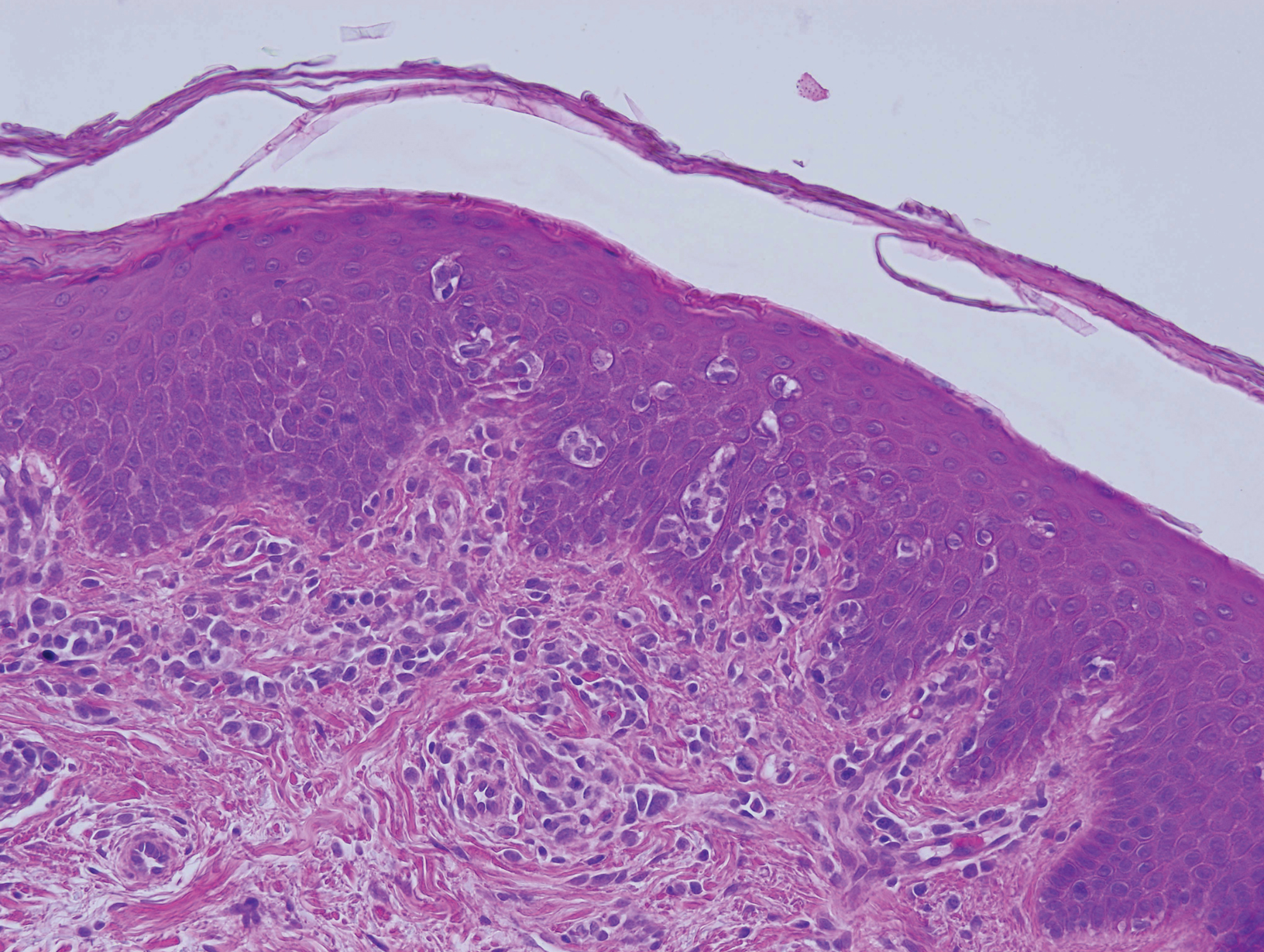
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
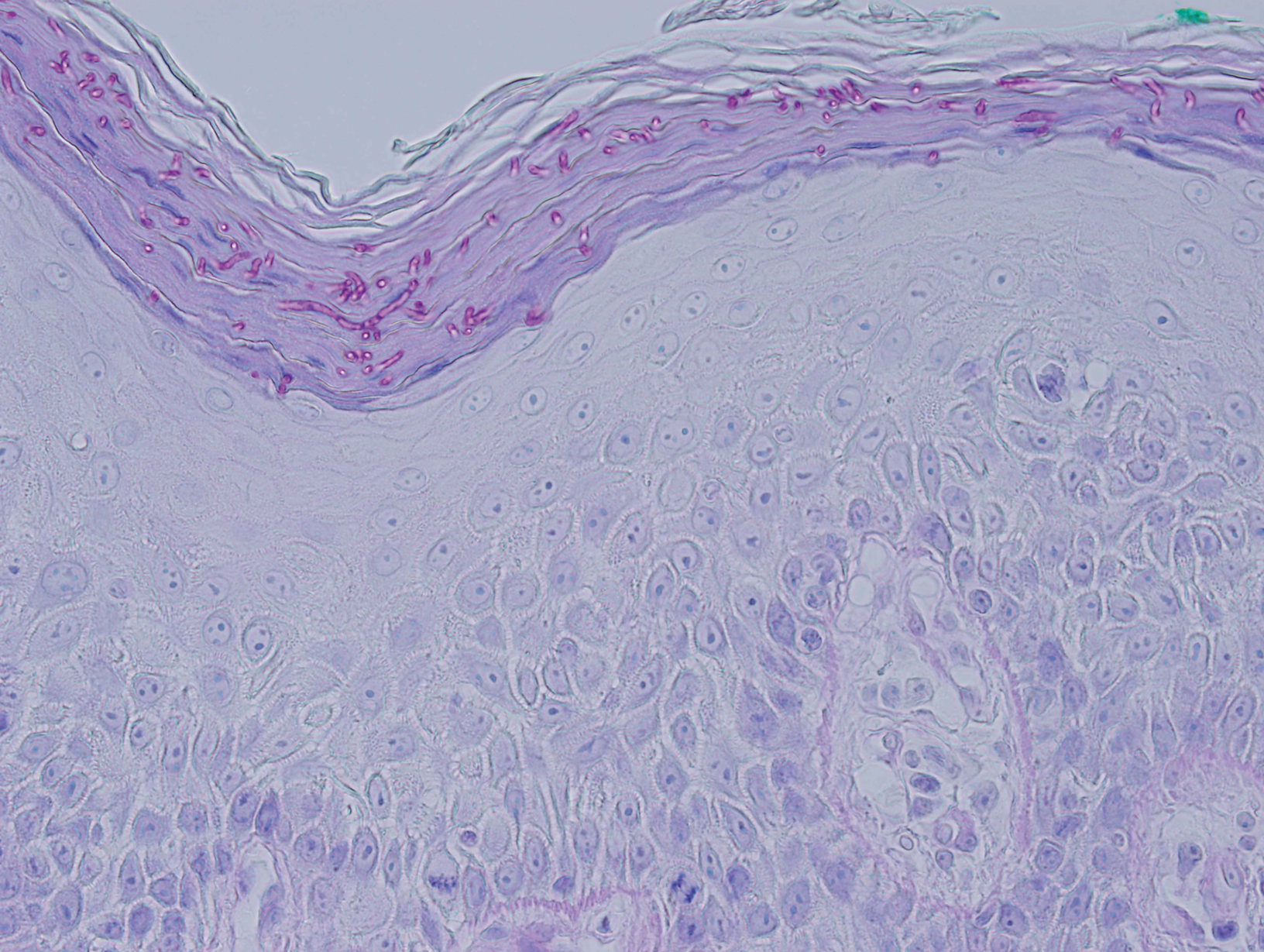
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
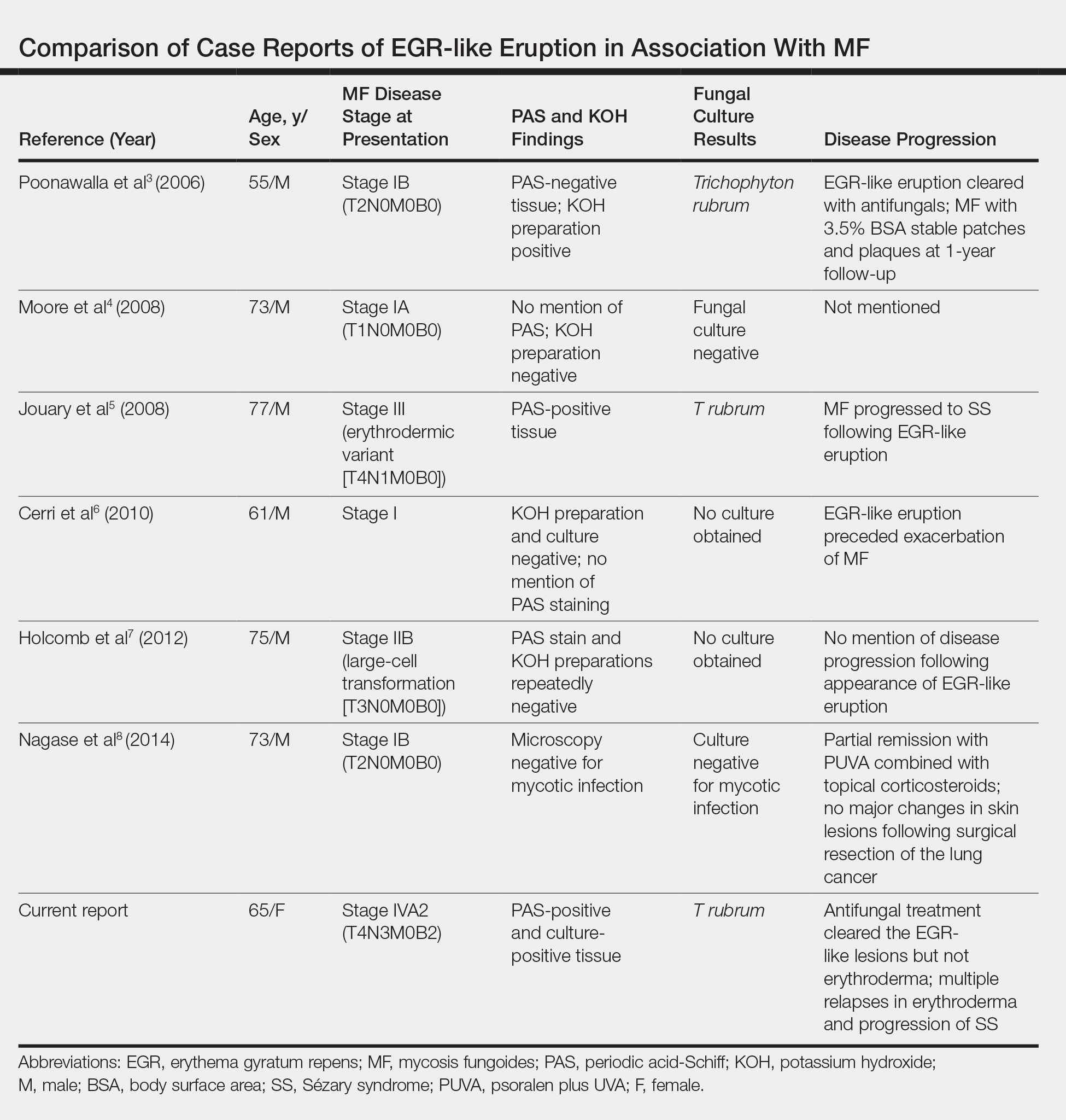
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Practice Points
- Erythema gyratum repens (EGR) presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with fine trailing scale.
- Although EGR typically is associated with underlying malignancy, it is not an obligate paraneoplastic syndrome. There are numerous cases that are not associated with underlying neoplasms.
- An EGR-like eruption may be observed in Sézary syndrome, and an overlying superficial dermatophyte infection may play a role.
Addressing Health Literacy for Miscommunication in Dermatology
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
Ocular Chemical Burns in the Dermatology Office: A Practical Approach to Managing Safety Precautions
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Practice Points
- Dermatologists should be cognizant of potential hazards to the eyes during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- If a patient’s eye(s) becomes exposed to a chemical during a dermatologic procedure, immediate copious irrigation for at least 15 to 30 minutes (longer for alkaline burns) is crucial, followed by prompt evaluation by an ophthalmologist.
- The patient should be instructed to manually hold open the eye and move the eyeball in all directions to achieve the most effective irrigation of the chemical.
- If the patient is wearing contact lenses, they should be removed promptly, but do not delay the irrigation to do so. Lenses should be removed once irrigation is underway.
Acquired Digital Fibrokeratoma Presenting as a Painless Nodule on the Right Fifth Fingernail
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Practice Points
- Acquired digital fibrokeratoma is a benign tumor of the nail bed and periungual area.
- Histopathology shows epidermal acanthosis and hyperkeratosis, and collagen bundles are arranged in a vertical orientation to the long axis of the epidermis.
- Acquired digital fibrokeratoma should be considered in the differential diagnosis of flesh-colored papules on the nail unit associated with longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.
Treatment Consideration for US Military Members With Skin Disease
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
Practice Points
- Certain conditions and treatments are incompatible with military service and may result in separation.
- Dermatologists must consider a patient’s profession when choosing a treatment modality.
Trends in Nail Services May Cause Dermatitis: Not Your Mother’s Nail Polish
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
Practice Points
- Changing trends in nail services mean new exposures for consumers. Traditional nail polish has been replaced by semipermanent nail polish, which contains acrylates.
- Acrylates are a common cause of allergic contact dermatitis from nail polish. Acrylates can be found in gel, dip, and shellac nail polishes, among others.
- Patch testing with 2-hydroxyethyl methacrylate and ethyl cyanoacrylate can screen many patients for allergy due to nail services.
Pazopanib extended PFS in patients with carcinoid tumors
CHICAGO – Pazopanib significantly improved progression-free survival (PFS) in patients with progressive carcinoid tumors who were enrolled in a prospective randomized phase 2 trial.*
Median PFS was 11.6 months for patients receiving the small molecule VEGF inhibitor, versus 8.5 months for those receiving placebo (P = .0005), according to results of the Alliance A021202 trial.
This is the first randomized study to show that the vascular endothelial growth factor (VEGF) pathway may be a valid therapeutic target in carcinoid tumors, said investigator Emily K. Bergsland, MD, of the University of California, San Francisco.
However, the potential benefits of pazopanib need to be viewed in light of toxicity risks, including an excess of hypertension, Dr. Bergsland said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
The efficacy results suggest that pazopanib is another promising systemic option for carcinoid tumors, according to William P. Harris, MD, of the University of Washington and Fred Hutchinson Cancer Research Center, Seattle.
“Pazopanib does inhibit other targets, including [fibroblast growth factor receptors], which may be of relevance in carcinoid,” Dr. Harris noted in a podium discussion of the A021202 trial results.
Other promising systemic options under investigation include cabozantinib, which he said will be evaluated in phase 3 trials, lenvatinib, and ramucirumab plus a somatostatin analogue.
The CDK4/6 inhibition is of interest and will be pursued in future trials, though unfortunately the role of checkpoint inhibitors is “unclear” in this tumor type, he said.
In the present randomized study of pazopanib, a computer error resulted in slightly more patients being randomized to the pazopanib arm – 89 patients – while 72 were randomized to placebo, according to the investigator.
The rate of grade 3 or greater toxicities was 73.0% for pazopanib, and 7.9% for placebo in the study. Notably, there was a relatively high rate of grade 3 or greater hypertension, at 26.9% versus 4.2% for placebo, though only one case in the pazopanib arm was grade 4, Dr. Bergsland said.
Pazopanib was also associated with more symptoms such as diarrhea, appetite loss, and fatigue, but the overall quality of life was similar between the groups in preliminary analyses, said Dr. Bergsland.
Despite the improved PFS in the pazopanib arm, there was no improvement in overall survival. That’s likely because placebo-treated patients were allowed to cross over to pazopanib upon progression, and two-thirds of them did so, said Dr. Bergsland. The resulting median overall survival was 41.3 months for pazopanib and 42.4 months for placebo.
“Additional work is needed to identify strategies for mitigating toxicity and/or selecting patients most likely to benefit,” Dr. Bergsland said in her presentation.
Toward that end, investigators are looking at strategies including angiome profiling, assessment of tumor growth rate, and textural image analysis, she added.
Dr. Bergsland reported disclosures related to More Health, UpToDate, Advanced Accelerator Applications, Lexicon, Merck, and Novartis.
SOURCE: Bergsland EK, et al. ASCO 2019. Abstract 4005.
Correction, 6/17/19: An earlier version of this article mischaracterized the study type.
CHICAGO – Pazopanib significantly improved progression-free survival (PFS) in patients with progressive carcinoid tumors who were enrolled in a prospective randomized phase 2 trial.*
Median PFS was 11.6 months for patients receiving the small molecule VEGF inhibitor, versus 8.5 months for those receiving placebo (P = .0005), according to results of the Alliance A021202 trial.
This is the first randomized study to show that the vascular endothelial growth factor (VEGF) pathway may be a valid therapeutic target in carcinoid tumors, said investigator Emily K. Bergsland, MD, of the University of California, San Francisco.
However, the potential benefits of pazopanib need to be viewed in light of toxicity risks, including an excess of hypertension, Dr. Bergsland said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
The efficacy results suggest that pazopanib is another promising systemic option for carcinoid tumors, according to William P. Harris, MD, of the University of Washington and Fred Hutchinson Cancer Research Center, Seattle.
“Pazopanib does inhibit other targets, including [fibroblast growth factor receptors], which may be of relevance in carcinoid,” Dr. Harris noted in a podium discussion of the A021202 trial results.
Other promising systemic options under investigation include cabozantinib, which he said will be evaluated in phase 3 trials, lenvatinib, and ramucirumab plus a somatostatin analogue.
The CDK4/6 inhibition is of interest and will be pursued in future trials, though unfortunately the role of checkpoint inhibitors is “unclear” in this tumor type, he said.
In the present randomized study of pazopanib, a computer error resulted in slightly more patients being randomized to the pazopanib arm – 89 patients – while 72 were randomized to placebo, according to the investigator.
The rate of grade 3 or greater toxicities was 73.0% for pazopanib, and 7.9% for placebo in the study. Notably, there was a relatively high rate of grade 3 or greater hypertension, at 26.9% versus 4.2% for placebo, though only one case in the pazopanib arm was grade 4, Dr. Bergsland said.
Pazopanib was also associated with more symptoms such as diarrhea, appetite loss, and fatigue, but the overall quality of life was similar between the groups in preliminary analyses, said Dr. Bergsland.
Despite the improved PFS in the pazopanib arm, there was no improvement in overall survival. That’s likely because placebo-treated patients were allowed to cross over to pazopanib upon progression, and two-thirds of them did so, said Dr. Bergsland. The resulting median overall survival was 41.3 months for pazopanib and 42.4 months for placebo.
“Additional work is needed to identify strategies for mitigating toxicity and/or selecting patients most likely to benefit,” Dr. Bergsland said in her presentation.
Toward that end, investigators are looking at strategies including angiome profiling, assessment of tumor growth rate, and textural image analysis, she added.
Dr. Bergsland reported disclosures related to More Health, UpToDate, Advanced Accelerator Applications, Lexicon, Merck, and Novartis.
SOURCE: Bergsland EK, et al. ASCO 2019. Abstract 4005.
Correction, 6/17/19: An earlier version of this article mischaracterized the study type.
CHICAGO – Pazopanib significantly improved progression-free survival (PFS) in patients with progressive carcinoid tumors who were enrolled in a prospective randomized phase 2 trial.*
Median PFS was 11.6 months for patients receiving the small molecule VEGF inhibitor, versus 8.5 months for those receiving placebo (P = .0005), according to results of the Alliance A021202 trial.
This is the first randomized study to show that the vascular endothelial growth factor (VEGF) pathway may be a valid therapeutic target in carcinoid tumors, said investigator Emily K. Bergsland, MD, of the University of California, San Francisco.
However, the potential benefits of pazopanib need to be viewed in light of toxicity risks, including an excess of hypertension, Dr. Bergsland said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
The efficacy results suggest that pazopanib is another promising systemic option for carcinoid tumors, according to William P. Harris, MD, of the University of Washington and Fred Hutchinson Cancer Research Center, Seattle.
“Pazopanib does inhibit other targets, including [fibroblast growth factor receptors], which may be of relevance in carcinoid,” Dr. Harris noted in a podium discussion of the A021202 trial results.
Other promising systemic options under investigation include cabozantinib, which he said will be evaluated in phase 3 trials, lenvatinib, and ramucirumab plus a somatostatin analogue.
The CDK4/6 inhibition is of interest and will be pursued in future trials, though unfortunately the role of checkpoint inhibitors is “unclear” in this tumor type, he said.
In the present randomized study of pazopanib, a computer error resulted in slightly more patients being randomized to the pazopanib arm – 89 patients – while 72 were randomized to placebo, according to the investigator.
The rate of grade 3 or greater toxicities was 73.0% for pazopanib, and 7.9% for placebo in the study. Notably, there was a relatively high rate of grade 3 or greater hypertension, at 26.9% versus 4.2% for placebo, though only one case in the pazopanib arm was grade 4, Dr. Bergsland said.
Pazopanib was also associated with more symptoms such as diarrhea, appetite loss, and fatigue, but the overall quality of life was similar between the groups in preliminary analyses, said Dr. Bergsland.
Despite the improved PFS in the pazopanib arm, there was no improvement in overall survival. That’s likely because placebo-treated patients were allowed to cross over to pazopanib upon progression, and two-thirds of them did so, said Dr. Bergsland. The resulting median overall survival was 41.3 months for pazopanib and 42.4 months for placebo.
“Additional work is needed to identify strategies for mitigating toxicity and/or selecting patients most likely to benefit,” Dr. Bergsland said in her presentation.
Toward that end, investigators are looking at strategies including angiome profiling, assessment of tumor growth rate, and textural image analysis, she added.
Dr. Bergsland reported disclosures related to More Health, UpToDate, Advanced Accelerator Applications, Lexicon, Merck, and Novartis.
SOURCE: Bergsland EK, et al. ASCO 2019. Abstract 4005.
Correction, 6/17/19: An earlier version of this article mischaracterized the study type.
REPORTING FROM ASCO 2019
Review clarifies depression, anxiety risk among hidradenitis suppurativa patients
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
FROM JAMA DERMATOLOGY