User login
REIMBURSEMENT ADVISER
- Patient is a virgin, takes no hormones, and refuses a pelvic exam and Pap smear. Blood pressure is in the normal range. Body mass index is 21. She reports no problems and has no questions.
- Examination of breasts reveals normal skin and nipples, no masses or tenderness, and no lymph-node swelling.
- Patient is given a slip for a routine mammogram and instructions on performing breast self-exam, and is instructed to return in 1 year, barring problems or concerns.
If you report this visit as a problem E/M service using only this diagnosis, on the other hand, you are more than likely to be denied by Medicare.
For Medicare to consider this a covered service billed as a problem E/M service, you would also have to list diagnostic codes that indicate a complaint, a history of a breast condition, or a strong family history of breast cancer. Medicare will pay for the screening mammogram, but the screening breast exam by itself may not be considered a covered service.
You have a few options:
- Contact the Medicare carrier and explain the situation. See if they propose a coding solution that they will accept. Get their answer in writing!
- Bill Medicare using a low-level E/M code (eg, 99212, problem focused exam with straightforward medical decision making) linked to the diagnosis code V76.19. If you choose this option, have the patient sign a waiver that she is responsible for payment should Medicare deny the service. Add the modifier –GA (waiver of liability statement on file) to the problem E/M code. This will allow you to collect payment from the patient.
- Submit the unlisted code or preventive services 99429 because you performed an exam—although not one that meets the criteria of age-specific preventive codes. This code is never reimbursed by Medicare, but once you get a denial, you either can collect from the patient or are able to submit the charge to any secondary insurance she might have. A modifier –GY (item or service statutorily excluded or does not meet the definition of any Medicare benefit) would also need to be added to the preventive medicine code.
Fern testing: CLIA-waived but payer might not cover
Code 87210, in addition to requiring addition of saline or potassium chloride, is not a CLIA-waived test. You would not be able to bill for it unless you have an advanced lab certificate.
Code 89060 is assigned when looking for crystals in synovial fluid. It is also not a CLIA-waived or physician-performed microscopy test, so billing using this code would require an advanced lab certificate as well.
The advent of the national code set has meant that your payers are required to recognize all codes, although they can determine whether to cover a service or not. It may be that this test isn’t covered by your payer, rather than the code not being recognized as correct.
Two voiding studies: Bill together but specify parts
The “9” indicator used by Medicare for bundled codes means that the edit was deleted. In this case, it was deleted on the same date it was added. For some reason, Medicare elects not to remove deleted code pairs from the master database. Although you will get paid for both of these codes, the code order is different depending on whether you are using your own equipment (because of differences in relative value units).
If you bill each test with a modifier -26 (professional component only), you do not own the equipment and the place of service is a facility. In that case, list 51797-26 first and 51795-26-51 second. The modifier -51 is used on the second code because this is a multiple procedure. If you are billing both professional and technical components (ie, you are using your equipment, in the office), billing order is reversed: List 51797 first and 51795-51 second. Do not use a modifier -59 with this code combination.
Fetal genetic abnormality inferred from US; code for further study
At the time of the sonogram, therefore, you can only suspect a problem with the fetal genes; further testing is required. In that case, report 655.13 (known or suspected chromosomal abnormality of the fetus affecting management of mother; antepartum condition or complication) with a secondary diagnosis of 793.99 (other nonspecific abnormal findings on radiological and other examinations of body structure).
Positive ANA—don’t leap to “autoimmune disorder”
Because you have not eliminated the other possibilities for the positive ANA, it is premature to assign the code for an autoimmune condition. Instead, report 648.93 as your primary code (Other current conditions in the mother classifiable elsewhere, complicating pregnancy, childbirth, or the puerperium; antepartum condition or complication), with the secondary diagnosis code 795.79 (Other and unspecified nonspecific immunological findings).
Coding Zoladex depends on the patient’s condition
The drug is supplied as tiny pellets, which are injected under the skin of the abdomen using a small, “trocar-like” needle and syringe. The procedure constitutes an injection. If you are treating breast cancer with this drug, the correct code would be 96402 (Chemotherapy administration, subcutaneous or intramuscular; hormonal antineoplastic). The code for the pellets is J9202 (Goserelin acetate implant, per 3.6 mg). If you administer more than 3.6 mg at a time, remember to adjust the quantity you bill for. If you are using this drug to treat endometriosis or fibroids, CPT directs you to report 90772 for the injection because it is then considered a nonantineoplastic hormone injection.
Call a contraceptive a contraceptive when coding
Implanon’s manufacturer thinks the correct code is 11981 (Insertion, nonbiodegradable drug delivery implant), but I disagree: This is a contraceptive that is implanted under the skin and, under CPT rules, you must use the code that most closely describes the procedure.
Note also that, although Implanon involves insertion of one rod (other systems require insertion of several), the code 11981 has greater relative value units than 11975. This payment difference will not be lost on most payers because the diagnostic link for the procedure, whichever code is reported, is V25.5 (insertion of implantable subdermal contraceptive).
- Patient is a virgin, takes no hormones, and refuses a pelvic exam and Pap smear. Blood pressure is in the normal range. Body mass index is 21. She reports no problems and has no questions.
- Examination of breasts reveals normal skin and nipples, no masses or tenderness, and no lymph-node swelling.
- Patient is given a slip for a routine mammogram and instructions on performing breast self-exam, and is instructed to return in 1 year, barring problems or concerns.
If you report this visit as a problem E/M service using only this diagnosis, on the other hand, you are more than likely to be denied by Medicare.
For Medicare to consider this a covered service billed as a problem E/M service, you would also have to list diagnostic codes that indicate a complaint, a history of a breast condition, or a strong family history of breast cancer. Medicare will pay for the screening mammogram, but the screening breast exam by itself may not be considered a covered service.
You have a few options:
- Contact the Medicare carrier and explain the situation. See if they propose a coding solution that they will accept. Get their answer in writing!
- Bill Medicare using a low-level E/M code (eg, 99212, problem focused exam with straightforward medical decision making) linked to the diagnosis code V76.19. If you choose this option, have the patient sign a waiver that she is responsible for payment should Medicare deny the service. Add the modifier –GA (waiver of liability statement on file) to the problem E/M code. This will allow you to collect payment from the patient.
- Submit the unlisted code or preventive services 99429 because you performed an exam—although not one that meets the criteria of age-specific preventive codes. This code is never reimbursed by Medicare, but once you get a denial, you either can collect from the patient or are able to submit the charge to any secondary insurance she might have. A modifier –GY (item or service statutorily excluded or does not meet the definition of any Medicare benefit) would also need to be added to the preventive medicine code.
Fern testing: CLIA-waived but payer might not cover
Code 87210, in addition to requiring addition of saline or potassium chloride, is not a CLIA-waived test. You would not be able to bill for it unless you have an advanced lab certificate.
Code 89060 is assigned when looking for crystals in synovial fluid. It is also not a CLIA-waived or physician-performed microscopy test, so billing using this code would require an advanced lab certificate as well.
The advent of the national code set has meant that your payers are required to recognize all codes, although they can determine whether to cover a service or not. It may be that this test isn’t covered by your payer, rather than the code not being recognized as correct.
Two voiding studies: Bill together but specify parts
The “9” indicator used by Medicare for bundled codes means that the edit was deleted. In this case, it was deleted on the same date it was added. For some reason, Medicare elects not to remove deleted code pairs from the master database. Although you will get paid for both of these codes, the code order is different depending on whether you are using your own equipment (because of differences in relative value units).
If you bill each test with a modifier -26 (professional component only), you do not own the equipment and the place of service is a facility. In that case, list 51797-26 first and 51795-26-51 second. The modifier -51 is used on the second code because this is a multiple procedure. If you are billing both professional and technical components (ie, you are using your equipment, in the office), billing order is reversed: List 51797 first and 51795-51 second. Do not use a modifier -59 with this code combination.
Fetal genetic abnormality inferred from US; code for further study
At the time of the sonogram, therefore, you can only suspect a problem with the fetal genes; further testing is required. In that case, report 655.13 (known or suspected chromosomal abnormality of the fetus affecting management of mother; antepartum condition or complication) with a secondary diagnosis of 793.99 (other nonspecific abnormal findings on radiological and other examinations of body structure).
Positive ANA—don’t leap to “autoimmune disorder”
Because you have not eliminated the other possibilities for the positive ANA, it is premature to assign the code for an autoimmune condition. Instead, report 648.93 as your primary code (Other current conditions in the mother classifiable elsewhere, complicating pregnancy, childbirth, or the puerperium; antepartum condition or complication), with the secondary diagnosis code 795.79 (Other and unspecified nonspecific immunological findings).
Coding Zoladex depends on the patient’s condition
The drug is supplied as tiny pellets, which are injected under the skin of the abdomen using a small, “trocar-like” needle and syringe. The procedure constitutes an injection. If you are treating breast cancer with this drug, the correct code would be 96402 (Chemotherapy administration, subcutaneous or intramuscular; hormonal antineoplastic). The code for the pellets is J9202 (Goserelin acetate implant, per 3.6 mg). If you administer more than 3.6 mg at a time, remember to adjust the quantity you bill for. If you are using this drug to treat endometriosis or fibroids, CPT directs you to report 90772 for the injection because it is then considered a nonantineoplastic hormone injection.
Call a contraceptive a contraceptive when coding
Implanon’s manufacturer thinks the correct code is 11981 (Insertion, nonbiodegradable drug delivery implant), but I disagree: This is a contraceptive that is implanted under the skin and, under CPT rules, you must use the code that most closely describes the procedure.
Note also that, although Implanon involves insertion of one rod (other systems require insertion of several), the code 11981 has greater relative value units than 11975. This payment difference will not be lost on most payers because the diagnostic link for the procedure, whichever code is reported, is V25.5 (insertion of implantable subdermal contraceptive).
- Patient is a virgin, takes no hormones, and refuses a pelvic exam and Pap smear. Blood pressure is in the normal range. Body mass index is 21. She reports no problems and has no questions.
- Examination of breasts reveals normal skin and nipples, no masses or tenderness, and no lymph-node swelling.
- Patient is given a slip for a routine mammogram and instructions on performing breast self-exam, and is instructed to return in 1 year, barring problems or concerns.
If you report this visit as a problem E/M service using only this diagnosis, on the other hand, you are more than likely to be denied by Medicare.
For Medicare to consider this a covered service billed as a problem E/M service, you would also have to list diagnostic codes that indicate a complaint, a history of a breast condition, or a strong family history of breast cancer. Medicare will pay for the screening mammogram, but the screening breast exam by itself may not be considered a covered service.
You have a few options:
- Contact the Medicare carrier and explain the situation. See if they propose a coding solution that they will accept. Get their answer in writing!
- Bill Medicare using a low-level E/M code (eg, 99212, problem focused exam with straightforward medical decision making) linked to the diagnosis code V76.19. If you choose this option, have the patient sign a waiver that she is responsible for payment should Medicare deny the service. Add the modifier –GA (waiver of liability statement on file) to the problem E/M code. This will allow you to collect payment from the patient.
- Submit the unlisted code or preventive services 99429 because you performed an exam—although not one that meets the criteria of age-specific preventive codes. This code is never reimbursed by Medicare, but once you get a denial, you either can collect from the patient or are able to submit the charge to any secondary insurance she might have. A modifier –GY (item or service statutorily excluded or does not meet the definition of any Medicare benefit) would also need to be added to the preventive medicine code.
Fern testing: CLIA-waived but payer might not cover
Code 87210, in addition to requiring addition of saline or potassium chloride, is not a CLIA-waived test. You would not be able to bill for it unless you have an advanced lab certificate.
Code 89060 is assigned when looking for crystals in synovial fluid. It is also not a CLIA-waived or physician-performed microscopy test, so billing using this code would require an advanced lab certificate as well.
The advent of the national code set has meant that your payers are required to recognize all codes, although they can determine whether to cover a service or not. It may be that this test isn’t covered by your payer, rather than the code not being recognized as correct.
Two voiding studies: Bill together but specify parts
The “9” indicator used by Medicare for bundled codes means that the edit was deleted. In this case, it was deleted on the same date it was added. For some reason, Medicare elects not to remove deleted code pairs from the master database. Although you will get paid for both of these codes, the code order is different depending on whether you are using your own equipment (because of differences in relative value units).
If you bill each test with a modifier -26 (professional component only), you do not own the equipment and the place of service is a facility. In that case, list 51797-26 first and 51795-26-51 second. The modifier -51 is used on the second code because this is a multiple procedure. If you are billing both professional and technical components (ie, you are using your equipment, in the office), billing order is reversed: List 51797 first and 51795-51 second. Do not use a modifier -59 with this code combination.
Fetal genetic abnormality inferred from US; code for further study
At the time of the sonogram, therefore, you can only suspect a problem with the fetal genes; further testing is required. In that case, report 655.13 (known or suspected chromosomal abnormality of the fetus affecting management of mother; antepartum condition or complication) with a secondary diagnosis of 793.99 (other nonspecific abnormal findings on radiological and other examinations of body structure).
Positive ANA—don’t leap to “autoimmune disorder”
Because you have not eliminated the other possibilities for the positive ANA, it is premature to assign the code for an autoimmune condition. Instead, report 648.93 as your primary code (Other current conditions in the mother classifiable elsewhere, complicating pregnancy, childbirth, or the puerperium; antepartum condition or complication), with the secondary diagnosis code 795.79 (Other and unspecified nonspecific immunological findings).
Coding Zoladex depends on the patient’s condition
The drug is supplied as tiny pellets, which are injected under the skin of the abdomen using a small, “trocar-like” needle and syringe. The procedure constitutes an injection. If you are treating breast cancer with this drug, the correct code would be 96402 (Chemotherapy administration, subcutaneous or intramuscular; hormonal antineoplastic). The code for the pellets is J9202 (Goserelin acetate implant, per 3.6 mg). If you administer more than 3.6 mg at a time, remember to adjust the quantity you bill for. If you are using this drug to treat endometriosis or fibroids, CPT directs you to report 90772 for the injection because it is then considered a nonantineoplastic hormone injection.
Call a contraceptive a contraceptive when coding
Implanon’s manufacturer thinks the correct code is 11981 (Insertion, nonbiodegradable drug delivery implant), but I disagree: This is a contraceptive that is implanted under the skin and, under CPT rules, you must use the code that most closely describes the procedure.
Note also that, although Implanon involves insertion of one rod (other systems require insertion of several), the code 11981 has greater relative value units than 11975. This payment difference will not be lost on most payers because the diagnostic link for the procedure, whichever code is reported, is V25.5 (insertion of implantable subdermal contraceptive).
Symptoms Postdischarge
The Institute of Medicine reports To Err is Human and Crossing the Quality Chasm have drawn great attention to quality improvement and patient safety in the hospital setting.13 With the growth of the hospitalist field over the past several years,4 there has been increasing discussion about the importance of assuring quality of care, and some have argued that improving health care quality and reducing avoidable errors may be among the hospitalist's most important functions.5 Most discussions about the quality of hospital care have concerned the inpatient stay itself. However, the growth of hospital medicine, with its inherent discontinuity between inpatient and outpatient physicians, has intensified interest in the transition period from hospital discharge until first outpatient appointment.
At discharge, physicians may prescribe medications, order home health services, and arrange follow‐up appointments. It is often assumed a patient will remain stable after discharge and will follow up at the outpatient physician's office. Previous research has shown there may be problems with these assumptions. A patient may not understand the postdischarge treatment plan as well as the physician thinks.6 A recent study found that adverse events after discharge were common and often preventable.7 A follow‐up study confirmed that approximately 25% of patients had an adverse event after hospital discharge and that most adverse events caused symptoms but did not result in an emergency department visit, hospitalization, or death.8 Another study also found the prevalence of medical errors following hospitalization was high because of the discontinuity of care from the inpatient to the outpatient setting.9 These errors resulted in an increased rate of rehospitalization.
Telephone follow‐up may be a useful method of bridging the gap in care between discharge and the first outpatient appointment.10, 11 In most previous studies it was 2 or 3 weeks after discharge before patients were contacted or their records studied. By this point, patients who had done poorly may already have been readmitted or sought care at alternative locations. In one small study, pharmacists found that 12 of 79 patients (15%) contacted by telephone within 2 days of discharge12 had symptoms there were new or had worsened since discharge. The purpose of the present investigation was to extend these previous findings through a large multicenter study of how frequently patients had new or worsened symptoms within several days of discharge.
METHODS
Settings and Participants
IPCThe Hospitalist Company has hospitalist practices at more than 150 health care facilities in 10 health care markets. At the time of the study, IPC employed more than 300 internal medicine and family practice physicians and discharged approximately 11,000 adult patients per month. The study is a retrospective analysis of data collected from May 1, 2003, to October 31, 2003.
Data Acquisition
Physicians entered clinical and financial information on all hospitalized patients into a handheld personal digital assistant (PDA) utilizing functions of IPC‐LINK software. At the time of discharge, a physician completed a discharge summary on the PDA that was transmitted electronically to a centralized data center. Copies of the discharge summary were also faxed to the outpatient physician's office. Patients were first interviewed by call‐center patient representatives, unlicensed staff with medical backgrounds. Call‐center representatives made several attempts by telephone to reach all patients discharged home within several days of discharge. Using a scripted survey instrument (Appendix A), they asked patients or family members a series of questions about clinical status, new or worsening symptoms, problems with medications or prescribed home health care services, follow‐up appointments with their outpatient physician, and satisfaction with the care received. Call‐center nurses, licensed personnel with extensive medical/surgical and case management experience, contacted patients whose answers to questions on the scripted survey instrument (see last section of Appendix A) indicated a high risk of postdischarge problems, intervening as necessary to resolve the health care issues.
Health status was self rated on a 5‐point Likert scale from excellent to poor in response to the health status question on the SF‐12.13, 14 Patient age was calculated using birth date and admit date from the IPC‐Link discharge summary. With clinical data from the IPC‐Link discharge summary, the 3M DRG Grouper was used to assign each patient a DRG and severity of illness (SOI) score.15 Reported symptoms were grouped in clinically meaningful categories by the lead author.
Statistical Analysis
Logistic regression analysis was performed to analyze the effects of sex, health insurance, inpatient severity of illness, and self‐reported health status on the proportion of patients with symptoms. Sex, health status, and severity of illness were treated as ordered variables. Because insurance type is a nominal variable, HMO was used as the reference category, and the other categories were converted to indicator variables. Pearson chi‐square testing was used for all other analyses. The large number of planned analyses necessitated adjustment of the P values computed for the tests to maintain the type I error rate at 0.05. Therefore, a step‐down Bonferroni procedure was used.16
Role of the Funding Source
Data collection, analysis, and interpretation were funded by IPC and performed by employees of IPC.
RESULTS
During the study period, 48,236 patients were discharged to their homes from an acute care hospital. The IPC call center successfully contacted 16,135 patients after discharge, of whom 368 patients (2.3%) were excluded because of incomplete answers, leaving 15,767 as the valid study population (effective response rate = 32.4%). Of these, 98.9% were contacted within the first 5 days. The primary reasons for nonresponse or noninclusion in the present analysis were no answer after 2 attempts (52%) and missing or incorrect phone numbers (16%). If there was an answering machine, a message was left for the patient to call back. Those who called back accounted for fewer than 1% of all the patients.
A comparison of participants versus nonparticipants is shown in Table 1. The mean age of surveyed patients was 60.1 years, and 57% were female. The most common categories of insurance coverage were Medicare and HMO. The inpatient severity of illness of most patients was minor to moderate. Self‐reported health status was normally distributed, with the greatest percentage of patients rating their health as fair or good. On average, nonparticipants were younger than participants, more likely to be male, had a different pattern of health insurance, and a slightly lower severity of illness. The top 10 DRGs were the same for the respondents and nonrespondents, and the order of these 10 diagnoses was very similar.
| Characteristic | Patients in Study | Patients Not in Study | P Value* | ||
|---|---|---|---|---|---|
| Number of Patients | Percentage of All Patients | Number of Patients | Percentage of All Patients | ||
| |||||
| All patients | 15,767 | 32,101 | |||
| Mean age (years) | 60.1 | 54.1 | <.0001 | ||
| Sex | |||||
| Female | 8985 | 57.0% | 17220 | 53.7% | <.0001 |
| Male | 6515 | 41.3% | 14337 | 44.7% | <.0001 |
| Unknown | 267 | 1.7% | 544 | 1.7% | .897 |
| Insurance type | |||||
| HMO | 6391 | 40.5% | 12540 | 39.1% | <.001 |
| Medicaid | 1066 | 6.8% | 2815 | 8.8% | <.0001 |
| Medicare | 6055 | 38.4% | 9777 | 30.4% | <.0001 |
| Commercial and other | 1370 | 8.7% | 3490 | 10.9% | <.0001 |
| Self‐pay | 885 | 5.6% | 3479 | 10.9% | <.0001 |
| Severity of illness | |||||
| Minor | 6740 | 42.7% | 14679 | 45.7% | <.0001 |
| Moderate | 6854 | 43.5% | 13197 | 41.1% | |
| Major | 1688 | 10.7% | 3091 | 9.6% | <.0001 |
| Extreme | 118 | 0.7% | 219 | 0.7% | .571 |
| Unknown | 367 | 2.3% | 915 | 2.9% | .001 |
| Health status | |||||
| Excellent | 343 | 2.2% | N/A | ||
| Very good | 1392 | 8.8% | N/A | ||
| Good | 5505 | 34.9% | N/A | ||
| Fair | 5901 | 37.4% | N/A | ||
| Poor | 1468 | 9.3% | N/A | ||
| Unknown | 1158 | 7.3% | N/A | ||
Of the 15,767 patients contacted, 11.9% (N = 1876) reported symptoms that were new or had worsened since leaving the hospital. Sixty‐four percent of these patients had new symptoms, and 36% had worsening symptoms. These two groups were combined for analysis in this study because for both groups, identification and action are important. Of the patients with new or worse symptoms, 37% required no assistance from the nurse because they had already notified a doctor and/or were doing something about it. The other 63% either had not notified their doctor or had concerns about their signs and symptoms. The most common action taken by the nurse was patient education regarding the symptoms. Of those requiring nurse intervention in addition to education, the most common intervention was to contact the patient's primary care provider or specialist about the patient's symptoms, followed by contacting the hospitalist. In 72% of nurse interventions, the patient's primary care physician or a specialist was contacted about the new or worsened symptoms. Other interventions included contacting the physician's office to obtain a prescription for a medication for the symptom, to get an appointment for the patient, or to reschedule an appointment to be earlier. A referral to an emergency room or urgent care center was given to 4% of patients.
Mean age of the patients with new or worsened symptoms was 60.5 years. The age distribution of symptomatic and asymptomatic patients was not significantly different, whether comparing by mean, median, or decades. Table 2 illustrates factors associated with the increased rate of new or worsened symptoms. Women were more likely than men to report symptoms (13.0% vs. 10.3%, P < .0001). As health status worsened, the percentage of patients with new or worsened symptoms increased (P < .0001), as it did with increased inpatient SOI (P < .0001). There was no correlation between self‐rated health status and SOI score based on DRG score, suggesting they measured different parameters. Table 3 lists the percentages of patients reporting new or worsened symptoms for the most common DRGs. The only significant distinction was that patients discharged with a DRG of chest pain were less likely to report symptoms than were all patients.
| Characteristic | Number of Patients with New or Worsening Symptoms | Percentage of All Patients with New or Worsening Symptoms | P Value for Difference or Trend* |
|---|---|---|---|
| |||
| All Patients | 1876 | 11.9% | |
| Sex | <.0001 | ||
| Female | 1170 | 13.0% | |
| Male | 672 | 10.3% | |
| Insurance Type | |||
| HMO | 722 | 11.3% | .89 |
| Medicare | 748 | 12.4% | .21 |
| Commercial and other | 165 | 12.0% | .53 |
| Medicaid | 128 | 12.0% | .27 |
| Self‐pay | 113 | 12.8% | |
| Severity of illness | .17 | ||
| Minor | 748 | 11.1% | |
| Moderate | 814 | 11.9% | |
| Major | 247 | 14.6% | |
| Extreme | 19 | 16.1% | |
| Health Status | <.0001 | ||
| Excellent | 22 | 6.4% | |
| Very good | 85 | 6.1% | |
| Good | 429 | 7.8% | |
| Fair | 725 | 12.3% | |
| Poor | 384 | 26.2% | |
| DRG | Description | Number of Patients | Percentage of Patients | Patients with New or Worsening Symptoms | Rate of New or Worsening Symptoms | P value |
|---|---|---|---|---|---|---|
| ||||||
| Total patients in Study | 15,767 | 1876 | 11.9% | |||
| 143 | Chest pain | 1306 | 8.3% | 128 | 9.8% | 0.027 |
| 182 | Digest disorders with complications | 801 | 5.1% | 92 | 11.5% | 0.767 |
| 183 | Digest disorders without complications | 632 | 4.0% | 78 | 12.3% | 0.783 |
| 127 | Heart failure and shock | 544 | 3.5% | 55 | 10.1% | 0.230 |
| 89 | Pneumonia with complications | 426 | 2.7% | 39 | 9.2% | 0.098 |
| 88 | COPD | 380 | 2.4% | 44 | 11.6% | 0.913 |
| 278 | Cellulitis | 323 | 2.0% | 32 | 9.9% | 0.313 |
| 174 | GI hemorrhage with complications | 320 | 2.0% | 40 | 12.5% | 0.809 |
| 15 | CVA | 302 | 1.9% | 25 | 8.3% | 0.066 |
| 175 | GI hemorrhage without complications | 287 | 1.8% | 34 | 11.8% | 0.948 |
The symptoms the patients reported are categorized in Table 4 without distinction as to whether they are primary or secondary. Gastrointestinal symptoms were the most common category of symptoms, followed by general symptoms, cardiovascular symptoms, and pain. The most common symptoms reported were fatigue/weakness, nausea/vomiting, and edema.
| Category | Specific Symptom | Number | % 0f Total |
|---|---|---|---|
| |||
| Gastrointestinal | 771 | 24.1% | |
| Nausea/vomiting | 245 | 7.7% | |
| Abdominal pain | 162 | 5.1% | |
| Diarrhea | 146 | 4.6% | |
| Eating problems | 107 | 3.3% | |
| Constipation | 71 | 2.2% | |
| General | 527 | 16.5% | |
| Fatigue or weakness | 360 | 11.3% | |
| Dizziness | 167 | 5.2% | |
| Cardiovascular | 388 | 12.1% | |
| Edema | 219 | 6.8% | |
| Chest pain | 101 | 3.2% | |
| Pain | 382 | 11.9% | |
| Back and neck | 118 | 3.7% | |
| Lower exttremity (including hip) | 115 | 3.6% | |
| Generalized | 76 | 2.4% | |
| Psychological | 209 | 6.5% | |
| Sleeping problems | 125 | 3.9% | |
| Change in mental status/psychiatric symptoms | 84 | 2.6% | |
| Pulmonary | 382 | 11.9% | |
| Dyspnea | 134 | 4.2% | |
| Neurological | 199 | 6.2% | |
| Headache | 118 | 3.7% | |
| Infectious | 192 | 6.0% | |
| Fever | 82 | 2.6% | |
| Dermatological | 65 | 2.0% | |
| Urological | 62 | 1.9% | |
| ENT | 50 | 1.6% | |
| Diabetic (problems with blood sugar) | 45 | 1.4% | |
| Postoperative wound problems | 39 | 1.2% | |
| Problems with intravenous sites | 17 | 0.5% | |
| Medication Reaction | 14 | 0.4% | |
| Bleeding (other than above locations) | 14 | 0.4% | |
| Gynecological | 9 | 0.3% | |
| Others | 89 | 2.8% | |
The call center assessed whether the patient had difficulty making a follow‐up appointment and whether an appointment was scheduled within 2 weeks of discharge. Patients with new or worsening symptoms were only minimally more likely to have scheduled follow‐up (61.0% vs. 58.4% for patients not reporting new or worsening symptoms, P < .05). Symptomatic patients had a higher prevalence of medication issues, defined as not picking up their prescriptions or not understanding how to take their medication (22.2% vs. 6.8% for asymptomatic patients; P < .0001). Likewise, the prevalence of symptomatic patients having problems receiving scheduled home health care services was 5.8%, compared with a prevalence of 3.6% for asymptomatic patients (P < .0001).
DISCUSSION
Enhancing the quality of care provided by hospitalists means not only improving care during hospitalization but also assuring patient stability between discharge and outpatient follow‐up. As part of efforts to improve transition management, the call center at IPC attempted to contact all patients discharged home within several days of discharge. Of 15,767 patients surveyed between May 1, 2003, and October 31, 2003, 11.9% (N = 1876) had new or worsening symptoms since leaving the hospital only 2 or 3 days earlier. We had hypothesized that older patients might be more symptomatic than younger ones, but found no difference in the prevalence of new or worsening symptoms based on age. Women were more likely to be symptomatic than men.
We defined appropriate postdischarge follow‐up as having an appointment with an outpatient physician within 2 weeks. A previous study of psychiatric patients documented that keeping a follow‐up appointment significantly reduces the risk of rehospitalization.17 Similar data do not exist for medical patients. Our data demonstrated that symptomatic patients were only minimally more likely to have made a follow‐up appointment with their outpatient physician within the first 2 weeks than were those patients who were not symptomatic. As part of patient education at discharge, clinicians routinely counsel patients to call their outpatient physician should they experience new or worsening symptoms once at home. Inpatient physicians may assume this recommendation provides a safety net for the patients should they develop problems after discharge. However, our finding that almost 40% of patients with new or worsening symptoms within 2‐3 days of discharge had not made a follow‐up appointment with their physician suggests many patients fall through this safety net. Although there was a slight statistically significant difference between the groups, this difference was not clinically significant. One potential limitation of our data is that we did not examine whether there was a correlation between the day of the week that a patient was discharged and inability to make a follow‐up appointment.
As part of the survey script (see Appendix), we inquired whether patients were able to pick up their prescriptions and whether they understood how to take their medication. A high percentage of patients in our study reported having one of these medication issues in the first several days following hospital discharge, providing an opportunity for early intervention and prevention of medical error. Forster and others have demonstrated that adverse events and medical errors are common in the postdischarge period, affecting 23%‐49% of patients.79 Errors in the transition from inpatient to outpatient care increased the 3‐month rate of rehospitalization.9 New or worsening symptoms represented the most common adverse event.8 Noting that many of these postdischarge complications could be preventable if detected early, Forster suggested system changes such as earlier follow‐up with the outpatient physician or a postdischarge telephone call to check on the patient's status.7, 18 Future studies are planned to further analyze our data on medication issues and to determine if these problems are more prevalent for certain medications or diagnoses.
Comprehensive discharge planning remains an essential step in the discharge process. This may involve prescribing medications, arranging home health care services, and arranging outpatient follow‐up. The traditional hospital discharge process does not adequately ensure that patients understand their discharge plan and are able to comply with it. Calkins et al. compared physicians' perceptions of patients' understanding of medication side effects and activity restrictions with patients' actual understanding.6 They found that, compared with what was reported by patients, physicians overestimated the time spent discussing discharge plans and how well patients understood medication side effects and activity restrictions.
An important method for reducing patient problems is to contact patients by telephone after discharge in order to identify any health care issues. Previous research has confirmed that follow‐up telephone calls improve health outcomes and decrease resource utilization of patients, mainly those discharged from the emergency department.10, 11, 1923 A study of telephone follow‐up after ambulatory care visits did not find significant benefits of this procedure.24 In one of the few studies of telephone calls after hospitalization, pharmacists contacted patients 2 days after discharge and were able to detect and resolve medication‐related problems in 19% of patients and learned of new or worsening symptoms in 15%. Patient satisfaction was improved, and the intervention resulted in a lower rate of repeat visits to the emergency room within 30 days of discharge.12 Another study of telephone follow‐up following hospital discharge compared proactively calling all patients with providing a phone number that patients can call if they have questions. The study demonstrated that very few patients called the number provided, but of those patients called by the nursing service, more than 90% had questions about self‐care and recovery.25 These findings demonstrate the value of proactively contacting patients in the first several days after discharge, when problems can be detected and interventions initiated earlier.
One potential concern with this study was the low response rate. This was a retrospective analysis of an existing discharge management call‐center system, not a prospective study. We were not able to reach 52% of the patients discharged after 2 attempts by telephone. To have our call center make additional attempts to reach each patient by telephone would require a significant increase in the size of the call center, because at the time of the study, the staff was handling more than 370 patients discharged home a day. The telephone number of 16% of patients was missing or incorrect. We have since developed internal quality improvement mechanisms to decrease this percentage. After subtracting the patients we were unable to reach and those whose phone number was missing or incorrect, we were able to contact 32.4% of all the patients discharged home.
Several reasons explain the response rate found by many prospective research studies. In most studies of telephone follow‐up, patients must be able to consent to participate in order to be considered eligible inclusion. This raises the response rate because patients who do not consent to participate, have language barriers, or have no telephone are excluded from the study. In our study none of these types of patients were excluded. There are 2 additional differences between our study and many published studies that involve telephone surveys. Ours was not a prospective research study, and we contacted many more patients than did other studies. For example, a study by Forster et al. involved only 581 patients, and the research staff was diligent in its efforts to reach the patients,7 making up to 20 attempts for each patient. They reported a response rate of 69%. If they had included patients who were non‐English speakers or had no phone in their study, the response rate would have been 59%. Shesser et al. were able to reach 144 of 297 patients in their study of emergency room follow‐up, for a 48.4% response rate.25 The response rate in a study of telephone consultation with asthma patients was quite similar to ours. They enrolled 932 eligible patients, of whom they were able to reach 278, for a response rate of 30%.
It is possible that the rate of symptoms and the other variables we measured relative to this would have been different if we had been able to reach 100% of patients. There were some demographic differences between the patients we were able to reach and those we were not (Table 1). The nonresponders were slightly younger and slightly more likely to be female. Nonresponders were more likely to have Medicaid or commercial insurance or be self‐pay and were less likely to have Medicare. In addition, nonresponders had less severe illness. Although this scenario is highly unlikely, if none of the nonresponders had new or worsening symptoms, the rate of symptoms would only have been 3.86%. Conversely, it is possible but also very unlikely that a greater percentage of the nonresponders had new or worsening symptoms. Given the demographics of our study participants, we would expect a potentially slightly lower rate of signs and symptoms.
The present study had several other limitations. First, all patients surveyed were cared for by IPC‐employed physicians. It is possible that reported rates of symptoms and other postdischarge issues are not generalizable to other hospitalist practices. However, the present data were collected at more than 100 health care facilities in 10 health care markets, and the patients were cared for by more than 200 physicians. Therefore, it is unlikely these results would have been significantly influenced by a particular physician's or institution's practice patterns.
Second, because of the large number of facilities involved and that we could only track readmissions to facilities where our own hospitalists practice, we were not able to report 30‐ or 90‐day readmission rates or emergency room visit rates. In a prospective study, these would be important variables to track in order to assess the clinical relevance of the symptoms. We could track this data for some institutions, but for most of them, the quality of data was not sufficient to be meaningful or to make conclusions.
An additional limitation is that the call center did not differentiate between clinically minor and major symptoms. The inclusion of symptoms perhaps considered minor might have elevated the reported symptom frequency. However, the definitions of minor and major symptoms are very subjective, and a clinician's definitions might differ from those of a patient who is at home and uncomfortable. For example, nausea or loss of appetite related to new medications may be considered minor clinically but could be devastating to the patient experiencing them, leading the patient to stop taking the medication. Conversely, symptoms that may be considered nonsignificant by the patient may be interpreted as indicating clinically significant disease by a physician. Therefore, we would argue that, regardless of the severity of the symptom, follow‐up with a clinician is important.
Another limitation is based on our definition of an adequate follow‐up appointment as one scheduled within 2 weeks of discharge. It might be argued that if a patient's new symptoms were considered minor clinically, then a follow‐up interval greater than 2 weeks might be considered adequate. However, as already noted, a patient's criteria for considering a symptom minor and not requiring follow‐up may differ from a clinician's criteria. Also, the standardized discharge process requires that the hospitalist identify a physician for outpatient follow‐up and specify the period when the patient is to see the physician. Because of the inherent variability in having a many hospitalists practicing in many hospitals, not all patients had a scheduled appointment at discharge. We were not able to determine whether patients had an appointment date and time for follow‐up before discharge or had only received instructions to call the office for an appointment.
The Institute of Medicine, in its report Crossing the Quality Chasm, identified the coordination of care across services and sites of care as one of the health care system's redesign imperatives.2 Hospitalists are in a unique position to address transition care issues. Managing the transition from inpatient to outpatient care is vitally important, and hospitalists should play an essential role in designing a transition management system for discharged patients. Although individual efforts by hospitalists are essential to assuring postdischarge contact with patients, there is increasing agreement that system solutions are needed to improve the quality of care in the transition period following hospitalization. Improving a health care process involves more than working harder; it involves working differently.3 It is therefore imperative that hospitalist programs develop effective systems to manage the transition period until safe arrival by the patient in the outpatient physician's office.
In summary, 11.9% of patients contacted by a telephone call center within several days of discharge had new or worsening symptoms since discharge. There was no difference by age in the prevalence of symptoms. Patients who rated their health status as fair to poor were more likely to be symptomatic. Symptomatic patients were also more likely to have difficulty obtaining or understanding how to take their medications and receiving home health services. Patients who felt poorly were only minimally more likely to have made an appointment for follow‐up with their outpatient physician. It is hoped that by identifying patients who are doing poorly after discharge and intervening as necessary, we can improve the health outcome of our patients, as well as reduce the number of emergency room visits and readmission rates. Although actions by individual physicians are important, a system to manage the postdischarge transition period is essential for improving posthospitalization outcomes.
Acknowledgements
The authors thank Rahul M. Dodhia for his assistance in the statistical analysis of the data and Sunil Kripalani for his thoughtful review of the manuscript.
APPENDIX
- Kohn LT,Corrigan J,Donaldson M, editors. To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2000:xxi,287.
- Institute of Medicine (U.S.).Committee on Quality of Health Care in America. Crossing the Quality Chasm: a New Health System for the 21st Century.Washington, DC:National Academy Press;2001:xx,337.
- ,,.An approach to hospital quality improvement.Med Clin North Am.2002;86:825–845.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- .Making health care safe. Supplement on hospital medicine and patient safety.The Hospitalist.2004:3–4.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from the hospital.CMAJ.2004;170:345–349.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,,,.Telephone care as a substitute for routine clinic follow‐up.JAMA.1992;267:1788–1793.
- ,,,,,.Effect of a standardized nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- Medical Outcomes Trust.How to Score the SF‐12 Short Form Health Survey.Boston:The Medical Outcomes Trust;1992.
- ,,.Examining emotional, physical, social, and spiritual health as determinants of self‐rated health status.Am J Health Promot.1998;12:275–282.
- 3M Health Information Systems, 3M All Patient Refined DRG Software. Available at: http://3m.com/market/healthcare/his/us/products/apr_drg/brochure.html.
- .A simple sequentially rejective Bonferroni test procedure.Scand J Stat.1979;6:65–70.
- ,,.Effects of discharge planning and compliance with outpatient appointments on readmission rates.Psychiatr Serv.2000;51:885–889.
- .J. Can you prevent adverse drug events after hospital discharge?CMAJ.2006;174:921–922.
- ,.Follow‐up phone calls after an emergency department visit.Pediatrics.1994;93:513–514.
- ,,,.Efficacy of a telephone follow‐up system in the emergency department.J Emerg Med.1988;6:249–254.
- ,,.A randomized trial to improve compliance in urinary tract infection patients in the emergency department.Ann Emerg Med.1990;19:16–20.
- ,,,,.The effectiveness of an organized emergency department follow‐up system.Ann Emerg Med.1986;15:911–915.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,.Telephone care as an adjunct to routine medical follow‐up. A negative randomized trial.Eff Clin Pract.2000;3:123–130.
- ,,.Telephone follow‐up after discharge from the hospital: does it make a difference?Appl Nurs Res.1996;9:47–52.
The Institute of Medicine reports To Err is Human and Crossing the Quality Chasm have drawn great attention to quality improvement and patient safety in the hospital setting.13 With the growth of the hospitalist field over the past several years,4 there has been increasing discussion about the importance of assuring quality of care, and some have argued that improving health care quality and reducing avoidable errors may be among the hospitalist's most important functions.5 Most discussions about the quality of hospital care have concerned the inpatient stay itself. However, the growth of hospital medicine, with its inherent discontinuity between inpatient and outpatient physicians, has intensified interest in the transition period from hospital discharge until first outpatient appointment.
At discharge, physicians may prescribe medications, order home health services, and arrange follow‐up appointments. It is often assumed a patient will remain stable after discharge and will follow up at the outpatient physician's office. Previous research has shown there may be problems with these assumptions. A patient may not understand the postdischarge treatment plan as well as the physician thinks.6 A recent study found that adverse events after discharge were common and often preventable.7 A follow‐up study confirmed that approximately 25% of patients had an adverse event after hospital discharge and that most adverse events caused symptoms but did not result in an emergency department visit, hospitalization, or death.8 Another study also found the prevalence of medical errors following hospitalization was high because of the discontinuity of care from the inpatient to the outpatient setting.9 These errors resulted in an increased rate of rehospitalization.
Telephone follow‐up may be a useful method of bridging the gap in care between discharge and the first outpatient appointment.10, 11 In most previous studies it was 2 or 3 weeks after discharge before patients were contacted or their records studied. By this point, patients who had done poorly may already have been readmitted or sought care at alternative locations. In one small study, pharmacists found that 12 of 79 patients (15%) contacted by telephone within 2 days of discharge12 had symptoms there were new or had worsened since discharge. The purpose of the present investigation was to extend these previous findings through a large multicenter study of how frequently patients had new or worsened symptoms within several days of discharge.
METHODS
Settings and Participants
IPCThe Hospitalist Company has hospitalist practices at more than 150 health care facilities in 10 health care markets. At the time of the study, IPC employed more than 300 internal medicine and family practice physicians and discharged approximately 11,000 adult patients per month. The study is a retrospective analysis of data collected from May 1, 2003, to October 31, 2003.
Data Acquisition
Physicians entered clinical and financial information on all hospitalized patients into a handheld personal digital assistant (PDA) utilizing functions of IPC‐LINK software. At the time of discharge, a physician completed a discharge summary on the PDA that was transmitted electronically to a centralized data center. Copies of the discharge summary were also faxed to the outpatient physician's office. Patients were first interviewed by call‐center patient representatives, unlicensed staff with medical backgrounds. Call‐center representatives made several attempts by telephone to reach all patients discharged home within several days of discharge. Using a scripted survey instrument (Appendix A), they asked patients or family members a series of questions about clinical status, new or worsening symptoms, problems with medications or prescribed home health care services, follow‐up appointments with their outpatient physician, and satisfaction with the care received. Call‐center nurses, licensed personnel with extensive medical/surgical and case management experience, contacted patients whose answers to questions on the scripted survey instrument (see last section of Appendix A) indicated a high risk of postdischarge problems, intervening as necessary to resolve the health care issues.
Health status was self rated on a 5‐point Likert scale from excellent to poor in response to the health status question on the SF‐12.13, 14 Patient age was calculated using birth date and admit date from the IPC‐Link discharge summary. With clinical data from the IPC‐Link discharge summary, the 3M DRG Grouper was used to assign each patient a DRG and severity of illness (SOI) score.15 Reported symptoms were grouped in clinically meaningful categories by the lead author.
Statistical Analysis
Logistic regression analysis was performed to analyze the effects of sex, health insurance, inpatient severity of illness, and self‐reported health status on the proportion of patients with symptoms. Sex, health status, and severity of illness were treated as ordered variables. Because insurance type is a nominal variable, HMO was used as the reference category, and the other categories were converted to indicator variables. Pearson chi‐square testing was used for all other analyses. The large number of planned analyses necessitated adjustment of the P values computed for the tests to maintain the type I error rate at 0.05. Therefore, a step‐down Bonferroni procedure was used.16
Role of the Funding Source
Data collection, analysis, and interpretation were funded by IPC and performed by employees of IPC.
RESULTS
During the study period, 48,236 patients were discharged to their homes from an acute care hospital. The IPC call center successfully contacted 16,135 patients after discharge, of whom 368 patients (2.3%) were excluded because of incomplete answers, leaving 15,767 as the valid study population (effective response rate = 32.4%). Of these, 98.9% were contacted within the first 5 days. The primary reasons for nonresponse or noninclusion in the present analysis were no answer after 2 attempts (52%) and missing or incorrect phone numbers (16%). If there was an answering machine, a message was left for the patient to call back. Those who called back accounted for fewer than 1% of all the patients.
A comparison of participants versus nonparticipants is shown in Table 1. The mean age of surveyed patients was 60.1 years, and 57% were female. The most common categories of insurance coverage were Medicare and HMO. The inpatient severity of illness of most patients was minor to moderate. Self‐reported health status was normally distributed, with the greatest percentage of patients rating their health as fair or good. On average, nonparticipants were younger than participants, more likely to be male, had a different pattern of health insurance, and a slightly lower severity of illness. The top 10 DRGs were the same for the respondents and nonrespondents, and the order of these 10 diagnoses was very similar.
| Characteristic | Patients in Study | Patients Not in Study | P Value* | ||
|---|---|---|---|---|---|
| Number of Patients | Percentage of All Patients | Number of Patients | Percentage of All Patients | ||
| |||||
| All patients | 15,767 | 32,101 | |||
| Mean age (years) | 60.1 | 54.1 | <.0001 | ||
| Sex | |||||
| Female | 8985 | 57.0% | 17220 | 53.7% | <.0001 |
| Male | 6515 | 41.3% | 14337 | 44.7% | <.0001 |
| Unknown | 267 | 1.7% | 544 | 1.7% | .897 |
| Insurance type | |||||
| HMO | 6391 | 40.5% | 12540 | 39.1% | <.001 |
| Medicaid | 1066 | 6.8% | 2815 | 8.8% | <.0001 |
| Medicare | 6055 | 38.4% | 9777 | 30.4% | <.0001 |
| Commercial and other | 1370 | 8.7% | 3490 | 10.9% | <.0001 |
| Self‐pay | 885 | 5.6% | 3479 | 10.9% | <.0001 |
| Severity of illness | |||||
| Minor | 6740 | 42.7% | 14679 | 45.7% | <.0001 |
| Moderate | 6854 | 43.5% | 13197 | 41.1% | |
| Major | 1688 | 10.7% | 3091 | 9.6% | <.0001 |
| Extreme | 118 | 0.7% | 219 | 0.7% | .571 |
| Unknown | 367 | 2.3% | 915 | 2.9% | .001 |
| Health status | |||||
| Excellent | 343 | 2.2% | N/A | ||
| Very good | 1392 | 8.8% | N/A | ||
| Good | 5505 | 34.9% | N/A | ||
| Fair | 5901 | 37.4% | N/A | ||
| Poor | 1468 | 9.3% | N/A | ||
| Unknown | 1158 | 7.3% | N/A | ||
Of the 15,767 patients contacted, 11.9% (N = 1876) reported symptoms that were new or had worsened since leaving the hospital. Sixty‐four percent of these patients had new symptoms, and 36% had worsening symptoms. These two groups were combined for analysis in this study because for both groups, identification and action are important. Of the patients with new or worse symptoms, 37% required no assistance from the nurse because they had already notified a doctor and/or were doing something about it. The other 63% either had not notified their doctor or had concerns about their signs and symptoms. The most common action taken by the nurse was patient education regarding the symptoms. Of those requiring nurse intervention in addition to education, the most common intervention was to contact the patient's primary care provider or specialist about the patient's symptoms, followed by contacting the hospitalist. In 72% of nurse interventions, the patient's primary care physician or a specialist was contacted about the new or worsened symptoms. Other interventions included contacting the physician's office to obtain a prescription for a medication for the symptom, to get an appointment for the patient, or to reschedule an appointment to be earlier. A referral to an emergency room or urgent care center was given to 4% of patients.
Mean age of the patients with new or worsened symptoms was 60.5 years. The age distribution of symptomatic and asymptomatic patients was not significantly different, whether comparing by mean, median, or decades. Table 2 illustrates factors associated with the increased rate of new or worsened symptoms. Women were more likely than men to report symptoms (13.0% vs. 10.3%, P < .0001). As health status worsened, the percentage of patients with new or worsened symptoms increased (P < .0001), as it did with increased inpatient SOI (P < .0001). There was no correlation between self‐rated health status and SOI score based on DRG score, suggesting they measured different parameters. Table 3 lists the percentages of patients reporting new or worsened symptoms for the most common DRGs. The only significant distinction was that patients discharged with a DRG of chest pain were less likely to report symptoms than were all patients.
| Characteristic | Number of Patients with New or Worsening Symptoms | Percentage of All Patients with New or Worsening Symptoms | P Value for Difference or Trend* |
|---|---|---|---|
| |||
| All Patients | 1876 | 11.9% | |
| Sex | <.0001 | ||
| Female | 1170 | 13.0% | |
| Male | 672 | 10.3% | |
| Insurance Type | |||
| HMO | 722 | 11.3% | .89 |
| Medicare | 748 | 12.4% | .21 |
| Commercial and other | 165 | 12.0% | .53 |
| Medicaid | 128 | 12.0% | .27 |
| Self‐pay | 113 | 12.8% | |
| Severity of illness | .17 | ||
| Minor | 748 | 11.1% | |
| Moderate | 814 | 11.9% | |
| Major | 247 | 14.6% | |
| Extreme | 19 | 16.1% | |
| Health Status | <.0001 | ||
| Excellent | 22 | 6.4% | |
| Very good | 85 | 6.1% | |
| Good | 429 | 7.8% | |
| Fair | 725 | 12.3% | |
| Poor | 384 | 26.2% | |
| DRG | Description | Number of Patients | Percentage of Patients | Patients with New or Worsening Symptoms | Rate of New or Worsening Symptoms | P value |
|---|---|---|---|---|---|---|
| ||||||
| Total patients in Study | 15,767 | 1876 | 11.9% | |||
| 143 | Chest pain | 1306 | 8.3% | 128 | 9.8% | 0.027 |
| 182 | Digest disorders with complications | 801 | 5.1% | 92 | 11.5% | 0.767 |
| 183 | Digest disorders without complications | 632 | 4.0% | 78 | 12.3% | 0.783 |
| 127 | Heart failure and shock | 544 | 3.5% | 55 | 10.1% | 0.230 |
| 89 | Pneumonia with complications | 426 | 2.7% | 39 | 9.2% | 0.098 |
| 88 | COPD | 380 | 2.4% | 44 | 11.6% | 0.913 |
| 278 | Cellulitis | 323 | 2.0% | 32 | 9.9% | 0.313 |
| 174 | GI hemorrhage with complications | 320 | 2.0% | 40 | 12.5% | 0.809 |
| 15 | CVA | 302 | 1.9% | 25 | 8.3% | 0.066 |
| 175 | GI hemorrhage without complications | 287 | 1.8% | 34 | 11.8% | 0.948 |
The symptoms the patients reported are categorized in Table 4 without distinction as to whether they are primary or secondary. Gastrointestinal symptoms were the most common category of symptoms, followed by general symptoms, cardiovascular symptoms, and pain. The most common symptoms reported were fatigue/weakness, nausea/vomiting, and edema.
| Category | Specific Symptom | Number | % 0f Total |
|---|---|---|---|
| |||
| Gastrointestinal | 771 | 24.1% | |
| Nausea/vomiting | 245 | 7.7% | |
| Abdominal pain | 162 | 5.1% | |
| Diarrhea | 146 | 4.6% | |
| Eating problems | 107 | 3.3% | |
| Constipation | 71 | 2.2% | |
| General | 527 | 16.5% | |
| Fatigue or weakness | 360 | 11.3% | |
| Dizziness | 167 | 5.2% | |
| Cardiovascular | 388 | 12.1% | |
| Edema | 219 | 6.8% | |
| Chest pain | 101 | 3.2% | |
| Pain | 382 | 11.9% | |
| Back and neck | 118 | 3.7% | |
| Lower exttremity (including hip) | 115 | 3.6% | |
| Generalized | 76 | 2.4% | |
| Psychological | 209 | 6.5% | |
| Sleeping problems | 125 | 3.9% | |
| Change in mental status/psychiatric symptoms | 84 | 2.6% | |
| Pulmonary | 382 | 11.9% | |
| Dyspnea | 134 | 4.2% | |
| Neurological | 199 | 6.2% | |
| Headache | 118 | 3.7% | |
| Infectious | 192 | 6.0% | |
| Fever | 82 | 2.6% | |
| Dermatological | 65 | 2.0% | |
| Urological | 62 | 1.9% | |
| ENT | 50 | 1.6% | |
| Diabetic (problems with blood sugar) | 45 | 1.4% | |
| Postoperative wound problems | 39 | 1.2% | |
| Problems with intravenous sites | 17 | 0.5% | |
| Medication Reaction | 14 | 0.4% | |
| Bleeding (other than above locations) | 14 | 0.4% | |
| Gynecological | 9 | 0.3% | |
| Others | 89 | 2.8% | |
The call center assessed whether the patient had difficulty making a follow‐up appointment and whether an appointment was scheduled within 2 weeks of discharge. Patients with new or worsening symptoms were only minimally more likely to have scheduled follow‐up (61.0% vs. 58.4% for patients not reporting new or worsening symptoms, P < .05). Symptomatic patients had a higher prevalence of medication issues, defined as not picking up their prescriptions or not understanding how to take their medication (22.2% vs. 6.8% for asymptomatic patients; P < .0001). Likewise, the prevalence of symptomatic patients having problems receiving scheduled home health care services was 5.8%, compared with a prevalence of 3.6% for asymptomatic patients (P < .0001).
DISCUSSION
Enhancing the quality of care provided by hospitalists means not only improving care during hospitalization but also assuring patient stability between discharge and outpatient follow‐up. As part of efforts to improve transition management, the call center at IPC attempted to contact all patients discharged home within several days of discharge. Of 15,767 patients surveyed between May 1, 2003, and October 31, 2003, 11.9% (N = 1876) had new or worsening symptoms since leaving the hospital only 2 or 3 days earlier. We had hypothesized that older patients might be more symptomatic than younger ones, but found no difference in the prevalence of new or worsening symptoms based on age. Women were more likely to be symptomatic than men.
We defined appropriate postdischarge follow‐up as having an appointment with an outpatient physician within 2 weeks. A previous study of psychiatric patients documented that keeping a follow‐up appointment significantly reduces the risk of rehospitalization.17 Similar data do not exist for medical patients. Our data demonstrated that symptomatic patients were only minimally more likely to have made a follow‐up appointment with their outpatient physician within the first 2 weeks than were those patients who were not symptomatic. As part of patient education at discharge, clinicians routinely counsel patients to call their outpatient physician should they experience new or worsening symptoms once at home. Inpatient physicians may assume this recommendation provides a safety net for the patients should they develop problems after discharge. However, our finding that almost 40% of patients with new or worsening symptoms within 2‐3 days of discharge had not made a follow‐up appointment with their physician suggests many patients fall through this safety net. Although there was a slight statistically significant difference between the groups, this difference was not clinically significant. One potential limitation of our data is that we did not examine whether there was a correlation between the day of the week that a patient was discharged and inability to make a follow‐up appointment.
As part of the survey script (see Appendix), we inquired whether patients were able to pick up their prescriptions and whether they understood how to take their medication. A high percentage of patients in our study reported having one of these medication issues in the first several days following hospital discharge, providing an opportunity for early intervention and prevention of medical error. Forster and others have demonstrated that adverse events and medical errors are common in the postdischarge period, affecting 23%‐49% of patients.79 Errors in the transition from inpatient to outpatient care increased the 3‐month rate of rehospitalization.9 New or worsening symptoms represented the most common adverse event.8 Noting that many of these postdischarge complications could be preventable if detected early, Forster suggested system changes such as earlier follow‐up with the outpatient physician or a postdischarge telephone call to check on the patient's status.7, 18 Future studies are planned to further analyze our data on medication issues and to determine if these problems are more prevalent for certain medications or diagnoses.
Comprehensive discharge planning remains an essential step in the discharge process. This may involve prescribing medications, arranging home health care services, and arranging outpatient follow‐up. The traditional hospital discharge process does not adequately ensure that patients understand their discharge plan and are able to comply with it. Calkins et al. compared physicians' perceptions of patients' understanding of medication side effects and activity restrictions with patients' actual understanding.6 They found that, compared with what was reported by patients, physicians overestimated the time spent discussing discharge plans and how well patients understood medication side effects and activity restrictions.
An important method for reducing patient problems is to contact patients by telephone after discharge in order to identify any health care issues. Previous research has confirmed that follow‐up telephone calls improve health outcomes and decrease resource utilization of patients, mainly those discharged from the emergency department.10, 11, 1923 A study of telephone follow‐up after ambulatory care visits did not find significant benefits of this procedure.24 In one of the few studies of telephone calls after hospitalization, pharmacists contacted patients 2 days after discharge and were able to detect and resolve medication‐related problems in 19% of patients and learned of new or worsening symptoms in 15%. Patient satisfaction was improved, and the intervention resulted in a lower rate of repeat visits to the emergency room within 30 days of discharge.12 Another study of telephone follow‐up following hospital discharge compared proactively calling all patients with providing a phone number that patients can call if they have questions. The study demonstrated that very few patients called the number provided, but of those patients called by the nursing service, more than 90% had questions about self‐care and recovery.25 These findings demonstrate the value of proactively contacting patients in the first several days after discharge, when problems can be detected and interventions initiated earlier.
One potential concern with this study was the low response rate. This was a retrospective analysis of an existing discharge management call‐center system, not a prospective study. We were not able to reach 52% of the patients discharged after 2 attempts by telephone. To have our call center make additional attempts to reach each patient by telephone would require a significant increase in the size of the call center, because at the time of the study, the staff was handling more than 370 patients discharged home a day. The telephone number of 16% of patients was missing or incorrect. We have since developed internal quality improvement mechanisms to decrease this percentage. After subtracting the patients we were unable to reach and those whose phone number was missing or incorrect, we were able to contact 32.4% of all the patients discharged home.
Several reasons explain the response rate found by many prospective research studies. In most studies of telephone follow‐up, patients must be able to consent to participate in order to be considered eligible inclusion. This raises the response rate because patients who do not consent to participate, have language barriers, or have no telephone are excluded from the study. In our study none of these types of patients were excluded. There are 2 additional differences between our study and many published studies that involve telephone surveys. Ours was not a prospective research study, and we contacted many more patients than did other studies. For example, a study by Forster et al. involved only 581 patients, and the research staff was diligent in its efforts to reach the patients,7 making up to 20 attempts for each patient. They reported a response rate of 69%. If they had included patients who were non‐English speakers or had no phone in their study, the response rate would have been 59%. Shesser et al. were able to reach 144 of 297 patients in their study of emergency room follow‐up, for a 48.4% response rate.25 The response rate in a study of telephone consultation with asthma patients was quite similar to ours. They enrolled 932 eligible patients, of whom they were able to reach 278, for a response rate of 30%.
It is possible that the rate of symptoms and the other variables we measured relative to this would have been different if we had been able to reach 100% of patients. There were some demographic differences between the patients we were able to reach and those we were not (Table 1). The nonresponders were slightly younger and slightly more likely to be female. Nonresponders were more likely to have Medicaid or commercial insurance or be self‐pay and were less likely to have Medicare. In addition, nonresponders had less severe illness. Although this scenario is highly unlikely, if none of the nonresponders had new or worsening symptoms, the rate of symptoms would only have been 3.86%. Conversely, it is possible but also very unlikely that a greater percentage of the nonresponders had new or worsening symptoms. Given the demographics of our study participants, we would expect a potentially slightly lower rate of signs and symptoms.
The present study had several other limitations. First, all patients surveyed were cared for by IPC‐employed physicians. It is possible that reported rates of symptoms and other postdischarge issues are not generalizable to other hospitalist practices. However, the present data were collected at more than 100 health care facilities in 10 health care markets, and the patients were cared for by more than 200 physicians. Therefore, it is unlikely these results would have been significantly influenced by a particular physician's or institution's practice patterns.
Second, because of the large number of facilities involved and that we could only track readmissions to facilities where our own hospitalists practice, we were not able to report 30‐ or 90‐day readmission rates or emergency room visit rates. In a prospective study, these would be important variables to track in order to assess the clinical relevance of the symptoms. We could track this data for some institutions, but for most of them, the quality of data was not sufficient to be meaningful or to make conclusions.
An additional limitation is that the call center did not differentiate between clinically minor and major symptoms. The inclusion of symptoms perhaps considered minor might have elevated the reported symptom frequency. However, the definitions of minor and major symptoms are very subjective, and a clinician's definitions might differ from those of a patient who is at home and uncomfortable. For example, nausea or loss of appetite related to new medications may be considered minor clinically but could be devastating to the patient experiencing them, leading the patient to stop taking the medication. Conversely, symptoms that may be considered nonsignificant by the patient may be interpreted as indicating clinically significant disease by a physician. Therefore, we would argue that, regardless of the severity of the symptom, follow‐up with a clinician is important.
Another limitation is based on our definition of an adequate follow‐up appointment as one scheduled within 2 weeks of discharge. It might be argued that if a patient's new symptoms were considered minor clinically, then a follow‐up interval greater than 2 weeks might be considered adequate. However, as already noted, a patient's criteria for considering a symptom minor and not requiring follow‐up may differ from a clinician's criteria. Also, the standardized discharge process requires that the hospitalist identify a physician for outpatient follow‐up and specify the period when the patient is to see the physician. Because of the inherent variability in having a many hospitalists practicing in many hospitals, not all patients had a scheduled appointment at discharge. We were not able to determine whether patients had an appointment date and time for follow‐up before discharge or had only received instructions to call the office for an appointment.
The Institute of Medicine, in its report Crossing the Quality Chasm, identified the coordination of care across services and sites of care as one of the health care system's redesign imperatives.2 Hospitalists are in a unique position to address transition care issues. Managing the transition from inpatient to outpatient care is vitally important, and hospitalists should play an essential role in designing a transition management system for discharged patients. Although individual efforts by hospitalists are essential to assuring postdischarge contact with patients, there is increasing agreement that system solutions are needed to improve the quality of care in the transition period following hospitalization. Improving a health care process involves more than working harder; it involves working differently.3 It is therefore imperative that hospitalist programs develop effective systems to manage the transition period until safe arrival by the patient in the outpatient physician's office.
In summary, 11.9% of patients contacted by a telephone call center within several days of discharge had new or worsening symptoms since discharge. There was no difference by age in the prevalence of symptoms. Patients who rated their health status as fair to poor were more likely to be symptomatic. Symptomatic patients were also more likely to have difficulty obtaining or understanding how to take their medications and receiving home health services. Patients who felt poorly were only minimally more likely to have made an appointment for follow‐up with their outpatient physician. It is hoped that by identifying patients who are doing poorly after discharge and intervening as necessary, we can improve the health outcome of our patients, as well as reduce the number of emergency room visits and readmission rates. Although actions by individual physicians are important, a system to manage the postdischarge transition period is essential for improving posthospitalization outcomes.
Acknowledgements
The authors thank Rahul M. Dodhia for his assistance in the statistical analysis of the data and Sunil Kripalani for his thoughtful review of the manuscript.
APPENDIX
The Institute of Medicine reports To Err is Human and Crossing the Quality Chasm have drawn great attention to quality improvement and patient safety in the hospital setting.13 With the growth of the hospitalist field over the past several years,4 there has been increasing discussion about the importance of assuring quality of care, and some have argued that improving health care quality and reducing avoidable errors may be among the hospitalist's most important functions.5 Most discussions about the quality of hospital care have concerned the inpatient stay itself. However, the growth of hospital medicine, with its inherent discontinuity between inpatient and outpatient physicians, has intensified interest in the transition period from hospital discharge until first outpatient appointment.
At discharge, physicians may prescribe medications, order home health services, and arrange follow‐up appointments. It is often assumed a patient will remain stable after discharge and will follow up at the outpatient physician's office. Previous research has shown there may be problems with these assumptions. A patient may not understand the postdischarge treatment plan as well as the physician thinks.6 A recent study found that adverse events after discharge were common and often preventable.7 A follow‐up study confirmed that approximately 25% of patients had an adverse event after hospital discharge and that most adverse events caused symptoms but did not result in an emergency department visit, hospitalization, or death.8 Another study also found the prevalence of medical errors following hospitalization was high because of the discontinuity of care from the inpatient to the outpatient setting.9 These errors resulted in an increased rate of rehospitalization.
Telephone follow‐up may be a useful method of bridging the gap in care between discharge and the first outpatient appointment.10, 11 In most previous studies it was 2 or 3 weeks after discharge before patients were contacted or their records studied. By this point, patients who had done poorly may already have been readmitted or sought care at alternative locations. In one small study, pharmacists found that 12 of 79 patients (15%) contacted by telephone within 2 days of discharge12 had symptoms there were new or had worsened since discharge. The purpose of the present investigation was to extend these previous findings through a large multicenter study of how frequently patients had new or worsened symptoms within several days of discharge.
METHODS
Settings and Participants
IPCThe Hospitalist Company has hospitalist practices at more than 150 health care facilities in 10 health care markets. At the time of the study, IPC employed more than 300 internal medicine and family practice physicians and discharged approximately 11,000 adult patients per month. The study is a retrospective analysis of data collected from May 1, 2003, to October 31, 2003.
Data Acquisition
Physicians entered clinical and financial information on all hospitalized patients into a handheld personal digital assistant (PDA) utilizing functions of IPC‐LINK software. At the time of discharge, a physician completed a discharge summary on the PDA that was transmitted electronically to a centralized data center. Copies of the discharge summary were also faxed to the outpatient physician's office. Patients were first interviewed by call‐center patient representatives, unlicensed staff with medical backgrounds. Call‐center representatives made several attempts by telephone to reach all patients discharged home within several days of discharge. Using a scripted survey instrument (Appendix A), they asked patients or family members a series of questions about clinical status, new or worsening symptoms, problems with medications or prescribed home health care services, follow‐up appointments with their outpatient physician, and satisfaction with the care received. Call‐center nurses, licensed personnel with extensive medical/surgical and case management experience, contacted patients whose answers to questions on the scripted survey instrument (see last section of Appendix A) indicated a high risk of postdischarge problems, intervening as necessary to resolve the health care issues.
Health status was self rated on a 5‐point Likert scale from excellent to poor in response to the health status question on the SF‐12.13, 14 Patient age was calculated using birth date and admit date from the IPC‐Link discharge summary. With clinical data from the IPC‐Link discharge summary, the 3M DRG Grouper was used to assign each patient a DRG and severity of illness (SOI) score.15 Reported symptoms were grouped in clinically meaningful categories by the lead author.
Statistical Analysis
Logistic regression analysis was performed to analyze the effects of sex, health insurance, inpatient severity of illness, and self‐reported health status on the proportion of patients with symptoms. Sex, health status, and severity of illness were treated as ordered variables. Because insurance type is a nominal variable, HMO was used as the reference category, and the other categories were converted to indicator variables. Pearson chi‐square testing was used for all other analyses. The large number of planned analyses necessitated adjustment of the P values computed for the tests to maintain the type I error rate at 0.05. Therefore, a step‐down Bonferroni procedure was used.16
Role of the Funding Source
Data collection, analysis, and interpretation were funded by IPC and performed by employees of IPC.
RESULTS
During the study period, 48,236 patients were discharged to their homes from an acute care hospital. The IPC call center successfully contacted 16,135 patients after discharge, of whom 368 patients (2.3%) were excluded because of incomplete answers, leaving 15,767 as the valid study population (effective response rate = 32.4%). Of these, 98.9% were contacted within the first 5 days. The primary reasons for nonresponse or noninclusion in the present analysis were no answer after 2 attempts (52%) and missing or incorrect phone numbers (16%). If there was an answering machine, a message was left for the patient to call back. Those who called back accounted for fewer than 1% of all the patients.
A comparison of participants versus nonparticipants is shown in Table 1. The mean age of surveyed patients was 60.1 years, and 57% were female. The most common categories of insurance coverage were Medicare and HMO. The inpatient severity of illness of most patients was minor to moderate. Self‐reported health status was normally distributed, with the greatest percentage of patients rating their health as fair or good. On average, nonparticipants were younger than participants, more likely to be male, had a different pattern of health insurance, and a slightly lower severity of illness. The top 10 DRGs were the same for the respondents and nonrespondents, and the order of these 10 diagnoses was very similar.
| Characteristic | Patients in Study | Patients Not in Study | P Value* | ||
|---|---|---|---|---|---|
| Number of Patients | Percentage of All Patients | Number of Patients | Percentage of All Patients | ||
| |||||
| All patients | 15,767 | 32,101 | |||
| Mean age (years) | 60.1 | 54.1 | <.0001 | ||
| Sex | |||||
| Female | 8985 | 57.0% | 17220 | 53.7% | <.0001 |
| Male | 6515 | 41.3% | 14337 | 44.7% | <.0001 |
| Unknown | 267 | 1.7% | 544 | 1.7% | .897 |
| Insurance type | |||||
| HMO | 6391 | 40.5% | 12540 | 39.1% | <.001 |
| Medicaid | 1066 | 6.8% | 2815 | 8.8% | <.0001 |
| Medicare | 6055 | 38.4% | 9777 | 30.4% | <.0001 |
| Commercial and other | 1370 | 8.7% | 3490 | 10.9% | <.0001 |
| Self‐pay | 885 | 5.6% | 3479 | 10.9% | <.0001 |
| Severity of illness | |||||
| Minor | 6740 | 42.7% | 14679 | 45.7% | <.0001 |
| Moderate | 6854 | 43.5% | 13197 | 41.1% | |
| Major | 1688 | 10.7% | 3091 | 9.6% | <.0001 |
| Extreme | 118 | 0.7% | 219 | 0.7% | .571 |
| Unknown | 367 | 2.3% | 915 | 2.9% | .001 |
| Health status | |||||
| Excellent | 343 | 2.2% | N/A | ||
| Very good | 1392 | 8.8% | N/A | ||
| Good | 5505 | 34.9% | N/A | ||
| Fair | 5901 | 37.4% | N/A | ||
| Poor | 1468 | 9.3% | N/A | ||
| Unknown | 1158 | 7.3% | N/A | ||
Of the 15,767 patients contacted, 11.9% (N = 1876) reported symptoms that were new or had worsened since leaving the hospital. Sixty‐four percent of these patients had new symptoms, and 36% had worsening symptoms. These two groups were combined for analysis in this study because for both groups, identification and action are important. Of the patients with new or worse symptoms, 37% required no assistance from the nurse because they had already notified a doctor and/or were doing something about it. The other 63% either had not notified their doctor or had concerns about their signs and symptoms. The most common action taken by the nurse was patient education regarding the symptoms. Of those requiring nurse intervention in addition to education, the most common intervention was to contact the patient's primary care provider or specialist about the patient's symptoms, followed by contacting the hospitalist. In 72% of nurse interventions, the patient's primary care physician or a specialist was contacted about the new or worsened symptoms. Other interventions included contacting the physician's office to obtain a prescription for a medication for the symptom, to get an appointment for the patient, or to reschedule an appointment to be earlier. A referral to an emergency room or urgent care center was given to 4% of patients.
Mean age of the patients with new or worsened symptoms was 60.5 years. The age distribution of symptomatic and asymptomatic patients was not significantly different, whether comparing by mean, median, or decades. Table 2 illustrates factors associated with the increased rate of new or worsened symptoms. Women were more likely than men to report symptoms (13.0% vs. 10.3%, P < .0001). As health status worsened, the percentage of patients with new or worsened symptoms increased (P < .0001), as it did with increased inpatient SOI (P < .0001). There was no correlation between self‐rated health status and SOI score based on DRG score, suggesting they measured different parameters. Table 3 lists the percentages of patients reporting new or worsened symptoms for the most common DRGs. The only significant distinction was that patients discharged with a DRG of chest pain were less likely to report symptoms than were all patients.
| Characteristic | Number of Patients with New or Worsening Symptoms | Percentage of All Patients with New or Worsening Symptoms | P Value for Difference or Trend* |
|---|---|---|---|
| |||
| All Patients | 1876 | 11.9% | |
| Sex | <.0001 | ||
| Female | 1170 | 13.0% | |
| Male | 672 | 10.3% | |
| Insurance Type | |||
| HMO | 722 | 11.3% | .89 |
| Medicare | 748 | 12.4% | .21 |
| Commercial and other | 165 | 12.0% | .53 |
| Medicaid | 128 | 12.0% | .27 |
| Self‐pay | 113 | 12.8% | |
| Severity of illness | .17 | ||
| Minor | 748 | 11.1% | |
| Moderate | 814 | 11.9% | |
| Major | 247 | 14.6% | |
| Extreme | 19 | 16.1% | |
| Health Status | <.0001 | ||
| Excellent | 22 | 6.4% | |
| Very good | 85 | 6.1% | |
| Good | 429 | 7.8% | |
| Fair | 725 | 12.3% | |
| Poor | 384 | 26.2% | |
| DRG | Description | Number of Patients | Percentage of Patients | Patients with New or Worsening Symptoms | Rate of New or Worsening Symptoms | P value |
|---|---|---|---|---|---|---|
| ||||||
| Total patients in Study | 15,767 | 1876 | 11.9% | |||
| 143 | Chest pain | 1306 | 8.3% | 128 | 9.8% | 0.027 |
| 182 | Digest disorders with complications | 801 | 5.1% | 92 | 11.5% | 0.767 |
| 183 | Digest disorders without complications | 632 | 4.0% | 78 | 12.3% | 0.783 |
| 127 | Heart failure and shock | 544 | 3.5% | 55 | 10.1% | 0.230 |
| 89 | Pneumonia with complications | 426 | 2.7% | 39 | 9.2% | 0.098 |
| 88 | COPD | 380 | 2.4% | 44 | 11.6% | 0.913 |
| 278 | Cellulitis | 323 | 2.0% | 32 | 9.9% | 0.313 |
| 174 | GI hemorrhage with complications | 320 | 2.0% | 40 | 12.5% | 0.809 |
| 15 | CVA | 302 | 1.9% | 25 | 8.3% | 0.066 |
| 175 | GI hemorrhage without complications | 287 | 1.8% | 34 | 11.8% | 0.948 |
The symptoms the patients reported are categorized in Table 4 without distinction as to whether they are primary or secondary. Gastrointestinal symptoms were the most common category of symptoms, followed by general symptoms, cardiovascular symptoms, and pain. The most common symptoms reported were fatigue/weakness, nausea/vomiting, and edema.
| Category | Specific Symptom | Number | % 0f Total |
|---|---|---|---|
| |||
| Gastrointestinal | 771 | 24.1% | |
| Nausea/vomiting | 245 | 7.7% | |
| Abdominal pain | 162 | 5.1% | |
| Diarrhea | 146 | 4.6% | |
| Eating problems | 107 | 3.3% | |
| Constipation | 71 | 2.2% | |
| General | 527 | 16.5% | |
| Fatigue or weakness | 360 | 11.3% | |
| Dizziness | 167 | 5.2% | |
| Cardiovascular | 388 | 12.1% | |
| Edema | 219 | 6.8% | |
| Chest pain | 101 | 3.2% | |
| Pain | 382 | 11.9% | |
| Back and neck | 118 | 3.7% | |
| Lower exttremity (including hip) | 115 | 3.6% | |
| Generalized | 76 | 2.4% | |
| Psychological | 209 | 6.5% | |
| Sleeping problems | 125 | 3.9% | |
| Change in mental status/psychiatric symptoms | 84 | 2.6% | |
| Pulmonary | 382 | 11.9% | |
| Dyspnea | 134 | 4.2% | |
| Neurological | 199 | 6.2% | |
| Headache | 118 | 3.7% | |
| Infectious | 192 | 6.0% | |
| Fever | 82 | 2.6% | |
| Dermatological | 65 | 2.0% | |
| Urological | 62 | 1.9% | |
| ENT | 50 | 1.6% | |
| Diabetic (problems with blood sugar) | 45 | 1.4% | |
| Postoperative wound problems | 39 | 1.2% | |
| Problems with intravenous sites | 17 | 0.5% | |
| Medication Reaction | 14 | 0.4% | |
| Bleeding (other than above locations) | 14 | 0.4% | |
| Gynecological | 9 | 0.3% | |
| Others | 89 | 2.8% | |
The call center assessed whether the patient had difficulty making a follow‐up appointment and whether an appointment was scheduled within 2 weeks of discharge. Patients with new or worsening symptoms were only minimally more likely to have scheduled follow‐up (61.0% vs. 58.4% for patients not reporting new or worsening symptoms, P < .05). Symptomatic patients had a higher prevalence of medication issues, defined as not picking up their prescriptions or not understanding how to take their medication (22.2% vs. 6.8% for asymptomatic patients; P < .0001). Likewise, the prevalence of symptomatic patients having problems receiving scheduled home health care services was 5.8%, compared with a prevalence of 3.6% for asymptomatic patients (P < .0001).
DISCUSSION
Enhancing the quality of care provided by hospitalists means not only improving care during hospitalization but also assuring patient stability between discharge and outpatient follow‐up. As part of efforts to improve transition management, the call center at IPC attempted to contact all patients discharged home within several days of discharge. Of 15,767 patients surveyed between May 1, 2003, and October 31, 2003, 11.9% (N = 1876) had new or worsening symptoms since leaving the hospital only 2 or 3 days earlier. We had hypothesized that older patients might be more symptomatic than younger ones, but found no difference in the prevalence of new or worsening symptoms based on age. Women were more likely to be symptomatic than men.
We defined appropriate postdischarge follow‐up as having an appointment with an outpatient physician within 2 weeks. A previous study of psychiatric patients documented that keeping a follow‐up appointment significantly reduces the risk of rehospitalization.17 Similar data do not exist for medical patients. Our data demonstrated that symptomatic patients were only minimally more likely to have made a follow‐up appointment with their outpatient physician within the first 2 weeks than were those patients who were not symptomatic. As part of patient education at discharge, clinicians routinely counsel patients to call their outpatient physician should they experience new or worsening symptoms once at home. Inpatient physicians may assume this recommendation provides a safety net for the patients should they develop problems after discharge. However, our finding that almost 40% of patients with new or worsening symptoms within 2‐3 days of discharge had not made a follow‐up appointment with their physician suggests many patients fall through this safety net. Although there was a slight statistically significant difference between the groups, this difference was not clinically significant. One potential limitation of our data is that we did not examine whether there was a correlation between the day of the week that a patient was discharged and inability to make a follow‐up appointment.
As part of the survey script (see Appendix), we inquired whether patients were able to pick up their prescriptions and whether they understood how to take their medication. A high percentage of patients in our study reported having one of these medication issues in the first several days following hospital discharge, providing an opportunity for early intervention and prevention of medical error. Forster and others have demonstrated that adverse events and medical errors are common in the postdischarge period, affecting 23%‐49% of patients.79 Errors in the transition from inpatient to outpatient care increased the 3‐month rate of rehospitalization.9 New or worsening symptoms represented the most common adverse event.8 Noting that many of these postdischarge complications could be preventable if detected early, Forster suggested system changes such as earlier follow‐up with the outpatient physician or a postdischarge telephone call to check on the patient's status.7, 18 Future studies are planned to further analyze our data on medication issues and to determine if these problems are more prevalent for certain medications or diagnoses.
Comprehensive discharge planning remains an essential step in the discharge process. This may involve prescribing medications, arranging home health care services, and arranging outpatient follow‐up. The traditional hospital discharge process does not adequately ensure that patients understand their discharge plan and are able to comply with it. Calkins et al. compared physicians' perceptions of patients' understanding of medication side effects and activity restrictions with patients' actual understanding.6 They found that, compared with what was reported by patients, physicians overestimated the time spent discussing discharge plans and how well patients understood medication side effects and activity restrictions.
An important method for reducing patient problems is to contact patients by telephone after discharge in order to identify any health care issues. Previous research has confirmed that follow‐up telephone calls improve health outcomes and decrease resource utilization of patients, mainly those discharged from the emergency department.10, 11, 1923 A study of telephone follow‐up after ambulatory care visits did not find significant benefits of this procedure.24 In one of the few studies of telephone calls after hospitalization, pharmacists contacted patients 2 days after discharge and were able to detect and resolve medication‐related problems in 19% of patients and learned of new or worsening symptoms in 15%. Patient satisfaction was improved, and the intervention resulted in a lower rate of repeat visits to the emergency room within 30 days of discharge.12 Another study of telephone follow‐up following hospital discharge compared proactively calling all patients with providing a phone number that patients can call if they have questions. The study demonstrated that very few patients called the number provided, but of those patients called by the nursing service, more than 90% had questions about self‐care and recovery.25 These findings demonstrate the value of proactively contacting patients in the first several days after discharge, when problems can be detected and interventions initiated earlier.
One potential concern with this study was the low response rate. This was a retrospective analysis of an existing discharge management call‐center system, not a prospective study. We were not able to reach 52% of the patients discharged after 2 attempts by telephone. To have our call center make additional attempts to reach each patient by telephone would require a significant increase in the size of the call center, because at the time of the study, the staff was handling more than 370 patients discharged home a day. The telephone number of 16% of patients was missing or incorrect. We have since developed internal quality improvement mechanisms to decrease this percentage. After subtracting the patients we were unable to reach and those whose phone number was missing or incorrect, we were able to contact 32.4% of all the patients discharged home.
Several reasons explain the response rate found by many prospective research studies. In most studies of telephone follow‐up, patients must be able to consent to participate in order to be considered eligible inclusion. This raises the response rate because patients who do not consent to participate, have language barriers, or have no telephone are excluded from the study. In our study none of these types of patients were excluded. There are 2 additional differences between our study and many published studies that involve telephone surveys. Ours was not a prospective research study, and we contacted many more patients than did other studies. For example, a study by Forster et al. involved only 581 patients, and the research staff was diligent in its efforts to reach the patients,7 making up to 20 attempts for each patient. They reported a response rate of 69%. If they had included patients who were non‐English speakers or had no phone in their study, the response rate would have been 59%. Shesser et al. were able to reach 144 of 297 patients in their study of emergency room follow‐up, for a 48.4% response rate.25 The response rate in a study of telephone consultation with asthma patients was quite similar to ours. They enrolled 932 eligible patients, of whom they were able to reach 278, for a response rate of 30%.
It is possible that the rate of symptoms and the other variables we measured relative to this would have been different if we had been able to reach 100% of patients. There were some demographic differences between the patients we were able to reach and those we were not (Table 1). The nonresponders were slightly younger and slightly more likely to be female. Nonresponders were more likely to have Medicaid or commercial insurance or be self‐pay and were less likely to have Medicare. In addition, nonresponders had less severe illness. Although this scenario is highly unlikely, if none of the nonresponders had new or worsening symptoms, the rate of symptoms would only have been 3.86%. Conversely, it is possible but also very unlikely that a greater percentage of the nonresponders had new or worsening symptoms. Given the demographics of our study participants, we would expect a potentially slightly lower rate of signs and symptoms.
The present study had several other limitations. First, all patients surveyed were cared for by IPC‐employed physicians. It is possible that reported rates of symptoms and other postdischarge issues are not generalizable to other hospitalist practices. However, the present data were collected at more than 100 health care facilities in 10 health care markets, and the patients were cared for by more than 200 physicians. Therefore, it is unlikely these results would have been significantly influenced by a particular physician's or institution's practice patterns.
Second, because of the large number of facilities involved and that we could only track readmissions to facilities where our own hospitalists practice, we were not able to report 30‐ or 90‐day readmission rates or emergency room visit rates. In a prospective study, these would be important variables to track in order to assess the clinical relevance of the symptoms. We could track this data for some institutions, but for most of them, the quality of data was not sufficient to be meaningful or to make conclusions.
An additional limitation is that the call center did not differentiate between clinically minor and major symptoms. The inclusion of symptoms perhaps considered minor might have elevated the reported symptom frequency. However, the definitions of minor and major symptoms are very subjective, and a clinician's definitions might differ from those of a patient who is at home and uncomfortable. For example, nausea or loss of appetite related to new medications may be considered minor clinically but could be devastating to the patient experiencing them, leading the patient to stop taking the medication. Conversely, symptoms that may be considered nonsignificant by the patient may be interpreted as indicating clinically significant disease by a physician. Therefore, we would argue that, regardless of the severity of the symptom, follow‐up with a clinician is important.
Another limitation is based on our definition of an adequate follow‐up appointment as one scheduled within 2 weeks of discharge. It might be argued that if a patient's new symptoms were considered minor clinically, then a follow‐up interval greater than 2 weeks might be considered adequate. However, as already noted, a patient's criteria for considering a symptom minor and not requiring follow‐up may differ from a clinician's criteria. Also, the standardized discharge process requires that the hospitalist identify a physician for outpatient follow‐up and specify the period when the patient is to see the physician. Because of the inherent variability in having a many hospitalists practicing in many hospitals, not all patients had a scheduled appointment at discharge. We were not able to determine whether patients had an appointment date and time for follow‐up before discharge or had only received instructions to call the office for an appointment.
The Institute of Medicine, in its report Crossing the Quality Chasm, identified the coordination of care across services and sites of care as one of the health care system's redesign imperatives.2 Hospitalists are in a unique position to address transition care issues. Managing the transition from inpatient to outpatient care is vitally important, and hospitalists should play an essential role in designing a transition management system for discharged patients. Although individual efforts by hospitalists are essential to assuring postdischarge contact with patients, there is increasing agreement that system solutions are needed to improve the quality of care in the transition period following hospitalization. Improving a health care process involves more than working harder; it involves working differently.3 It is therefore imperative that hospitalist programs develop effective systems to manage the transition period until safe arrival by the patient in the outpatient physician's office.
In summary, 11.9% of patients contacted by a telephone call center within several days of discharge had new or worsening symptoms since discharge. There was no difference by age in the prevalence of symptoms. Patients who rated their health status as fair to poor were more likely to be symptomatic. Symptomatic patients were also more likely to have difficulty obtaining or understanding how to take their medications and receiving home health services. Patients who felt poorly were only minimally more likely to have made an appointment for follow‐up with their outpatient physician. It is hoped that by identifying patients who are doing poorly after discharge and intervening as necessary, we can improve the health outcome of our patients, as well as reduce the number of emergency room visits and readmission rates. Although actions by individual physicians are important, a system to manage the postdischarge transition period is essential for improving posthospitalization outcomes.
Acknowledgements
The authors thank Rahul M. Dodhia for his assistance in the statistical analysis of the data and Sunil Kripalani for his thoughtful review of the manuscript.
APPENDIX
- Kohn LT,Corrigan J,Donaldson M, editors. To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2000:xxi,287.
- Institute of Medicine (U.S.).Committee on Quality of Health Care in America. Crossing the Quality Chasm: a New Health System for the 21st Century.Washington, DC:National Academy Press;2001:xx,337.
- ,,.An approach to hospital quality improvement.Med Clin North Am.2002;86:825–845.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- .Making health care safe. Supplement on hospital medicine and patient safety.The Hospitalist.2004:3–4.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from the hospital.CMAJ.2004;170:345–349.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,,,.Telephone care as a substitute for routine clinic follow‐up.JAMA.1992;267:1788–1793.
- ,,,,,.Effect of a standardized nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- Medical Outcomes Trust.How to Score the SF‐12 Short Form Health Survey.Boston:The Medical Outcomes Trust;1992.
- ,,.Examining emotional, physical, social, and spiritual health as determinants of self‐rated health status.Am J Health Promot.1998;12:275–282.
- 3M Health Information Systems, 3M All Patient Refined DRG Software. Available at: http://3m.com/market/healthcare/his/us/products/apr_drg/brochure.html.
- .A simple sequentially rejective Bonferroni test procedure.Scand J Stat.1979;6:65–70.
- ,,.Effects of discharge planning and compliance with outpatient appointments on readmission rates.Psychiatr Serv.2000;51:885–889.
- .J. Can you prevent adverse drug events after hospital discharge?CMAJ.2006;174:921–922.
- ,.Follow‐up phone calls after an emergency department visit.Pediatrics.1994;93:513–514.
- ,,,.Efficacy of a telephone follow‐up system in the emergency department.J Emerg Med.1988;6:249–254.
- ,,.A randomized trial to improve compliance in urinary tract infection patients in the emergency department.Ann Emerg Med.1990;19:16–20.
- ,,,,.The effectiveness of an organized emergency department follow‐up system.Ann Emerg Med.1986;15:911–915.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,.Telephone care as an adjunct to routine medical follow‐up. A negative randomized trial.Eff Clin Pract.2000;3:123–130.
- ,,.Telephone follow‐up after discharge from the hospital: does it make a difference?Appl Nurs Res.1996;9:47–52.
- Kohn LT,Corrigan J,Donaldson M, editors. To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2000:xxi,287.
- Institute of Medicine (U.S.).Committee on Quality of Health Care in America. Crossing the Quality Chasm: a New Health System for the 21st Century.Washington, DC:National Academy Press;2001:xx,337.
- ,,.An approach to hospital quality improvement.Med Clin North Am.2002;86:825–845.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- .Making health care safe. Supplement on hospital medicine and patient safety.The Hospitalist.2004:3–4.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Adverse events among medical patients after discharge from the hospital.CMAJ.2004;170:345–349.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,,,,.Telephone care as a substitute for routine clinic follow‐up.JAMA.1992;267:1788–1793.
- ,,,,,.Effect of a standardized nurse case‐management telephone intervention on resource use in patients with chronic heart failure.Arch Intern Med.2002;162:705–712.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- Medical Outcomes Trust.How to Score the SF‐12 Short Form Health Survey.Boston:The Medical Outcomes Trust;1992.
- ,,.Examining emotional, physical, social, and spiritual health as determinants of self‐rated health status.Am J Health Promot.1998;12:275–282.
- 3M Health Information Systems, 3M All Patient Refined DRG Software. Available at: http://3m.com/market/healthcare/his/us/products/apr_drg/brochure.html.
- .A simple sequentially rejective Bonferroni test procedure.Scand J Stat.1979;6:65–70.
- ,,.Effects of discharge planning and compliance with outpatient appointments on readmission rates.Psychiatr Serv.2000;51:885–889.
- .J. Can you prevent adverse drug events after hospital discharge?CMAJ.2006;174:921–922.
- ,.Follow‐up phone calls after an emergency department visit.Pediatrics.1994;93:513–514.
- ,,,.Efficacy of a telephone follow‐up system in the emergency department.J Emerg Med.1988;6:249–254.
- ,,.A randomized trial to improve compliance in urinary tract infection patients in the emergency department.Ann Emerg Med.1990;19:16–20.
- ,,,,.The effectiveness of an organized emergency department follow‐up system.Ann Emerg Med.1986;15:911–915.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,.Telephone care as an adjunct to routine medical follow‐up. A negative randomized trial.Eff Clin Pract.2000;3:123–130.
- ,,.Telephone follow‐up after discharge from the hospital: does it make a difference?Appl Nurs Res.1996;9:47–52.
Copyright © 2007 Society of Hospital Medicine
Penetrating Point
Soon after they form, most new medical fields begin agitating for a special certification, something that says, We're here, and we're different. As I've noted previously in the Journal of Hospital Medicine, the field of hospital medicine resisted this impulse in its early years, fearing that any special designation or certification would actually harm the field's growth and status.1 The concern was that managed‐care organizationsconvinced by the evidence that hospitalists improve efficiency and might improve quality2would react to any new hospitalist sheepskin by mandating that anyone providing hospital care to its covered patients have one. The backlash from primary care physicians locked out of the hospital by such a mandate would have been swift and ultimately damaging to hospitalists. In addition to these political considerations, the early field of hospital medicine lacked the academic credibility and scientific underpinning needed for specialty designation.3
Times have changed. There are now more than 15,000 hospitalists in the United States, and nearly half of American hospitals have hospitalists on their medical staffs. In many markets, including my own, hospitalists care for most internal medicine inpatients, as well as significant numbers of pediatric and surgical patients. The field has achieved academic legitimacy, with this journal, several textbooks, large and flourishing groups in every academic medical center, and several residency tracks and fellowship programs.4, 5 The Society of Hospital Medicine (SHM) has grown to more than 6000 members, become a widely respected and dynamic member of the community of professional societies, and published its core competencies.6
With this as a background, in 2004 SHM asked the American Board of Internal Medicine (ABIM) to consider a program of certification for hospitalists. As a past SHM president and now a member of the ABIM Board of Directors, I am privileged to have a bird's‐eye view of the process. In this article, I reflect on some of the key issues it raises.
THE NUTS AND BOLTS OF BOARD CERTIFICATION
Since the first board (ophthalmology) was formed in 1917, 24 specialty boards have emerged, all under the umbrella of the American Board of Medical Specialties (ABMS).7 Because no one type of physician can do it all, certifying boards have had to struggle not only with how to assess competency in existing disciplines, but with the dynamic and often controversial questions raised when new fields emerge. In the past few decades, certifying boards have grappled with specialties formed around new procedures (such as cardiac electrophysiology), discrete populations (geriatrics, palliative care), complex diseases (HIV medicine), and sites of care (intensive care medicine, emergency medicine). It is this latter category that now includes hospital medicine.
In the past, it was relatively simple for a physician to obtain board certification. Residency or fellowship training was believed to confer on its graduates the presumption of competence and professionalismthe program director's attestation served as the graduate's Good Housekeeping seal of approval. Passing the board exam was the final step, ensuring that newly minted graduates had the requisite knowledge and judgment to practice in their fields.
Remarkably, for the first half century of the specialty boards, all certifications lasted for a physician's professional lifetime. Beginning with the 1969 decision of the American Board of Family Practice to limit the validity of its certificates to 7 years, all ABMS member boards now time limit their certifications, usually to 7‐10 years.7 Of course, in an environment of rapidly changing medical knowledge and new procedures, periodiceven continuousdemonstration of competence is increasingly expected by the public.
For ABIM, the mechanism to promote lifelong learning and demonstrate ongoing competence in the face of a rapidly changing environment is known as maintenance of certification (MOC).8 Through MOC, board‐certified internists demonstrate their ongoing clinical expertise and judgment, their involvement in lifelong learning and quality improvement activities, and their professionalism. Because MOC involves no new training requirements and includes an assessment of a physician's actual practice, it provides a potential mechanism, heretofore untapped, of demonstrating a unique professional focus that emerges after the completion of formal training.
HOSPITALIST CERTIFICATION AND THE MOC PROCESS
As ABIM considered a separate certification pathway for hospital medicine, it faced a conundrum. The vast majority of hospitalists are general internists (most of the rest are generalists in family medicine or pediatrics) who entered hospital medicine at the completion of their internal medicine training or after a period of primary care practice. Job opportunities for hospitalists are plentiful, andexcept for additional training in quality improvement, systems leadership, care transitions, palliative care, and communication9there is little clinical rationale to prolong internal medicine training for hospitalists (some individuals may opt for fellowships to enhance their leadership skills or to launch a research career,5 but few would argue for mandatory additional clinical training in hospital medicine at this time).
So, in the absence of formal training, how could the ABIM (or other boards) recognize the focused practice of hospitalists? This question must be framed within a broader challenge: Is it possible and appropriate for certifying boards to recognize expertise and focus that is accrued not through formal training, but through actual practice experience and accompanying self‐directed learning?
In 2006, the ABIM took up this question, producing a report (New and Emerging Disciplines in Internal Medicine II [NEDIM II]) that delineated several criteria to guide whether a new field merited focused recognition through MOC (Table 1). Judging by these criteria, hospital medicine appears to be a suitable first candidate for recognition of focused practice through MOC.
|
PRELIMINARY THOUGHTS ON FOCUSED RECOGNITION IN HOSPITAL MEDICINE
The ABIM has endorsed the concept of recognition of focused practice in hospital medicine and charged a subcommittee (that I chair) with working out the details. It would be premature to describe the committee's deliberations in detail (particularly because the final plan needs to be approved by both the ABIM and the ABMS), but the following are some key issues being discussed.
First, demonstration of focused practice requires some minimum volume of hospitalized patients. In the absence of hard data defining a threshold number of cases for hospitalists, we are likely to endorse a number that has face validity and that reliably separates self‐identified hospitalists from nonhospitalist generalists. As with all volume requirements, we will struggle over how to handle academic physicians, physician‐administrators, and physician‐researchers who limit their overall clinical practice but who spend most of their clinical time in hospital medicine and the bulk of their nonclinical time trying to improve hospital care.
The requirements to demonstrate performance in practice and lifelong learning may be more straightforward. As with all such MOC requirements, the ABIM is increasingly looking to use real practice data, trying to harmonize its data requirements with those of other organizations such as insurers, Medicare, the Joint Commission, or for pay‐for‐performance initiatives. Despite the operational challenges, this effort is vital: for MOC (including focused recognition) to be highly valued by patients, purchasers, and diplomates, it will increasingly need to measure not only what physicians know, but also what they do.
Finally, there is the test. It is likely that a secure exam for MOC with Recognition of Focused Practice in Hospital Medicine will involve core content in internal medicine (information that every internist should know), augmented by substantial and challenging content in hospital medicine. Because it will be vital that a competent hospitalist understand key elements of outpatient practice, the exam will not be stripped of ambulatory content but will likely have fewer questions on topics that hospitalists are unlikely to confront (osteoporosis, cancer screening).
ONGOING ISSUES
As hospital medicine continues its explosive growth, it is important to develop ways to make board certification relevant to hospitalists. The ABIM believes that modifying the MOC process to recognize physicians who have focused their practice and achieved special expertise in hospital medicine is a good way to launch this effort. Ultimately, this process is likely to evolve, particularly if separate training pathways for hospital medicine emerge. For now, the development of Recognition of Focused Practice in Hospital Medicine will further legitimize the new field, provide ABIM with insights into how to recognize physicians who have advanced through practice‐based learning rather than through training, and help to guide other certifying boards (particularly family medicine and pediatrics) considering hospitalist certification. In the end, the process will need to be user‐friendly for and satisfying to diplomates, flexible enough to allow for career transitions (both toward and away from hospital medicine), and sufficiently rigorous to be credible to all stakeholders, particularly patients.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- ,.The hospitalist movement 5 years later.JAMA2002;287:487–94.
- .The hospitalist: a new medical specialty?Ann Intern Med.1999;130:373–375.
- ,.Implications of the hospitalist movement for academic departments of medicine: lessons from the UCSF experience.Am J Med.1999;106:127–133.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72.e1–e7.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- .Recertification in the United States.BMJ.1999;319:1183–1185.
- ,.Professional standards in the USA: overview and new developments.Clin Med.2006;6:363–367.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
Soon after they form, most new medical fields begin agitating for a special certification, something that says, We're here, and we're different. As I've noted previously in the Journal of Hospital Medicine, the field of hospital medicine resisted this impulse in its early years, fearing that any special designation or certification would actually harm the field's growth and status.1 The concern was that managed‐care organizationsconvinced by the evidence that hospitalists improve efficiency and might improve quality2would react to any new hospitalist sheepskin by mandating that anyone providing hospital care to its covered patients have one. The backlash from primary care physicians locked out of the hospital by such a mandate would have been swift and ultimately damaging to hospitalists. In addition to these political considerations, the early field of hospital medicine lacked the academic credibility and scientific underpinning needed for specialty designation.3
Times have changed. There are now more than 15,000 hospitalists in the United States, and nearly half of American hospitals have hospitalists on their medical staffs. In many markets, including my own, hospitalists care for most internal medicine inpatients, as well as significant numbers of pediatric and surgical patients. The field has achieved academic legitimacy, with this journal, several textbooks, large and flourishing groups in every academic medical center, and several residency tracks and fellowship programs.4, 5 The Society of Hospital Medicine (SHM) has grown to more than 6000 members, become a widely respected and dynamic member of the community of professional societies, and published its core competencies.6
With this as a background, in 2004 SHM asked the American Board of Internal Medicine (ABIM) to consider a program of certification for hospitalists. As a past SHM president and now a member of the ABIM Board of Directors, I am privileged to have a bird's‐eye view of the process. In this article, I reflect on some of the key issues it raises.
THE NUTS AND BOLTS OF BOARD CERTIFICATION
Since the first board (ophthalmology) was formed in 1917, 24 specialty boards have emerged, all under the umbrella of the American Board of Medical Specialties (ABMS).7 Because no one type of physician can do it all, certifying boards have had to struggle not only with how to assess competency in existing disciplines, but with the dynamic and often controversial questions raised when new fields emerge. In the past few decades, certifying boards have grappled with specialties formed around new procedures (such as cardiac electrophysiology), discrete populations (geriatrics, palliative care), complex diseases (HIV medicine), and sites of care (intensive care medicine, emergency medicine). It is this latter category that now includes hospital medicine.
In the past, it was relatively simple for a physician to obtain board certification. Residency or fellowship training was believed to confer on its graduates the presumption of competence and professionalismthe program director's attestation served as the graduate's Good Housekeeping seal of approval. Passing the board exam was the final step, ensuring that newly minted graduates had the requisite knowledge and judgment to practice in their fields.
Remarkably, for the first half century of the specialty boards, all certifications lasted for a physician's professional lifetime. Beginning with the 1969 decision of the American Board of Family Practice to limit the validity of its certificates to 7 years, all ABMS member boards now time limit their certifications, usually to 7‐10 years.7 Of course, in an environment of rapidly changing medical knowledge and new procedures, periodiceven continuousdemonstration of competence is increasingly expected by the public.
For ABIM, the mechanism to promote lifelong learning and demonstrate ongoing competence in the face of a rapidly changing environment is known as maintenance of certification (MOC).8 Through MOC, board‐certified internists demonstrate their ongoing clinical expertise and judgment, their involvement in lifelong learning and quality improvement activities, and their professionalism. Because MOC involves no new training requirements and includes an assessment of a physician's actual practice, it provides a potential mechanism, heretofore untapped, of demonstrating a unique professional focus that emerges after the completion of formal training.
HOSPITALIST CERTIFICATION AND THE MOC PROCESS
As ABIM considered a separate certification pathway for hospital medicine, it faced a conundrum. The vast majority of hospitalists are general internists (most of the rest are generalists in family medicine or pediatrics) who entered hospital medicine at the completion of their internal medicine training or after a period of primary care practice. Job opportunities for hospitalists are plentiful, andexcept for additional training in quality improvement, systems leadership, care transitions, palliative care, and communication9there is little clinical rationale to prolong internal medicine training for hospitalists (some individuals may opt for fellowships to enhance their leadership skills or to launch a research career,5 but few would argue for mandatory additional clinical training in hospital medicine at this time).
So, in the absence of formal training, how could the ABIM (or other boards) recognize the focused practice of hospitalists? This question must be framed within a broader challenge: Is it possible and appropriate for certifying boards to recognize expertise and focus that is accrued not through formal training, but through actual practice experience and accompanying self‐directed learning?
In 2006, the ABIM took up this question, producing a report (New and Emerging Disciplines in Internal Medicine II [NEDIM II]) that delineated several criteria to guide whether a new field merited focused recognition through MOC (Table 1). Judging by these criteria, hospital medicine appears to be a suitable first candidate for recognition of focused practice through MOC.
|
PRELIMINARY THOUGHTS ON FOCUSED RECOGNITION IN HOSPITAL MEDICINE
The ABIM has endorsed the concept of recognition of focused practice in hospital medicine and charged a subcommittee (that I chair) with working out the details. It would be premature to describe the committee's deliberations in detail (particularly because the final plan needs to be approved by both the ABIM and the ABMS), but the following are some key issues being discussed.
First, demonstration of focused practice requires some minimum volume of hospitalized patients. In the absence of hard data defining a threshold number of cases for hospitalists, we are likely to endorse a number that has face validity and that reliably separates self‐identified hospitalists from nonhospitalist generalists. As with all volume requirements, we will struggle over how to handle academic physicians, physician‐administrators, and physician‐researchers who limit their overall clinical practice but who spend most of their clinical time in hospital medicine and the bulk of their nonclinical time trying to improve hospital care.
The requirements to demonstrate performance in practice and lifelong learning may be more straightforward. As with all such MOC requirements, the ABIM is increasingly looking to use real practice data, trying to harmonize its data requirements with those of other organizations such as insurers, Medicare, the Joint Commission, or for pay‐for‐performance initiatives. Despite the operational challenges, this effort is vital: for MOC (including focused recognition) to be highly valued by patients, purchasers, and diplomates, it will increasingly need to measure not only what physicians know, but also what they do.
Finally, there is the test. It is likely that a secure exam for MOC with Recognition of Focused Practice in Hospital Medicine will involve core content in internal medicine (information that every internist should know), augmented by substantial and challenging content in hospital medicine. Because it will be vital that a competent hospitalist understand key elements of outpatient practice, the exam will not be stripped of ambulatory content but will likely have fewer questions on topics that hospitalists are unlikely to confront (osteoporosis, cancer screening).
ONGOING ISSUES
As hospital medicine continues its explosive growth, it is important to develop ways to make board certification relevant to hospitalists. The ABIM believes that modifying the MOC process to recognize physicians who have focused their practice and achieved special expertise in hospital medicine is a good way to launch this effort. Ultimately, this process is likely to evolve, particularly if separate training pathways for hospital medicine emerge. For now, the development of Recognition of Focused Practice in Hospital Medicine will further legitimize the new field, provide ABIM with insights into how to recognize physicians who have advanced through practice‐based learning rather than through training, and help to guide other certifying boards (particularly family medicine and pediatrics) considering hospitalist certification. In the end, the process will need to be user‐friendly for and satisfying to diplomates, flexible enough to allow for career transitions (both toward and away from hospital medicine), and sufficiently rigorous to be credible to all stakeholders, particularly patients.
Soon after they form, most new medical fields begin agitating for a special certification, something that says, We're here, and we're different. As I've noted previously in the Journal of Hospital Medicine, the field of hospital medicine resisted this impulse in its early years, fearing that any special designation or certification would actually harm the field's growth and status.1 The concern was that managed‐care organizationsconvinced by the evidence that hospitalists improve efficiency and might improve quality2would react to any new hospitalist sheepskin by mandating that anyone providing hospital care to its covered patients have one. The backlash from primary care physicians locked out of the hospital by such a mandate would have been swift and ultimately damaging to hospitalists. In addition to these political considerations, the early field of hospital medicine lacked the academic credibility and scientific underpinning needed for specialty designation.3
Times have changed. There are now more than 15,000 hospitalists in the United States, and nearly half of American hospitals have hospitalists on their medical staffs. In many markets, including my own, hospitalists care for most internal medicine inpatients, as well as significant numbers of pediatric and surgical patients. The field has achieved academic legitimacy, with this journal, several textbooks, large and flourishing groups in every academic medical center, and several residency tracks and fellowship programs.4, 5 The Society of Hospital Medicine (SHM) has grown to more than 6000 members, become a widely respected and dynamic member of the community of professional societies, and published its core competencies.6
With this as a background, in 2004 SHM asked the American Board of Internal Medicine (ABIM) to consider a program of certification for hospitalists. As a past SHM president and now a member of the ABIM Board of Directors, I am privileged to have a bird's‐eye view of the process. In this article, I reflect on some of the key issues it raises.
THE NUTS AND BOLTS OF BOARD CERTIFICATION
Since the first board (ophthalmology) was formed in 1917, 24 specialty boards have emerged, all under the umbrella of the American Board of Medical Specialties (ABMS).7 Because no one type of physician can do it all, certifying boards have had to struggle not only with how to assess competency in existing disciplines, but with the dynamic and often controversial questions raised when new fields emerge. In the past few decades, certifying boards have grappled with specialties formed around new procedures (such as cardiac electrophysiology), discrete populations (geriatrics, palliative care), complex diseases (HIV medicine), and sites of care (intensive care medicine, emergency medicine). It is this latter category that now includes hospital medicine.
In the past, it was relatively simple for a physician to obtain board certification. Residency or fellowship training was believed to confer on its graduates the presumption of competence and professionalismthe program director's attestation served as the graduate's Good Housekeeping seal of approval. Passing the board exam was the final step, ensuring that newly minted graduates had the requisite knowledge and judgment to practice in their fields.
Remarkably, for the first half century of the specialty boards, all certifications lasted for a physician's professional lifetime. Beginning with the 1969 decision of the American Board of Family Practice to limit the validity of its certificates to 7 years, all ABMS member boards now time limit their certifications, usually to 7‐10 years.7 Of course, in an environment of rapidly changing medical knowledge and new procedures, periodiceven continuousdemonstration of competence is increasingly expected by the public.
For ABIM, the mechanism to promote lifelong learning and demonstrate ongoing competence in the face of a rapidly changing environment is known as maintenance of certification (MOC).8 Through MOC, board‐certified internists demonstrate their ongoing clinical expertise and judgment, their involvement in lifelong learning and quality improvement activities, and their professionalism. Because MOC involves no new training requirements and includes an assessment of a physician's actual practice, it provides a potential mechanism, heretofore untapped, of demonstrating a unique professional focus that emerges after the completion of formal training.
HOSPITALIST CERTIFICATION AND THE MOC PROCESS
As ABIM considered a separate certification pathway for hospital medicine, it faced a conundrum. The vast majority of hospitalists are general internists (most of the rest are generalists in family medicine or pediatrics) who entered hospital medicine at the completion of their internal medicine training or after a period of primary care practice. Job opportunities for hospitalists are plentiful, andexcept for additional training in quality improvement, systems leadership, care transitions, palliative care, and communication9there is little clinical rationale to prolong internal medicine training for hospitalists (some individuals may opt for fellowships to enhance their leadership skills or to launch a research career,5 but few would argue for mandatory additional clinical training in hospital medicine at this time).
So, in the absence of formal training, how could the ABIM (or other boards) recognize the focused practice of hospitalists? This question must be framed within a broader challenge: Is it possible and appropriate for certifying boards to recognize expertise and focus that is accrued not through formal training, but through actual practice experience and accompanying self‐directed learning?
In 2006, the ABIM took up this question, producing a report (New and Emerging Disciplines in Internal Medicine II [NEDIM II]) that delineated several criteria to guide whether a new field merited focused recognition through MOC (Table 1). Judging by these criteria, hospital medicine appears to be a suitable first candidate for recognition of focused practice through MOC.
|
PRELIMINARY THOUGHTS ON FOCUSED RECOGNITION IN HOSPITAL MEDICINE
The ABIM has endorsed the concept of recognition of focused practice in hospital medicine and charged a subcommittee (that I chair) with working out the details. It would be premature to describe the committee's deliberations in detail (particularly because the final plan needs to be approved by both the ABIM and the ABMS), but the following are some key issues being discussed.
First, demonstration of focused practice requires some minimum volume of hospitalized patients. In the absence of hard data defining a threshold number of cases for hospitalists, we are likely to endorse a number that has face validity and that reliably separates self‐identified hospitalists from nonhospitalist generalists. As with all volume requirements, we will struggle over how to handle academic physicians, physician‐administrators, and physician‐researchers who limit their overall clinical practice but who spend most of their clinical time in hospital medicine and the bulk of their nonclinical time trying to improve hospital care.
The requirements to demonstrate performance in practice and lifelong learning may be more straightforward. As with all such MOC requirements, the ABIM is increasingly looking to use real practice data, trying to harmonize its data requirements with those of other organizations such as insurers, Medicare, the Joint Commission, or for pay‐for‐performance initiatives. Despite the operational challenges, this effort is vital: for MOC (including focused recognition) to be highly valued by patients, purchasers, and diplomates, it will increasingly need to measure not only what physicians know, but also what they do.
Finally, there is the test. It is likely that a secure exam for MOC with Recognition of Focused Practice in Hospital Medicine will involve core content in internal medicine (information that every internist should know), augmented by substantial and challenging content in hospital medicine. Because it will be vital that a competent hospitalist understand key elements of outpatient practice, the exam will not be stripped of ambulatory content but will likely have fewer questions on topics that hospitalists are unlikely to confront (osteoporosis, cancer screening).
ONGOING ISSUES
As hospital medicine continues its explosive growth, it is important to develop ways to make board certification relevant to hospitalists. The ABIM believes that modifying the MOC process to recognize physicians who have focused their practice and achieved special expertise in hospital medicine is a good way to launch this effort. Ultimately, this process is likely to evolve, particularly if separate training pathways for hospital medicine emerge. For now, the development of Recognition of Focused Practice in Hospital Medicine will further legitimize the new field, provide ABIM with insights into how to recognize physicians who have advanced through practice‐based learning rather than through training, and help to guide other certifying boards (particularly family medicine and pediatrics) considering hospitalist certification. In the end, the process will need to be user‐friendly for and satisfying to diplomates, flexible enough to allow for career transitions (both toward and away from hospital medicine), and sufficiently rigorous to be credible to all stakeholders, particularly patients.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- ,.The hospitalist movement 5 years later.JAMA2002;287:487–94.
- .The hospitalist: a new medical specialty?Ann Intern Med.1999;130:373–375.
- ,.Implications of the hospitalist movement for academic departments of medicine: lessons from the UCSF experience.Am J Med.1999;106:127–133.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72.e1–e7.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- .Recertification in the United States.BMJ.1999;319:1183–1185.
- ,.Professional standards in the USA: overview and new developments.Clin Med.2006;6:363–367.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- ,.The hospitalist movement 5 years later.JAMA2002;287:487–94.
- .The hospitalist: a new medical specialty?Ann Intern Med.1999;130:373–375.
- ,.Implications of the hospitalist movement for academic departments of medicine: lessons from the UCSF experience.Am J Med.1999;106:127–133.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72.e1–e7.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- .Recertification in the United States.BMJ.1999;319:1183–1185.
- ,.Professional standards in the USA: overview and new developments.Clin Med.2006;6:363–367.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
View from the Hospital Bed
When I went back to Croatia this summer to visit family and friends, little did I think I would find myself standing in the hospital where I trained as an intern. At 1:00 AM I took my father to the ER for an acute episode of nausea, vomiting, dizziness, chest tightness, and dyspnea. Earlier that day, he had been watching the World Cup, not moving from his chair for several hours. The ER saw him promptly and transferred him to the main hospital in town.
Hours later, I was alone in the middle of a wide, marble corridor. Memories flowed back to when I was a young nursing student and later an intern, full of life, energy, and dreams. Now the walls were yellow and darker than I remembered. They were barren, almost sad: no pictures, no art, no life. On my left, patients were taken to a room for procedures such as thoracentesis and lumbar puncture. The next door led to a balcony where cigarettes glowed like orange fireflies. Dark shapes murmured to one another as they nursed their habit. At least smoking wasn't allowed in the patient rooms.
On my right were alternating male and female patient rooms: up to 7 beds per room. They would add an eighth and potentially squeeze in a ninth bed when needed. There were no private rooms, even if you had money or status. There were no dividers or curtains between patients. With no privacy and nothing else to do, patients entertained each other. Some were young, others older. Some had fought in the last war; others were tourists who were visiting. They shared their stories. They overheard each other's plans of care during morning rounds conducted by physicians and nurses.
Each hospital bed had a little nightstand, but that was about it. If you were lucky, you got pajamas. My dad got warm flannel pajamas in the middle of the summer, but at least he got something. We left the house in a hurry and didn't bring anything. No towels? You had to bring your own. No toothpaste or a toothbrush? There was running water but no cup to drink it in. There was not even toilet paper. You had to bring your own.
Everything was nice and clean but something was missing in this former military hospital, as if the life had been drained out of it. It was once a premier facility. Maybe it was getting older or worn out by the war, or lack of maintenance. The best equipment (including new ventilators) were reportedly stolen and sent to another city.
There was no army of people serving you. No medical assistants, physicians assistants, nurse‐practitioners, or technicians. No physical therapists or occupational therapists or even a front desk where people were greeted. No case managers. The hospital population was so much younger than in the United States. There was only 1 patient on the ward who was 90 years old, as opposed to the usual 10 patients on my service in their 80s and 90s. Most patients were discharged home with their families; very few were sent to nursing homes. There was no length of stay to worry about. There were no insurance hassles, preapproval for a test or for an additional day of hospitalization.
There were no daily blood draws. They are not watching him closely enough, I worried. The ER physician apparently ruled out cardiac causes before admitting my father to the neurology service for presumed vertebrobasilar insufficiency. Was the ER physician's history, cardiac bedside exam, 2 normal EKGs an hour apart, and CXR enough to assure him there was no need for a CPK or a troponin? I was told the CPK was normal but never saw the result. They used to do troponin but had stopped because it was too expensive. I cannot imagine admitting a patient in the United States with chest tightness without ordering cardiac enzymes. Are we scared we'll miss something or afraid of litigation? There was no such fear that I could detect in Croatia.
The inpatient workup was otherwise thorough, and everyone was courteous. Nonetheless, the money was tight. If a patient needed over the‐counter medications, a family member would be asked to buy it, sometimes at significant cost. Almost every patient had a peripheral IV but there were no unnecessary IV infusions, unlike the occasional TKO IVs we see here to justify hospitalization for a little old lady who didn't have a place to go or whose insurance would otherwise refuse to pay.
My father seemed dehydrated.
Could you put some normal saline in? I asked.
He already got some, the charge nurse replied.
What did he get?
Metoclopramide infusion in 500 cc of normal saline. He is not vomiting any longer, he can eat and drink, and he doesn't need any more IVs. It is expensive. Go buy some water and juices for him, the charge nurse said.
The meals were served 3 times a day. No snacks in‐between unless friends and family brought something.
Visiting hours were 2‐4 PM daily. Information time for families took place on Tuesday and Friday afternoons. I arrived 30 minutes early to speak with the neurologist. In less than 20 minutes the room was filled. Everyone patiently waited their turn. The time spent with the doctor was brief but was better than nothing. As hospitalists, we spend a significant amount of time on the telephone tracking down family members, talking to them, or arranging a meeting to accommodate their schedule. On the other hand, there are family members who become frustrated because they have difficulty catching the doctor. I wonder if it would be helpful for us to have a dedicated information time. It could prevent frustration, unnecessary phone calls and pages, and perhaps save us time. Given our shorter length of stay, information time only twice a week probably wouldn't be enough.
What are you doing here? Dr. T., a friend of mine, exclaimed. Come on, a doctor from America waiting for the information! Come here, you don't need to wait! Your father is already better. We did a head CT, and we'll do a cervical spine x‐ray and carotid ultrasound tomorrow. Although I was hesitant, my brother, a medical student, tried to question the current treatment.
Why are you giving him diazepam and diclofenac intramuscularly? he asked.
What do you mean why? Because this is how we do it, Dr. T. said.
You already have an IV access. Couldn't you give it to him IV? my brother persisted. Dr. T. looked at him like he had fallen off the apple cart.
I had never seen it done this way in Austria or Germany, my brother continued. Why cause unnecessary pain and complications?
What complications? said Dr. T., now impatient. We use it intramuscularly. This is how it is done. It is only 10 injections. That's nothing.
Ten injections? You can injure a nerve! my brother exclaimed.
What nerve? Not if you do it right. We've been doing it this way forever. Your dad had dizziness and vomiting, and now he is better.
My father was better. Something had worked. Was it the medications or that he had slept and was rested?
The night had sneaked up on us again. The ward was quiet. No call lights. No patient telephones. No TVs. No IV machines and their beeping. No vital signs were taken in the middle of the night. No early‐morning blood draws. No pagers. The hospital was a place to get some rest.
The following day I spoke to the on‐call physician. The head CT and labs were normal. After 4 days in the hospital, I needed to know what the weekend plans would be.
How much longer will he need to be in the hospital? I asked.
Oh, about 10 to 14 days.
Ten to 14 days? I couldn't hide my surprise. What for? I might have as well asked if pigs were falling from the sky. This is how it was done.
Documentation was scanty. No worries of audits to justify the work done. How much time was saved this way? No wonder each physician saw 20 to 30 patients or more. There were 2 forms at discharge typed into a computer by the physician: a short one containing discharge medications and follow‐up plans and a long one to be completed later. We got the short one immediately and made follow‐up appointments. But many weeks later, we are still waiting for the long one. Apparently not all the lab tests are back.
Looking back, I see that when I tried to push for more information, I was viewed as pushy. So I stopped pushing. When I asked some logical questions, I felt like I was showing off. So I stopped asking too many questions. When I asked dad to tell me what happened during the day, he did not know. He did not remember the name of the consultants or what they said. When I asked him to try harder, he refused. When I asked him to write things down, he hesitated. It would mark him as a troublemaker. He was concerned that he would be labeled as an outsider, despite having lived in Croatia his whole life. Years after the war, a person's last name could still conjure up barely concealed hatred or suspicion. My father wanted to be seen as a good and compliant patient. He felt too vulnerable to be pushing for answers. Somehow, against my better judgment, I fell into the same mode.
Coming back to my hometown curiously hampered me. I still don't understand why. Was it sadness in my heart and nostalgia? Or emotional remnants of the recent war lingering in the air? Or a more patriarchal mentality and the unwritten rules of thinking and behaving that I had forgotten about? I was both a daughter and a visitor. I grew up in Croatia, but I left 2 weeks before the fighting broke out, and my prewar memories were still pristine. I was both a native and an American physician with all the expectations of stateside care. When I was a student there, physicians were authoritative and almost never questioned.
In the end, my father received care that was professional and thorough. The hospital lacked some basic necessities. They were on a strict budget for medicines including IV solutions. On the other hand, they kept my father in for 9 days, consulted an internist, an ENT. and a urologist. They also did numerous studies, including a head CT, cervical spine x‐ray, carotid ultrasound, hearing test and vestibulogram, CXR, and abdominal ultrasound. He had only 2 blood draws: CBC and Chem 7 (one time, one stick) and thyroid tests and PSA (one time, second stick). If he had been my patient, I would have probably tried to discharge him within 2 days and have him complete the evaluation as an outpatient. They gave him 9 days of diclofenac and diazepam intramuscularly and 7 days of 500 cc of normal saline and metoclopramide IV. My father was released from the hospital several days early at his insistence and mine. He had no more nausea or vomiting, no chest pain or shortness of breath. He was calm and relaxed. He was well rested. He felt better. And he is fine today.
Acknowledgements
The author is grateful for S.R.C. and his support in writing this manuscript.
When I went back to Croatia this summer to visit family and friends, little did I think I would find myself standing in the hospital where I trained as an intern. At 1:00 AM I took my father to the ER for an acute episode of nausea, vomiting, dizziness, chest tightness, and dyspnea. Earlier that day, he had been watching the World Cup, not moving from his chair for several hours. The ER saw him promptly and transferred him to the main hospital in town.
Hours later, I was alone in the middle of a wide, marble corridor. Memories flowed back to when I was a young nursing student and later an intern, full of life, energy, and dreams. Now the walls were yellow and darker than I remembered. They were barren, almost sad: no pictures, no art, no life. On my left, patients were taken to a room for procedures such as thoracentesis and lumbar puncture. The next door led to a balcony where cigarettes glowed like orange fireflies. Dark shapes murmured to one another as they nursed their habit. At least smoking wasn't allowed in the patient rooms.
On my right were alternating male and female patient rooms: up to 7 beds per room. They would add an eighth and potentially squeeze in a ninth bed when needed. There were no private rooms, even if you had money or status. There were no dividers or curtains between patients. With no privacy and nothing else to do, patients entertained each other. Some were young, others older. Some had fought in the last war; others were tourists who were visiting. They shared their stories. They overheard each other's plans of care during morning rounds conducted by physicians and nurses.
Each hospital bed had a little nightstand, but that was about it. If you were lucky, you got pajamas. My dad got warm flannel pajamas in the middle of the summer, but at least he got something. We left the house in a hurry and didn't bring anything. No towels? You had to bring your own. No toothpaste or a toothbrush? There was running water but no cup to drink it in. There was not even toilet paper. You had to bring your own.
Everything was nice and clean but something was missing in this former military hospital, as if the life had been drained out of it. It was once a premier facility. Maybe it was getting older or worn out by the war, or lack of maintenance. The best equipment (including new ventilators) were reportedly stolen and sent to another city.
There was no army of people serving you. No medical assistants, physicians assistants, nurse‐practitioners, or technicians. No physical therapists or occupational therapists or even a front desk where people were greeted. No case managers. The hospital population was so much younger than in the United States. There was only 1 patient on the ward who was 90 years old, as opposed to the usual 10 patients on my service in their 80s and 90s. Most patients were discharged home with their families; very few were sent to nursing homes. There was no length of stay to worry about. There were no insurance hassles, preapproval for a test or for an additional day of hospitalization.
There were no daily blood draws. They are not watching him closely enough, I worried. The ER physician apparently ruled out cardiac causes before admitting my father to the neurology service for presumed vertebrobasilar insufficiency. Was the ER physician's history, cardiac bedside exam, 2 normal EKGs an hour apart, and CXR enough to assure him there was no need for a CPK or a troponin? I was told the CPK was normal but never saw the result. They used to do troponin but had stopped because it was too expensive. I cannot imagine admitting a patient in the United States with chest tightness without ordering cardiac enzymes. Are we scared we'll miss something or afraid of litigation? There was no such fear that I could detect in Croatia.
The inpatient workup was otherwise thorough, and everyone was courteous. Nonetheless, the money was tight. If a patient needed over the‐counter medications, a family member would be asked to buy it, sometimes at significant cost. Almost every patient had a peripheral IV but there were no unnecessary IV infusions, unlike the occasional TKO IVs we see here to justify hospitalization for a little old lady who didn't have a place to go or whose insurance would otherwise refuse to pay.
My father seemed dehydrated.
Could you put some normal saline in? I asked.
He already got some, the charge nurse replied.
What did he get?
Metoclopramide infusion in 500 cc of normal saline. He is not vomiting any longer, he can eat and drink, and he doesn't need any more IVs. It is expensive. Go buy some water and juices for him, the charge nurse said.
The meals were served 3 times a day. No snacks in‐between unless friends and family brought something.
Visiting hours were 2‐4 PM daily. Information time for families took place on Tuesday and Friday afternoons. I arrived 30 minutes early to speak with the neurologist. In less than 20 minutes the room was filled. Everyone patiently waited their turn. The time spent with the doctor was brief but was better than nothing. As hospitalists, we spend a significant amount of time on the telephone tracking down family members, talking to them, or arranging a meeting to accommodate their schedule. On the other hand, there are family members who become frustrated because they have difficulty catching the doctor. I wonder if it would be helpful for us to have a dedicated information time. It could prevent frustration, unnecessary phone calls and pages, and perhaps save us time. Given our shorter length of stay, information time only twice a week probably wouldn't be enough.
What are you doing here? Dr. T., a friend of mine, exclaimed. Come on, a doctor from America waiting for the information! Come here, you don't need to wait! Your father is already better. We did a head CT, and we'll do a cervical spine x‐ray and carotid ultrasound tomorrow. Although I was hesitant, my brother, a medical student, tried to question the current treatment.
Why are you giving him diazepam and diclofenac intramuscularly? he asked.
What do you mean why? Because this is how we do it, Dr. T. said.
You already have an IV access. Couldn't you give it to him IV? my brother persisted. Dr. T. looked at him like he had fallen off the apple cart.
I had never seen it done this way in Austria or Germany, my brother continued. Why cause unnecessary pain and complications?
What complications? said Dr. T., now impatient. We use it intramuscularly. This is how it is done. It is only 10 injections. That's nothing.
Ten injections? You can injure a nerve! my brother exclaimed.
What nerve? Not if you do it right. We've been doing it this way forever. Your dad had dizziness and vomiting, and now he is better.
My father was better. Something had worked. Was it the medications or that he had slept and was rested?
The night had sneaked up on us again. The ward was quiet. No call lights. No patient telephones. No TVs. No IV machines and their beeping. No vital signs were taken in the middle of the night. No early‐morning blood draws. No pagers. The hospital was a place to get some rest.
The following day I spoke to the on‐call physician. The head CT and labs were normal. After 4 days in the hospital, I needed to know what the weekend plans would be.
How much longer will he need to be in the hospital? I asked.
Oh, about 10 to 14 days.
Ten to 14 days? I couldn't hide my surprise. What for? I might have as well asked if pigs were falling from the sky. This is how it was done.
Documentation was scanty. No worries of audits to justify the work done. How much time was saved this way? No wonder each physician saw 20 to 30 patients or more. There were 2 forms at discharge typed into a computer by the physician: a short one containing discharge medications and follow‐up plans and a long one to be completed later. We got the short one immediately and made follow‐up appointments. But many weeks later, we are still waiting for the long one. Apparently not all the lab tests are back.
Looking back, I see that when I tried to push for more information, I was viewed as pushy. So I stopped pushing. When I asked some logical questions, I felt like I was showing off. So I stopped asking too many questions. When I asked dad to tell me what happened during the day, he did not know. He did not remember the name of the consultants or what they said. When I asked him to try harder, he refused. When I asked him to write things down, he hesitated. It would mark him as a troublemaker. He was concerned that he would be labeled as an outsider, despite having lived in Croatia his whole life. Years after the war, a person's last name could still conjure up barely concealed hatred or suspicion. My father wanted to be seen as a good and compliant patient. He felt too vulnerable to be pushing for answers. Somehow, against my better judgment, I fell into the same mode.
Coming back to my hometown curiously hampered me. I still don't understand why. Was it sadness in my heart and nostalgia? Or emotional remnants of the recent war lingering in the air? Or a more patriarchal mentality and the unwritten rules of thinking and behaving that I had forgotten about? I was both a daughter and a visitor. I grew up in Croatia, but I left 2 weeks before the fighting broke out, and my prewar memories were still pristine. I was both a native and an American physician with all the expectations of stateside care. When I was a student there, physicians were authoritative and almost never questioned.
In the end, my father received care that was professional and thorough. The hospital lacked some basic necessities. They were on a strict budget for medicines including IV solutions. On the other hand, they kept my father in for 9 days, consulted an internist, an ENT. and a urologist. They also did numerous studies, including a head CT, cervical spine x‐ray, carotid ultrasound, hearing test and vestibulogram, CXR, and abdominal ultrasound. He had only 2 blood draws: CBC and Chem 7 (one time, one stick) and thyroid tests and PSA (one time, second stick). If he had been my patient, I would have probably tried to discharge him within 2 days and have him complete the evaluation as an outpatient. They gave him 9 days of diclofenac and diazepam intramuscularly and 7 days of 500 cc of normal saline and metoclopramide IV. My father was released from the hospital several days early at his insistence and mine. He had no more nausea or vomiting, no chest pain or shortness of breath. He was calm and relaxed. He was well rested. He felt better. And he is fine today.
Acknowledgements
The author is grateful for S.R.C. and his support in writing this manuscript.
When I went back to Croatia this summer to visit family and friends, little did I think I would find myself standing in the hospital where I trained as an intern. At 1:00 AM I took my father to the ER for an acute episode of nausea, vomiting, dizziness, chest tightness, and dyspnea. Earlier that day, he had been watching the World Cup, not moving from his chair for several hours. The ER saw him promptly and transferred him to the main hospital in town.
Hours later, I was alone in the middle of a wide, marble corridor. Memories flowed back to when I was a young nursing student and later an intern, full of life, energy, and dreams. Now the walls were yellow and darker than I remembered. They were barren, almost sad: no pictures, no art, no life. On my left, patients were taken to a room for procedures such as thoracentesis and lumbar puncture. The next door led to a balcony where cigarettes glowed like orange fireflies. Dark shapes murmured to one another as they nursed their habit. At least smoking wasn't allowed in the patient rooms.
On my right were alternating male and female patient rooms: up to 7 beds per room. They would add an eighth and potentially squeeze in a ninth bed when needed. There were no private rooms, even if you had money or status. There were no dividers or curtains between patients. With no privacy and nothing else to do, patients entertained each other. Some were young, others older. Some had fought in the last war; others were tourists who were visiting. They shared their stories. They overheard each other's plans of care during morning rounds conducted by physicians and nurses.
Each hospital bed had a little nightstand, but that was about it. If you were lucky, you got pajamas. My dad got warm flannel pajamas in the middle of the summer, but at least he got something. We left the house in a hurry and didn't bring anything. No towels? You had to bring your own. No toothpaste or a toothbrush? There was running water but no cup to drink it in. There was not even toilet paper. You had to bring your own.
Everything was nice and clean but something was missing in this former military hospital, as if the life had been drained out of it. It was once a premier facility. Maybe it was getting older or worn out by the war, or lack of maintenance. The best equipment (including new ventilators) were reportedly stolen and sent to another city.
There was no army of people serving you. No medical assistants, physicians assistants, nurse‐practitioners, or technicians. No physical therapists or occupational therapists or even a front desk where people were greeted. No case managers. The hospital population was so much younger than in the United States. There was only 1 patient on the ward who was 90 years old, as opposed to the usual 10 patients on my service in their 80s and 90s. Most patients were discharged home with their families; very few were sent to nursing homes. There was no length of stay to worry about. There were no insurance hassles, preapproval for a test or for an additional day of hospitalization.
There were no daily blood draws. They are not watching him closely enough, I worried. The ER physician apparently ruled out cardiac causes before admitting my father to the neurology service for presumed vertebrobasilar insufficiency. Was the ER physician's history, cardiac bedside exam, 2 normal EKGs an hour apart, and CXR enough to assure him there was no need for a CPK or a troponin? I was told the CPK was normal but never saw the result. They used to do troponin but had stopped because it was too expensive. I cannot imagine admitting a patient in the United States with chest tightness without ordering cardiac enzymes. Are we scared we'll miss something or afraid of litigation? There was no such fear that I could detect in Croatia.
The inpatient workup was otherwise thorough, and everyone was courteous. Nonetheless, the money was tight. If a patient needed over the‐counter medications, a family member would be asked to buy it, sometimes at significant cost. Almost every patient had a peripheral IV but there were no unnecessary IV infusions, unlike the occasional TKO IVs we see here to justify hospitalization for a little old lady who didn't have a place to go or whose insurance would otherwise refuse to pay.
My father seemed dehydrated.
Could you put some normal saline in? I asked.
He already got some, the charge nurse replied.
What did he get?
Metoclopramide infusion in 500 cc of normal saline. He is not vomiting any longer, he can eat and drink, and he doesn't need any more IVs. It is expensive. Go buy some water and juices for him, the charge nurse said.
The meals were served 3 times a day. No snacks in‐between unless friends and family brought something.
Visiting hours were 2‐4 PM daily. Information time for families took place on Tuesday and Friday afternoons. I arrived 30 minutes early to speak with the neurologist. In less than 20 minutes the room was filled. Everyone patiently waited their turn. The time spent with the doctor was brief but was better than nothing. As hospitalists, we spend a significant amount of time on the telephone tracking down family members, talking to them, or arranging a meeting to accommodate their schedule. On the other hand, there are family members who become frustrated because they have difficulty catching the doctor. I wonder if it would be helpful for us to have a dedicated information time. It could prevent frustration, unnecessary phone calls and pages, and perhaps save us time. Given our shorter length of stay, information time only twice a week probably wouldn't be enough.
What are you doing here? Dr. T., a friend of mine, exclaimed. Come on, a doctor from America waiting for the information! Come here, you don't need to wait! Your father is already better. We did a head CT, and we'll do a cervical spine x‐ray and carotid ultrasound tomorrow. Although I was hesitant, my brother, a medical student, tried to question the current treatment.
Why are you giving him diazepam and diclofenac intramuscularly? he asked.
What do you mean why? Because this is how we do it, Dr. T. said.
You already have an IV access. Couldn't you give it to him IV? my brother persisted. Dr. T. looked at him like he had fallen off the apple cart.
I had never seen it done this way in Austria or Germany, my brother continued. Why cause unnecessary pain and complications?
What complications? said Dr. T., now impatient. We use it intramuscularly. This is how it is done. It is only 10 injections. That's nothing.
Ten injections? You can injure a nerve! my brother exclaimed.
What nerve? Not if you do it right. We've been doing it this way forever. Your dad had dizziness and vomiting, and now he is better.
My father was better. Something had worked. Was it the medications or that he had slept and was rested?
The night had sneaked up on us again. The ward was quiet. No call lights. No patient telephones. No TVs. No IV machines and their beeping. No vital signs were taken in the middle of the night. No early‐morning blood draws. No pagers. The hospital was a place to get some rest.
The following day I spoke to the on‐call physician. The head CT and labs were normal. After 4 days in the hospital, I needed to know what the weekend plans would be.
How much longer will he need to be in the hospital? I asked.
Oh, about 10 to 14 days.
Ten to 14 days? I couldn't hide my surprise. What for? I might have as well asked if pigs were falling from the sky. This is how it was done.
Documentation was scanty. No worries of audits to justify the work done. How much time was saved this way? No wonder each physician saw 20 to 30 patients or more. There were 2 forms at discharge typed into a computer by the physician: a short one containing discharge medications and follow‐up plans and a long one to be completed later. We got the short one immediately and made follow‐up appointments. But many weeks later, we are still waiting for the long one. Apparently not all the lab tests are back.
Looking back, I see that when I tried to push for more information, I was viewed as pushy. So I stopped pushing. When I asked some logical questions, I felt like I was showing off. So I stopped asking too many questions. When I asked dad to tell me what happened during the day, he did not know. He did not remember the name of the consultants or what they said. When I asked him to try harder, he refused. When I asked him to write things down, he hesitated. It would mark him as a troublemaker. He was concerned that he would be labeled as an outsider, despite having lived in Croatia his whole life. Years after the war, a person's last name could still conjure up barely concealed hatred or suspicion. My father wanted to be seen as a good and compliant patient. He felt too vulnerable to be pushing for answers. Somehow, against my better judgment, I fell into the same mode.
Coming back to my hometown curiously hampered me. I still don't understand why. Was it sadness in my heart and nostalgia? Or emotional remnants of the recent war lingering in the air? Or a more patriarchal mentality and the unwritten rules of thinking and behaving that I had forgotten about? I was both a daughter and a visitor. I grew up in Croatia, but I left 2 weeks before the fighting broke out, and my prewar memories were still pristine. I was both a native and an American physician with all the expectations of stateside care. When I was a student there, physicians were authoritative and almost never questioned.
In the end, my father received care that was professional and thorough. The hospital lacked some basic necessities. They were on a strict budget for medicines including IV solutions. On the other hand, they kept my father in for 9 days, consulted an internist, an ENT. and a urologist. They also did numerous studies, including a head CT, cervical spine x‐ray, carotid ultrasound, hearing test and vestibulogram, CXR, and abdominal ultrasound. He had only 2 blood draws: CBC and Chem 7 (one time, one stick) and thyroid tests and PSA (one time, second stick). If he had been my patient, I would have probably tried to discharge him within 2 days and have him complete the evaluation as an outpatient. They gave him 9 days of diclofenac and diazepam intramuscularly and 7 days of 500 cc of normal saline and metoclopramide IV. My father was released from the hospital several days early at his insistence and mine. He had no more nausea or vomiting, no chest pain or shortness of breath. He was calm and relaxed. He was well rested. He felt better. And he is fine today.
Acknowledgements
The author is grateful for S.R.C. and his support in writing this manuscript.
Clinical Conundrum
A 56‐year‐old woman from Colombia presented to the emergency department after 24 hours of abdominal pain. One week before, she had experienced similar pain that lasted for 4 hours and spontaneously resolved. She was nauseated but had no vomiting. She reported an unintentional 14‐pound weight loss over the preceding 3 weeks. She denied fever, chills, night sweats, diarrhea, constipation, dysuria, or jaundice.
In a middle‐aged woman with abdominal pain and nausea, diagnostic considerations include gallbladder disease, diseases of the bowel (such as a partial small‐bowel obstruction or inflammatory conditions), hepatic or pancreatic conditions, and nongastrointestinal ailments such as cardiac ischemia. Knowing the specific location of pain, its quality, precipitating factors, and accompanying systemic symptoms may help to narrow the diagnosis. The unintentional weight loss preceding the onset of pain may be an important clue because it suggests a systemic condition, and in a South American immigrantparticularly if she has traveled recentlyit is important to consider parasitic illnesses. The absence of fever makes some infections such as tuberculosis and malaria less likely. At this point, in addition to a thorough history and physical, laboratory tests should include a complete blood count (with quantification of eosinophils) and a metabolic panel with liver enzymes and albumin.
The patient described pain in the midline, just inferior to the umbilicus. The pain was constant, developed without any particular provocation, and was not related to meals or exertion. There were no constitutional symptoms aside from weight loss. She had a history of bipolar disorder, hypothyroidism, osteoarthritis, and chronic sinusitis and had previously undergone cholecystectomy and abdominal hysterectomy. She was taking levothyroxine, montelukast, bupropion, oxcarbazepine, fexofenadine, meloxicam, zolpidem, and, as needed, acetaminophen. She had recently completed a 10‐day course of levofloxacin for acute sinusitis. She had immigrated to the United States 10 years earlier and lived with her husband and daughter. She denied the use of tobacco, alcohol or illicit drugs. She had visited Colombia 6 months earlier but had no other recent travel history.
The history of cholecystectomy makes a biliary tract process unlikely. Its location reduces the likelihood of a hepatic or pancreatic process, but I would like to see the liver enzymes, especially given her recent acetaminophen use. The comorbid illnessesparticularly her bipolar disordermay be relevant because psychiatric illness might be associated with medication overuse or undisclosed toxic ingestions. For example, excess thyroxine might lead to weight loss while overuse of nonsteroidal anti‐inflammatory drugs such as meloxicam can cause intestinal ulceration, not only in the upper tract, but also in the colon. Undisclosed ingestions may also be associated with abdominal symptoms. Her surgical history makes adhesions with a secondary partial bowel obstruction possible. With no travel outside this country in the last 6 months, exotic infections are less likely. Finally, the recent course of levofloxacin may be relevant because many antibiotics are associated with nonspecific abdominal symptoms, and Clostridium difficile colitis occasionally presents without diarrhea.
The patient reported taking her medications as prescribed and denied ingesting other medications. On physical examination, she had a temperature of 98.9F, a pulse of 81 beats per minute, a blood pressure of 110/80 mm Hg, and a respiratory rate of 16 respirations per minute. She had a normal oxygen saturation while breathing ambient air. Her weight was 58 kg. There was no scleral icterus or jugular venous distension. She had a small painless ulcer involving the hard palate. Her lungs were clear to auscultation, and cardiac examination was normal. The abdomen was soft, bowel sounds were present, and there was moderate tenderness to palpation inferior to the umbilicus. There was no rebound or guarding, hepatosplenomegaly or other masses. There was no peripheral edema and no lymphadenopathy. Neurological examination was normal.
The oral ulcer may or may not be related to the clinical presentation because oral ulcers, whether painful or painless, are ubiquitous and may be isolated or may be associated with a wide range of infectious and noninfectious systemic diseases. Although some systemic causes of mucocutaneous ulcers are associated with weight loss (including Crohn's disease, Behcet's disease, celiac sprue, human immunodeficiency virus [HIV], herpesviruses, syphilis, and systemic lupus erythematosus [SLE], among others), the lack of specificity of this finding limits its diagnostic utility. However, it is reasonable to ask whether the patient has noted frequent ulceration in the mouth or genitalia, as recurrent or severe ulcerations may narrow the diagnostic considerations. On the other hand, the focal nature of the pain inferior to the umbilicus suggests a discrete process in the abdomen or pelvis, such as an abscess, mass, or localized area of bowel inflammation. A plain abdominal film is likely to be low yield in this situation, so pursuing computed tomography is appropriate. Not all patients with focal abdominal pain require abdominal imaging, but in the context of weight loss and persistent symptoms for more than a week, imaging is prudent in this case.
The patient denied genital ulceration but did report painless oral ulcers over the preceding months. Laboratory evaluation revealed a white‐cell count of 1000/mm3, of which 6% were neutrophils, 5% were band forms, 36% were lymphocytes, and 47% were monocytes. The absolute neutrophil count was 110/mm3. Hemoglobin level was 10.2 g/dL with a mean corpuscular volume of 90 m3, and the platelet count was 151,000/mm3. Other results of laboratory studies were: sodium, 140 mmol/L; potassium, 3.8 mmol/L; chloride, 96 mmol/L; bicarbonate, 23 mmol/L; blood urea nitrogen, 13 mg/dL; creatinine, 0.4 mg/dL; lipase, 32 U/L (normal range, 13‐60); amylase, 73 U/L (normal range, 30‐110); albumin, 4.0 g/dL; aspartate aminotransferase, 779 U/L (normal range, 13‐35); alanine aminotransferase, 330 U/L (normal range, 7‐35); alkaline phosphatase, 510 U/L (normal range, 35‐104); and total bilirubin, 0.9 mg/dL (normal range, 0.1‐1.2). The lactate dehydrogenase level was 200 U/L (normal range, 135‐214). The corrected reticulocyte count was 1.6% (normal range, 0.3‐2.3), and haptoglobin was 190 mg/dL (normal range, 43‐212). A direct Coomb's test was positive. The erythrocyte sedimentation rate was 113 mm/hour (normal range, 1‐25). Urinalysis was normal without evidence of protein or blood.
Laboratory abnormalities include elevated transaminases and alkaline phosphatase, a markedly elevated erythrocyte sedimentation rate, and profound leukopenia with neutropenia. The patient is anemic, which may elevate the sedimentation rate but not typically to this degree. The patient is not febrile, but if she were to develop a fever, empiric antibiotics would be prudent. The normal albumin and bilirubin suggest that hepatic synthetic and excretory functions remain intact. Although the direct Coombs test is positive, the reticulocyte and lactate dehydrogenase levels argue against brisk hemolysis; this abnormality may simply be a marker of nonspecific immune activation. A variety of infections can cause neutropenia and liver enzyme abnormalities including parasites (malaria or leishmaniasis), viruses (cytomegalovirus or Epstein‐Barr virus [EBV]), tick‐borne bacterial infections (ehrlichiosis or rickettsial infection), and granulomatous infections (tuberculosis). Malignant infiltration of the reticuloendothelial system can also lead to cytopenias and liver enzyme abnormalities. Autoimmunity remains a consideration, as SLE may lead to cytopenias, oral ulcers, and nonspecific immune phenomena. Rather than ordering a large number of blood tests, I favor a targeted approach with abdominal computed tomography followed by biopsy of either the liver or bone marrow.
Chest radiography revealed no abnormalities. Computed tomography of the chest, abdomen, and pelvis with intravenous and oral contrast demonstrated concentric wall thickening of the transverse colon, but no evidence of obstruction or free air. The patient was treated with intravenous fluids, morphine, and cefepime. Bone marrow biopsy was performed, which demonstrated a hypercellular marrow with increased myeloid precursors and a left shift and megakaryocytic hyperplasia. Flow cytometry revealed no abnormally restricted clonal populations. A concerted search for an infectious etiology of the patient's neutropenia was unrevealing, including tests for HIV, cytomegalovirus, hepatitis A, hepatitis B, hepatitis C, Mycoplasma pneumoniae, EBV, and parvovirus B19.
I hope blood cultures were drawn prior to the initiation of antibiotics. Hypercellularity of the bone marrow in the context of leukopenia raises concern that white blood cells are being destroyed peripherally. Autoimmunity against neutrophils can be transiently induced by viruses such as HIV, hepatitis B, and EBV, but these infections have been excluded. Testing for antinuclear antibodies is reasonable. A normal‐sized spleen on the abdominal CT excludes hypersplenism. Colonic thickening can be associated with infection, ischemia, inflammatory bowel disease, and malignancy. The question is whether the colonic thickening is part of the same disease process causing the leukopenia and liver enzyme elevation or whether it represents a secondary infectious process in the setting of neutropenia (such as Clostridium difficile infection or typhlitis). Testing for stool pathogens (including ova and parasites) is certainly appropriate, and consideration of a colonoscopy with biopsy is reasonable, provided that appropriate antimicrobial coverage remains in place.
Blood cultures obtained prior to starting antibiotics were negative. The patient's abdominal pain improved, and she was discharged home to have close follow‐up with a hematologist. The results of her liver function tests improved, and her absolute neutrophil count was 230/mm3 at the time of discharge. Her neutropenia was believed to be secondary to peripheral destruction from a viral, drug‐mediated, or autoimmune process. Oxcarbazepine (Trileptal) was discontinued, as it was believed to be the medication most likely to be responsible. She returned to the hospital 3 days later with recurrence of her abdominal pain and diarrhea. She remained afebrile. Additional history revealed arthralgias over the previous 2 months, mild alopecia, and prior symptoms suggestive of Raynaud's phenomenon. Stool studies failed to establish an infectious etiology for the diarrhea, and her continued neutropenia responded appropriately to treatment with subcutaneous filgrastim. Colonoscopy could be performed only to the hepatic flexure and revealed no abnormalities. A serologic test for antinuclear antibodies was positive at a titer of 1:640 in a homogenous pattern, and a test for antineutrophil cytoplasmic antibodies was negative. Complement levels were normal, and tests for cryoglobulins, rapid plasma reagin, anticardiolipin antibody, lupus anticoagulant, rheumatoid factor, and antibodies to extractable nuclear antigens were all negative.
Raynaud's phenomenon is consistent with lupus. Double‐stranded DNA antibodies should be sent, although the urine did not demonstrate protein or an active sediment. Systemic sclerosis and the CREST syndrome is strongly associated with Raynaud's phenomenon and high‐titer ANA, but the patient does not have sclerodactyly, which is generally the earliest skin involvement. Autoimmune hepatitis is often associated with high‐titer ANA but does not fit this clinical picture. Given that the patient's presentation included segmental bowel wall thickening and a transient but marked liver enzyme elevation with AST predominance, I am concerned about vasculitis of the abdominal vasculature and would strongly consider a mesenteric angiogram.
To exclude mesenteric vasculitis, the patient underwent magnetic resonance angiography of the abdomen, the results of which were normal. A repeat test for antinuclear antibodies was positive at a titer of 1:2560 in a uniform pattern. A test for anti‐double‐stranded DNA was positive at 1370 U/mL. The patient was diagnosed with systemic lupus and probable lupus enteritis, and therapy with oral prednisone (10 mg daily) and hydroxychloroquine was initiated. She had prompt improvement in her abdominal pain, and was discharged home. Five months later she developed proteinuria and underwent a renal biopsy, which showed minor, nonspecific glomerular abnormalities, suggesting possible mild lupus nephritis. Eight months after her initial presentation, she remains free of abdominal pain and has regained the weight she had initially lost. Her oral ulcers have resolved, and her blood counts have normalized. Her serum creatinine has remained normal. She is now maintained on prednisone (15 mg daily), hydroxychloroquine, and mycophenolate mofetil.
COMMENTARY
A diagnosis of systemic lupus erythematosus (SLE) provided a unifying explanation for the patient's findings. Indeed, she manifested 4 of the 11 American College of Rheumatology criteria for systemic lupus (oral ulcers, leukopenia, positive anti‐DNA, and positive ANA), meeting criteria for a definite diagnosis of SLE. She additionally had multiple other features suggestive of lupus including Raynaud's phenomenon, arthralgias, alopecia, mild thrombocytopenia, and a positive Coombs' test (although the normal reticulocyte count, lactate dehydrogenase, and haptoglobin were most consistent with anemia of a chronic disease).
The protean manifestations of SLE can present significant diagnostic challenges. In this case, physicians were immediately drawn to the patient's acute abdominal pain and severe neutropenia and failed to recognize more subtle disease manifestations that may have aided in establishing a unifying diagnosis sooner. The initial history and review of systems did not disclose arthralgias, alopecia, or Raynaud's phenomenon. In an era of increasing use of hospitalists, which creates potential discontinuity between inpatient and outpatient physicians, a thorough history and review of systems may be particularly important in diagnosing acute manifestations of chronic systemic disease. Inpatient physicians may be overly focused on the small subset of acute complaints leading to hospitalization, without considering the larger constellation of symptoms that may facilitate accurate diagnosis. Our discussant quickly recognized the multisystem nature of the patient's illness and appropriately focused on infectious, neoplastic, and autoimmune categories of disease as being most likely. When infectious and neoplastic conditions were excluded with reasonable certainty, a directed serologic investigation for autoimmune disease was requested, culminating in a diagnosis of SLE.
Involvement of the skin as well as hematologic, renal, and musculoskeletal systems in SLE is commonly recognized, whereas gastrointestinal involvement is perceived to occur much less frequently. However, abdominal pain occurs in up to 40% of patients with lupus.14 Abdominal pain in lupus patients can arise from non‐lupus‐related conditions as well as lupus‐related entities, including serositis, mesenteric vasculitis with or without infarction, mesenteric thrombosis, pancreatitis, inflammatory bowel disease, and adverse medication effects including peptic ulcer disease. Abnormal liver chemistries, as seen in our patient, occur in 20%‐50% of patients with lupus and may be due to lupus hepatitis, concomitant autoimmune hepatitis, or medications including NSAIDs.5, 6 Oral ulcers and leukopenia are likewise common in SLE, with each seen in up to half of patients.4, 7, 8 Leukopenia in SLE may a result of neutropenia, lymphocytopenia, or both. However, severe neutropenia (ie, absolute count less than 500/L), as seen in our case, is more often a result of myelotoxicity from immunosuppressive therapy, rather than SLE itself.9
Lupus enteritis represents bowel microischemia from small‐vessel arteritis or venulitis that often is not evident on conventional mesenteric angiography.4, 10, 11 The reported prevalence of intestinal vasculitis in patients with SLE varies widely, depending on the characteristics of lupus patients sampled in individual studies. Intestinal vasculitis affects 0.2%‐0.5% of SLE patients in general,4, 12 whereas among SLE patients with active disease and an acute abdomen, vasculitis has been reported in up to 53% of patients.10 Antiphospholipid antibodies, antibodies to extractable nuclear antigens, the SLE Disease Activity Index, complement levels, erythrocyte sedimentation rate, C‐reactive protein, and anti‐double‐stranded DNA do not reliably differentiate lupus enteritis from acute abdominal pain due to other etiologies in patients with SLE.11 However, a concomitant drop in the white blood cell count at the onset of symptoms may be useful in distinguishing lupus enteritis from other causes of acute abdominal pain among lupus patients.11 Computed tomography findings consistent with lupus enteritis are nonspecific and include bowel‐wall thickening, submucosal edema (eg, target sign), dilatation of intestinal segments, engorgement of mesenteric vessels, and increased attenuation of mesenteric fat.13 Colonoscopy may reveal areas of ischemia and ulceration, and biopsy can confirm intestinal vasculitis. However, intestinal involvement may be segmental, and pathologic confirmation may be difficult. Contrast enema, gallium scanning, and indium‐labeled white cell scanning may be useful, but lack specificity. No controlled trials to date have evaluated the optimal therapy for lupus enteritis, but pulsed methylprednisolone is often recommended.4 Cyclophosphamide, azathioprine, methotrexate, and cyclosporine have also been used as adjunctive agents. Patients may progress to intestinal infarction and perforation, which augurs a poor prognosis, and early surgical exploration should be considered in severely ill patients.10 Death may occur in more than two‐thirds of patients whose disease progresses to intestinal perforation.1
In summary, a multisystem disease such as SLE requires a comprehensive history, physical exam, and review of systems to establish a correct diagnosis. In our case, an extensive evaluation was necessary to exclude other etiologies of abdominal pain and systemic illness, particularly as infectious and neoplastic conditions occur far more often than lupus enteritis in the general population. However, profound laboratory abnormalities may have preoccupied the attention of treating physicians, leading them to overlook less obvious but important historical and physical findings suggestive of SLE. The cohesively abnormal forest may thus have been obscured by erratically abnormal individual trees. Gastrointestinal symptoms may be underrecognized in SLE. When these result for lupus enteritis, timely recognition may be lifesaving.
- ,.The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature.Semin Arthritis Rheum.1980;9:237.
- ,,.Acute abdominal complications of systemic lupus erythematosus and polyarteritis nodosa.Am J Med.1982;73:525–531.
- ,.Acute gastrointestinal manifestations of systemic lupus erythematosus.Can J Surg.1987;30:185–188.
- ,,.A review of gastrointestinal manifestations of systemic lupus erythematosus.Rheumatology.1999;38:917–932.
- ,.Connective tissue disease and the liver.J Clin Gastroenterol.2002;35:345–349.
- ,,.The spectrum of liver disease in systemic lupus erythematosus: report of 33 histologically‐proved cases and review of the literature.Am J Med.1980;69:187–194.
- ,.Hematologic aspects of systemic lupus erythematosus: current concepts.Ann Intern Med.1977;86:220–229.
- ,.Prevalence and significance of hematological abnormalities in patients with systemic lupus erythematosus.Q J Med1991;80:605–12.
- ,,, et al.Moderate and severe neutropenia in patients with systemic lupus erythematosus.Rheumatology.2006;45:994–998.
- ,,, et al.Acute abdomen in systemic lupus erythematosus: the importance of early laparotomy.Am J Med.1997;103:100–105.
- ,,, et al.Acute abdominal pain in systemic lupus erythematosus: focus on lupus enteritis (gastrointestinal vasculitis).Ann Rheum Dis,2002;61:547–550.
- ,,, et al.Vasculitis in systemic lupus erythematosus.Lupus.1997;6:235–242.
- ,,, et al.CT features of systemic lupus erythematosus in patients with acute abdominal pain: emphasis on ischemic bowel disease.Radiology.1999;211:203–209.
A 56‐year‐old woman from Colombia presented to the emergency department after 24 hours of abdominal pain. One week before, she had experienced similar pain that lasted for 4 hours and spontaneously resolved. She was nauseated but had no vomiting. She reported an unintentional 14‐pound weight loss over the preceding 3 weeks. She denied fever, chills, night sweats, diarrhea, constipation, dysuria, or jaundice.
In a middle‐aged woman with abdominal pain and nausea, diagnostic considerations include gallbladder disease, diseases of the bowel (such as a partial small‐bowel obstruction or inflammatory conditions), hepatic or pancreatic conditions, and nongastrointestinal ailments such as cardiac ischemia. Knowing the specific location of pain, its quality, precipitating factors, and accompanying systemic symptoms may help to narrow the diagnosis. The unintentional weight loss preceding the onset of pain may be an important clue because it suggests a systemic condition, and in a South American immigrantparticularly if she has traveled recentlyit is important to consider parasitic illnesses. The absence of fever makes some infections such as tuberculosis and malaria less likely. At this point, in addition to a thorough history and physical, laboratory tests should include a complete blood count (with quantification of eosinophils) and a metabolic panel with liver enzymes and albumin.
The patient described pain in the midline, just inferior to the umbilicus. The pain was constant, developed without any particular provocation, and was not related to meals or exertion. There were no constitutional symptoms aside from weight loss. She had a history of bipolar disorder, hypothyroidism, osteoarthritis, and chronic sinusitis and had previously undergone cholecystectomy and abdominal hysterectomy. She was taking levothyroxine, montelukast, bupropion, oxcarbazepine, fexofenadine, meloxicam, zolpidem, and, as needed, acetaminophen. She had recently completed a 10‐day course of levofloxacin for acute sinusitis. She had immigrated to the United States 10 years earlier and lived with her husband and daughter. She denied the use of tobacco, alcohol or illicit drugs. She had visited Colombia 6 months earlier but had no other recent travel history.
The history of cholecystectomy makes a biliary tract process unlikely. Its location reduces the likelihood of a hepatic or pancreatic process, but I would like to see the liver enzymes, especially given her recent acetaminophen use. The comorbid illnessesparticularly her bipolar disordermay be relevant because psychiatric illness might be associated with medication overuse or undisclosed toxic ingestions. For example, excess thyroxine might lead to weight loss while overuse of nonsteroidal anti‐inflammatory drugs such as meloxicam can cause intestinal ulceration, not only in the upper tract, but also in the colon. Undisclosed ingestions may also be associated with abdominal symptoms. Her surgical history makes adhesions with a secondary partial bowel obstruction possible. With no travel outside this country in the last 6 months, exotic infections are less likely. Finally, the recent course of levofloxacin may be relevant because many antibiotics are associated with nonspecific abdominal symptoms, and Clostridium difficile colitis occasionally presents without diarrhea.
The patient reported taking her medications as prescribed and denied ingesting other medications. On physical examination, she had a temperature of 98.9F, a pulse of 81 beats per minute, a blood pressure of 110/80 mm Hg, and a respiratory rate of 16 respirations per minute. She had a normal oxygen saturation while breathing ambient air. Her weight was 58 kg. There was no scleral icterus or jugular venous distension. She had a small painless ulcer involving the hard palate. Her lungs were clear to auscultation, and cardiac examination was normal. The abdomen was soft, bowel sounds were present, and there was moderate tenderness to palpation inferior to the umbilicus. There was no rebound or guarding, hepatosplenomegaly or other masses. There was no peripheral edema and no lymphadenopathy. Neurological examination was normal.
The oral ulcer may or may not be related to the clinical presentation because oral ulcers, whether painful or painless, are ubiquitous and may be isolated or may be associated with a wide range of infectious and noninfectious systemic diseases. Although some systemic causes of mucocutaneous ulcers are associated with weight loss (including Crohn's disease, Behcet's disease, celiac sprue, human immunodeficiency virus [HIV], herpesviruses, syphilis, and systemic lupus erythematosus [SLE], among others), the lack of specificity of this finding limits its diagnostic utility. However, it is reasonable to ask whether the patient has noted frequent ulceration in the mouth or genitalia, as recurrent or severe ulcerations may narrow the diagnostic considerations. On the other hand, the focal nature of the pain inferior to the umbilicus suggests a discrete process in the abdomen or pelvis, such as an abscess, mass, or localized area of bowel inflammation. A plain abdominal film is likely to be low yield in this situation, so pursuing computed tomography is appropriate. Not all patients with focal abdominal pain require abdominal imaging, but in the context of weight loss and persistent symptoms for more than a week, imaging is prudent in this case.
The patient denied genital ulceration but did report painless oral ulcers over the preceding months. Laboratory evaluation revealed a white‐cell count of 1000/mm3, of which 6% were neutrophils, 5% were band forms, 36% were lymphocytes, and 47% were monocytes. The absolute neutrophil count was 110/mm3. Hemoglobin level was 10.2 g/dL with a mean corpuscular volume of 90 m3, and the platelet count was 151,000/mm3. Other results of laboratory studies were: sodium, 140 mmol/L; potassium, 3.8 mmol/L; chloride, 96 mmol/L; bicarbonate, 23 mmol/L; blood urea nitrogen, 13 mg/dL; creatinine, 0.4 mg/dL; lipase, 32 U/L (normal range, 13‐60); amylase, 73 U/L (normal range, 30‐110); albumin, 4.0 g/dL; aspartate aminotransferase, 779 U/L (normal range, 13‐35); alanine aminotransferase, 330 U/L (normal range, 7‐35); alkaline phosphatase, 510 U/L (normal range, 35‐104); and total bilirubin, 0.9 mg/dL (normal range, 0.1‐1.2). The lactate dehydrogenase level was 200 U/L (normal range, 135‐214). The corrected reticulocyte count was 1.6% (normal range, 0.3‐2.3), and haptoglobin was 190 mg/dL (normal range, 43‐212). A direct Coomb's test was positive. The erythrocyte sedimentation rate was 113 mm/hour (normal range, 1‐25). Urinalysis was normal without evidence of protein or blood.
Laboratory abnormalities include elevated transaminases and alkaline phosphatase, a markedly elevated erythrocyte sedimentation rate, and profound leukopenia with neutropenia. The patient is anemic, which may elevate the sedimentation rate but not typically to this degree. The patient is not febrile, but if she were to develop a fever, empiric antibiotics would be prudent. The normal albumin and bilirubin suggest that hepatic synthetic and excretory functions remain intact. Although the direct Coombs test is positive, the reticulocyte and lactate dehydrogenase levels argue against brisk hemolysis; this abnormality may simply be a marker of nonspecific immune activation. A variety of infections can cause neutropenia and liver enzyme abnormalities including parasites (malaria or leishmaniasis), viruses (cytomegalovirus or Epstein‐Barr virus [EBV]), tick‐borne bacterial infections (ehrlichiosis or rickettsial infection), and granulomatous infections (tuberculosis). Malignant infiltration of the reticuloendothelial system can also lead to cytopenias and liver enzyme abnormalities. Autoimmunity remains a consideration, as SLE may lead to cytopenias, oral ulcers, and nonspecific immune phenomena. Rather than ordering a large number of blood tests, I favor a targeted approach with abdominal computed tomography followed by biopsy of either the liver or bone marrow.
Chest radiography revealed no abnormalities. Computed tomography of the chest, abdomen, and pelvis with intravenous and oral contrast demonstrated concentric wall thickening of the transverse colon, but no evidence of obstruction or free air. The patient was treated with intravenous fluids, morphine, and cefepime. Bone marrow biopsy was performed, which demonstrated a hypercellular marrow with increased myeloid precursors and a left shift and megakaryocytic hyperplasia. Flow cytometry revealed no abnormally restricted clonal populations. A concerted search for an infectious etiology of the patient's neutropenia was unrevealing, including tests for HIV, cytomegalovirus, hepatitis A, hepatitis B, hepatitis C, Mycoplasma pneumoniae, EBV, and parvovirus B19.
I hope blood cultures were drawn prior to the initiation of antibiotics. Hypercellularity of the bone marrow in the context of leukopenia raises concern that white blood cells are being destroyed peripherally. Autoimmunity against neutrophils can be transiently induced by viruses such as HIV, hepatitis B, and EBV, but these infections have been excluded. Testing for antinuclear antibodies is reasonable. A normal‐sized spleen on the abdominal CT excludes hypersplenism. Colonic thickening can be associated with infection, ischemia, inflammatory bowel disease, and malignancy. The question is whether the colonic thickening is part of the same disease process causing the leukopenia and liver enzyme elevation or whether it represents a secondary infectious process in the setting of neutropenia (such as Clostridium difficile infection or typhlitis). Testing for stool pathogens (including ova and parasites) is certainly appropriate, and consideration of a colonoscopy with biopsy is reasonable, provided that appropriate antimicrobial coverage remains in place.
Blood cultures obtained prior to starting antibiotics were negative. The patient's abdominal pain improved, and she was discharged home to have close follow‐up with a hematologist. The results of her liver function tests improved, and her absolute neutrophil count was 230/mm3 at the time of discharge. Her neutropenia was believed to be secondary to peripheral destruction from a viral, drug‐mediated, or autoimmune process. Oxcarbazepine (Trileptal) was discontinued, as it was believed to be the medication most likely to be responsible. She returned to the hospital 3 days later with recurrence of her abdominal pain and diarrhea. She remained afebrile. Additional history revealed arthralgias over the previous 2 months, mild alopecia, and prior symptoms suggestive of Raynaud's phenomenon. Stool studies failed to establish an infectious etiology for the diarrhea, and her continued neutropenia responded appropriately to treatment with subcutaneous filgrastim. Colonoscopy could be performed only to the hepatic flexure and revealed no abnormalities. A serologic test for antinuclear antibodies was positive at a titer of 1:640 in a homogenous pattern, and a test for antineutrophil cytoplasmic antibodies was negative. Complement levels were normal, and tests for cryoglobulins, rapid plasma reagin, anticardiolipin antibody, lupus anticoagulant, rheumatoid factor, and antibodies to extractable nuclear antigens were all negative.
Raynaud's phenomenon is consistent with lupus. Double‐stranded DNA antibodies should be sent, although the urine did not demonstrate protein or an active sediment. Systemic sclerosis and the CREST syndrome is strongly associated with Raynaud's phenomenon and high‐titer ANA, but the patient does not have sclerodactyly, which is generally the earliest skin involvement. Autoimmune hepatitis is often associated with high‐titer ANA but does not fit this clinical picture. Given that the patient's presentation included segmental bowel wall thickening and a transient but marked liver enzyme elevation with AST predominance, I am concerned about vasculitis of the abdominal vasculature and would strongly consider a mesenteric angiogram.
To exclude mesenteric vasculitis, the patient underwent magnetic resonance angiography of the abdomen, the results of which were normal. A repeat test for antinuclear antibodies was positive at a titer of 1:2560 in a uniform pattern. A test for anti‐double‐stranded DNA was positive at 1370 U/mL. The patient was diagnosed with systemic lupus and probable lupus enteritis, and therapy with oral prednisone (10 mg daily) and hydroxychloroquine was initiated. She had prompt improvement in her abdominal pain, and was discharged home. Five months later she developed proteinuria and underwent a renal biopsy, which showed minor, nonspecific glomerular abnormalities, suggesting possible mild lupus nephritis. Eight months after her initial presentation, she remains free of abdominal pain and has regained the weight she had initially lost. Her oral ulcers have resolved, and her blood counts have normalized. Her serum creatinine has remained normal. She is now maintained on prednisone (15 mg daily), hydroxychloroquine, and mycophenolate mofetil.
COMMENTARY
A diagnosis of systemic lupus erythematosus (SLE) provided a unifying explanation for the patient's findings. Indeed, she manifested 4 of the 11 American College of Rheumatology criteria for systemic lupus (oral ulcers, leukopenia, positive anti‐DNA, and positive ANA), meeting criteria for a definite diagnosis of SLE. She additionally had multiple other features suggestive of lupus including Raynaud's phenomenon, arthralgias, alopecia, mild thrombocytopenia, and a positive Coombs' test (although the normal reticulocyte count, lactate dehydrogenase, and haptoglobin were most consistent with anemia of a chronic disease).
The protean manifestations of SLE can present significant diagnostic challenges. In this case, physicians were immediately drawn to the patient's acute abdominal pain and severe neutropenia and failed to recognize more subtle disease manifestations that may have aided in establishing a unifying diagnosis sooner. The initial history and review of systems did not disclose arthralgias, alopecia, or Raynaud's phenomenon. In an era of increasing use of hospitalists, which creates potential discontinuity between inpatient and outpatient physicians, a thorough history and review of systems may be particularly important in diagnosing acute manifestations of chronic systemic disease. Inpatient physicians may be overly focused on the small subset of acute complaints leading to hospitalization, without considering the larger constellation of symptoms that may facilitate accurate diagnosis. Our discussant quickly recognized the multisystem nature of the patient's illness and appropriately focused on infectious, neoplastic, and autoimmune categories of disease as being most likely. When infectious and neoplastic conditions were excluded with reasonable certainty, a directed serologic investigation for autoimmune disease was requested, culminating in a diagnosis of SLE.
Involvement of the skin as well as hematologic, renal, and musculoskeletal systems in SLE is commonly recognized, whereas gastrointestinal involvement is perceived to occur much less frequently. However, abdominal pain occurs in up to 40% of patients with lupus.14 Abdominal pain in lupus patients can arise from non‐lupus‐related conditions as well as lupus‐related entities, including serositis, mesenteric vasculitis with or without infarction, mesenteric thrombosis, pancreatitis, inflammatory bowel disease, and adverse medication effects including peptic ulcer disease. Abnormal liver chemistries, as seen in our patient, occur in 20%‐50% of patients with lupus and may be due to lupus hepatitis, concomitant autoimmune hepatitis, or medications including NSAIDs.5, 6 Oral ulcers and leukopenia are likewise common in SLE, with each seen in up to half of patients.4, 7, 8 Leukopenia in SLE may a result of neutropenia, lymphocytopenia, or both. However, severe neutropenia (ie, absolute count less than 500/L), as seen in our case, is more often a result of myelotoxicity from immunosuppressive therapy, rather than SLE itself.9
Lupus enteritis represents bowel microischemia from small‐vessel arteritis or venulitis that often is not evident on conventional mesenteric angiography.4, 10, 11 The reported prevalence of intestinal vasculitis in patients with SLE varies widely, depending on the characteristics of lupus patients sampled in individual studies. Intestinal vasculitis affects 0.2%‐0.5% of SLE patients in general,4, 12 whereas among SLE patients with active disease and an acute abdomen, vasculitis has been reported in up to 53% of patients.10 Antiphospholipid antibodies, antibodies to extractable nuclear antigens, the SLE Disease Activity Index, complement levels, erythrocyte sedimentation rate, C‐reactive protein, and anti‐double‐stranded DNA do not reliably differentiate lupus enteritis from acute abdominal pain due to other etiologies in patients with SLE.11 However, a concomitant drop in the white blood cell count at the onset of symptoms may be useful in distinguishing lupus enteritis from other causes of acute abdominal pain among lupus patients.11 Computed tomography findings consistent with lupus enteritis are nonspecific and include bowel‐wall thickening, submucosal edema (eg, target sign), dilatation of intestinal segments, engorgement of mesenteric vessels, and increased attenuation of mesenteric fat.13 Colonoscopy may reveal areas of ischemia and ulceration, and biopsy can confirm intestinal vasculitis. However, intestinal involvement may be segmental, and pathologic confirmation may be difficult. Contrast enema, gallium scanning, and indium‐labeled white cell scanning may be useful, but lack specificity. No controlled trials to date have evaluated the optimal therapy for lupus enteritis, but pulsed methylprednisolone is often recommended.4 Cyclophosphamide, azathioprine, methotrexate, and cyclosporine have also been used as adjunctive agents. Patients may progress to intestinal infarction and perforation, which augurs a poor prognosis, and early surgical exploration should be considered in severely ill patients.10 Death may occur in more than two‐thirds of patients whose disease progresses to intestinal perforation.1
In summary, a multisystem disease such as SLE requires a comprehensive history, physical exam, and review of systems to establish a correct diagnosis. In our case, an extensive evaluation was necessary to exclude other etiologies of abdominal pain and systemic illness, particularly as infectious and neoplastic conditions occur far more often than lupus enteritis in the general population. However, profound laboratory abnormalities may have preoccupied the attention of treating physicians, leading them to overlook less obvious but important historical and physical findings suggestive of SLE. The cohesively abnormal forest may thus have been obscured by erratically abnormal individual trees. Gastrointestinal symptoms may be underrecognized in SLE. When these result for lupus enteritis, timely recognition may be lifesaving.
A 56‐year‐old woman from Colombia presented to the emergency department after 24 hours of abdominal pain. One week before, she had experienced similar pain that lasted for 4 hours and spontaneously resolved. She was nauseated but had no vomiting. She reported an unintentional 14‐pound weight loss over the preceding 3 weeks. She denied fever, chills, night sweats, diarrhea, constipation, dysuria, or jaundice.
In a middle‐aged woman with abdominal pain and nausea, diagnostic considerations include gallbladder disease, diseases of the bowel (such as a partial small‐bowel obstruction or inflammatory conditions), hepatic or pancreatic conditions, and nongastrointestinal ailments such as cardiac ischemia. Knowing the specific location of pain, its quality, precipitating factors, and accompanying systemic symptoms may help to narrow the diagnosis. The unintentional weight loss preceding the onset of pain may be an important clue because it suggests a systemic condition, and in a South American immigrantparticularly if she has traveled recentlyit is important to consider parasitic illnesses. The absence of fever makes some infections such as tuberculosis and malaria less likely. At this point, in addition to a thorough history and physical, laboratory tests should include a complete blood count (with quantification of eosinophils) and a metabolic panel with liver enzymes and albumin.
The patient described pain in the midline, just inferior to the umbilicus. The pain was constant, developed without any particular provocation, and was not related to meals or exertion. There were no constitutional symptoms aside from weight loss. She had a history of bipolar disorder, hypothyroidism, osteoarthritis, and chronic sinusitis and had previously undergone cholecystectomy and abdominal hysterectomy. She was taking levothyroxine, montelukast, bupropion, oxcarbazepine, fexofenadine, meloxicam, zolpidem, and, as needed, acetaminophen. She had recently completed a 10‐day course of levofloxacin for acute sinusitis. She had immigrated to the United States 10 years earlier and lived with her husband and daughter. She denied the use of tobacco, alcohol or illicit drugs. She had visited Colombia 6 months earlier but had no other recent travel history.
The history of cholecystectomy makes a biliary tract process unlikely. Its location reduces the likelihood of a hepatic or pancreatic process, but I would like to see the liver enzymes, especially given her recent acetaminophen use. The comorbid illnessesparticularly her bipolar disordermay be relevant because psychiatric illness might be associated with medication overuse or undisclosed toxic ingestions. For example, excess thyroxine might lead to weight loss while overuse of nonsteroidal anti‐inflammatory drugs such as meloxicam can cause intestinal ulceration, not only in the upper tract, but also in the colon. Undisclosed ingestions may also be associated with abdominal symptoms. Her surgical history makes adhesions with a secondary partial bowel obstruction possible. With no travel outside this country in the last 6 months, exotic infections are less likely. Finally, the recent course of levofloxacin may be relevant because many antibiotics are associated with nonspecific abdominal symptoms, and Clostridium difficile colitis occasionally presents without diarrhea.
The patient reported taking her medications as prescribed and denied ingesting other medications. On physical examination, she had a temperature of 98.9F, a pulse of 81 beats per minute, a blood pressure of 110/80 mm Hg, and a respiratory rate of 16 respirations per minute. She had a normal oxygen saturation while breathing ambient air. Her weight was 58 kg. There was no scleral icterus or jugular venous distension. She had a small painless ulcer involving the hard palate. Her lungs were clear to auscultation, and cardiac examination was normal. The abdomen was soft, bowel sounds were present, and there was moderate tenderness to palpation inferior to the umbilicus. There was no rebound or guarding, hepatosplenomegaly or other masses. There was no peripheral edema and no lymphadenopathy. Neurological examination was normal.
The oral ulcer may or may not be related to the clinical presentation because oral ulcers, whether painful or painless, are ubiquitous and may be isolated or may be associated with a wide range of infectious and noninfectious systemic diseases. Although some systemic causes of mucocutaneous ulcers are associated with weight loss (including Crohn's disease, Behcet's disease, celiac sprue, human immunodeficiency virus [HIV], herpesviruses, syphilis, and systemic lupus erythematosus [SLE], among others), the lack of specificity of this finding limits its diagnostic utility. However, it is reasonable to ask whether the patient has noted frequent ulceration in the mouth or genitalia, as recurrent or severe ulcerations may narrow the diagnostic considerations. On the other hand, the focal nature of the pain inferior to the umbilicus suggests a discrete process in the abdomen or pelvis, such as an abscess, mass, or localized area of bowel inflammation. A plain abdominal film is likely to be low yield in this situation, so pursuing computed tomography is appropriate. Not all patients with focal abdominal pain require abdominal imaging, but in the context of weight loss and persistent symptoms for more than a week, imaging is prudent in this case.
The patient denied genital ulceration but did report painless oral ulcers over the preceding months. Laboratory evaluation revealed a white‐cell count of 1000/mm3, of which 6% were neutrophils, 5% were band forms, 36% were lymphocytes, and 47% were monocytes. The absolute neutrophil count was 110/mm3. Hemoglobin level was 10.2 g/dL with a mean corpuscular volume of 90 m3, and the platelet count was 151,000/mm3. Other results of laboratory studies were: sodium, 140 mmol/L; potassium, 3.8 mmol/L; chloride, 96 mmol/L; bicarbonate, 23 mmol/L; blood urea nitrogen, 13 mg/dL; creatinine, 0.4 mg/dL; lipase, 32 U/L (normal range, 13‐60); amylase, 73 U/L (normal range, 30‐110); albumin, 4.0 g/dL; aspartate aminotransferase, 779 U/L (normal range, 13‐35); alanine aminotransferase, 330 U/L (normal range, 7‐35); alkaline phosphatase, 510 U/L (normal range, 35‐104); and total bilirubin, 0.9 mg/dL (normal range, 0.1‐1.2). The lactate dehydrogenase level was 200 U/L (normal range, 135‐214). The corrected reticulocyte count was 1.6% (normal range, 0.3‐2.3), and haptoglobin was 190 mg/dL (normal range, 43‐212). A direct Coomb's test was positive. The erythrocyte sedimentation rate was 113 mm/hour (normal range, 1‐25). Urinalysis was normal without evidence of protein or blood.
Laboratory abnormalities include elevated transaminases and alkaline phosphatase, a markedly elevated erythrocyte sedimentation rate, and profound leukopenia with neutropenia. The patient is anemic, which may elevate the sedimentation rate but not typically to this degree. The patient is not febrile, but if she were to develop a fever, empiric antibiotics would be prudent. The normal albumin and bilirubin suggest that hepatic synthetic and excretory functions remain intact. Although the direct Coombs test is positive, the reticulocyte and lactate dehydrogenase levels argue against brisk hemolysis; this abnormality may simply be a marker of nonspecific immune activation. A variety of infections can cause neutropenia and liver enzyme abnormalities including parasites (malaria or leishmaniasis), viruses (cytomegalovirus or Epstein‐Barr virus [EBV]), tick‐borne bacterial infections (ehrlichiosis or rickettsial infection), and granulomatous infections (tuberculosis). Malignant infiltration of the reticuloendothelial system can also lead to cytopenias and liver enzyme abnormalities. Autoimmunity remains a consideration, as SLE may lead to cytopenias, oral ulcers, and nonspecific immune phenomena. Rather than ordering a large number of blood tests, I favor a targeted approach with abdominal computed tomography followed by biopsy of either the liver or bone marrow.
Chest radiography revealed no abnormalities. Computed tomography of the chest, abdomen, and pelvis with intravenous and oral contrast demonstrated concentric wall thickening of the transverse colon, but no evidence of obstruction or free air. The patient was treated with intravenous fluids, morphine, and cefepime. Bone marrow biopsy was performed, which demonstrated a hypercellular marrow with increased myeloid precursors and a left shift and megakaryocytic hyperplasia. Flow cytometry revealed no abnormally restricted clonal populations. A concerted search for an infectious etiology of the patient's neutropenia was unrevealing, including tests for HIV, cytomegalovirus, hepatitis A, hepatitis B, hepatitis C, Mycoplasma pneumoniae, EBV, and parvovirus B19.
I hope blood cultures were drawn prior to the initiation of antibiotics. Hypercellularity of the bone marrow in the context of leukopenia raises concern that white blood cells are being destroyed peripherally. Autoimmunity against neutrophils can be transiently induced by viruses such as HIV, hepatitis B, and EBV, but these infections have been excluded. Testing for antinuclear antibodies is reasonable. A normal‐sized spleen on the abdominal CT excludes hypersplenism. Colonic thickening can be associated with infection, ischemia, inflammatory bowel disease, and malignancy. The question is whether the colonic thickening is part of the same disease process causing the leukopenia and liver enzyme elevation or whether it represents a secondary infectious process in the setting of neutropenia (such as Clostridium difficile infection or typhlitis). Testing for stool pathogens (including ova and parasites) is certainly appropriate, and consideration of a colonoscopy with biopsy is reasonable, provided that appropriate antimicrobial coverage remains in place.
Blood cultures obtained prior to starting antibiotics were negative. The patient's abdominal pain improved, and she was discharged home to have close follow‐up with a hematologist. The results of her liver function tests improved, and her absolute neutrophil count was 230/mm3 at the time of discharge. Her neutropenia was believed to be secondary to peripheral destruction from a viral, drug‐mediated, or autoimmune process. Oxcarbazepine (Trileptal) was discontinued, as it was believed to be the medication most likely to be responsible. She returned to the hospital 3 days later with recurrence of her abdominal pain and diarrhea. She remained afebrile. Additional history revealed arthralgias over the previous 2 months, mild alopecia, and prior symptoms suggestive of Raynaud's phenomenon. Stool studies failed to establish an infectious etiology for the diarrhea, and her continued neutropenia responded appropriately to treatment with subcutaneous filgrastim. Colonoscopy could be performed only to the hepatic flexure and revealed no abnormalities. A serologic test for antinuclear antibodies was positive at a titer of 1:640 in a homogenous pattern, and a test for antineutrophil cytoplasmic antibodies was negative. Complement levels were normal, and tests for cryoglobulins, rapid plasma reagin, anticardiolipin antibody, lupus anticoagulant, rheumatoid factor, and antibodies to extractable nuclear antigens were all negative.
Raynaud's phenomenon is consistent with lupus. Double‐stranded DNA antibodies should be sent, although the urine did not demonstrate protein or an active sediment. Systemic sclerosis and the CREST syndrome is strongly associated with Raynaud's phenomenon and high‐titer ANA, but the patient does not have sclerodactyly, which is generally the earliest skin involvement. Autoimmune hepatitis is often associated with high‐titer ANA but does not fit this clinical picture. Given that the patient's presentation included segmental bowel wall thickening and a transient but marked liver enzyme elevation with AST predominance, I am concerned about vasculitis of the abdominal vasculature and would strongly consider a mesenteric angiogram.
To exclude mesenteric vasculitis, the patient underwent magnetic resonance angiography of the abdomen, the results of which were normal. A repeat test for antinuclear antibodies was positive at a titer of 1:2560 in a uniform pattern. A test for anti‐double‐stranded DNA was positive at 1370 U/mL. The patient was diagnosed with systemic lupus and probable lupus enteritis, and therapy with oral prednisone (10 mg daily) and hydroxychloroquine was initiated. She had prompt improvement in her abdominal pain, and was discharged home. Five months later she developed proteinuria and underwent a renal biopsy, which showed minor, nonspecific glomerular abnormalities, suggesting possible mild lupus nephritis. Eight months after her initial presentation, she remains free of abdominal pain and has regained the weight she had initially lost. Her oral ulcers have resolved, and her blood counts have normalized. Her serum creatinine has remained normal. She is now maintained on prednisone (15 mg daily), hydroxychloroquine, and mycophenolate mofetil.
COMMENTARY
A diagnosis of systemic lupus erythematosus (SLE) provided a unifying explanation for the patient's findings. Indeed, she manifested 4 of the 11 American College of Rheumatology criteria for systemic lupus (oral ulcers, leukopenia, positive anti‐DNA, and positive ANA), meeting criteria for a definite diagnosis of SLE. She additionally had multiple other features suggestive of lupus including Raynaud's phenomenon, arthralgias, alopecia, mild thrombocytopenia, and a positive Coombs' test (although the normal reticulocyte count, lactate dehydrogenase, and haptoglobin were most consistent with anemia of a chronic disease).
The protean manifestations of SLE can present significant diagnostic challenges. In this case, physicians were immediately drawn to the patient's acute abdominal pain and severe neutropenia and failed to recognize more subtle disease manifestations that may have aided in establishing a unifying diagnosis sooner. The initial history and review of systems did not disclose arthralgias, alopecia, or Raynaud's phenomenon. In an era of increasing use of hospitalists, which creates potential discontinuity between inpatient and outpatient physicians, a thorough history and review of systems may be particularly important in diagnosing acute manifestations of chronic systemic disease. Inpatient physicians may be overly focused on the small subset of acute complaints leading to hospitalization, without considering the larger constellation of symptoms that may facilitate accurate diagnosis. Our discussant quickly recognized the multisystem nature of the patient's illness and appropriately focused on infectious, neoplastic, and autoimmune categories of disease as being most likely. When infectious and neoplastic conditions were excluded with reasonable certainty, a directed serologic investigation for autoimmune disease was requested, culminating in a diagnosis of SLE.
Involvement of the skin as well as hematologic, renal, and musculoskeletal systems in SLE is commonly recognized, whereas gastrointestinal involvement is perceived to occur much less frequently. However, abdominal pain occurs in up to 40% of patients with lupus.14 Abdominal pain in lupus patients can arise from non‐lupus‐related conditions as well as lupus‐related entities, including serositis, mesenteric vasculitis with or without infarction, mesenteric thrombosis, pancreatitis, inflammatory bowel disease, and adverse medication effects including peptic ulcer disease. Abnormal liver chemistries, as seen in our patient, occur in 20%‐50% of patients with lupus and may be due to lupus hepatitis, concomitant autoimmune hepatitis, or medications including NSAIDs.5, 6 Oral ulcers and leukopenia are likewise common in SLE, with each seen in up to half of patients.4, 7, 8 Leukopenia in SLE may a result of neutropenia, lymphocytopenia, or both. However, severe neutropenia (ie, absolute count less than 500/L), as seen in our case, is more often a result of myelotoxicity from immunosuppressive therapy, rather than SLE itself.9
Lupus enteritis represents bowel microischemia from small‐vessel arteritis or venulitis that often is not evident on conventional mesenteric angiography.4, 10, 11 The reported prevalence of intestinal vasculitis in patients with SLE varies widely, depending on the characteristics of lupus patients sampled in individual studies. Intestinal vasculitis affects 0.2%‐0.5% of SLE patients in general,4, 12 whereas among SLE patients with active disease and an acute abdomen, vasculitis has been reported in up to 53% of patients.10 Antiphospholipid antibodies, antibodies to extractable nuclear antigens, the SLE Disease Activity Index, complement levels, erythrocyte sedimentation rate, C‐reactive protein, and anti‐double‐stranded DNA do not reliably differentiate lupus enteritis from acute abdominal pain due to other etiologies in patients with SLE.11 However, a concomitant drop in the white blood cell count at the onset of symptoms may be useful in distinguishing lupus enteritis from other causes of acute abdominal pain among lupus patients.11 Computed tomography findings consistent with lupus enteritis are nonspecific and include bowel‐wall thickening, submucosal edema (eg, target sign), dilatation of intestinal segments, engorgement of mesenteric vessels, and increased attenuation of mesenteric fat.13 Colonoscopy may reveal areas of ischemia and ulceration, and biopsy can confirm intestinal vasculitis. However, intestinal involvement may be segmental, and pathologic confirmation may be difficult. Contrast enema, gallium scanning, and indium‐labeled white cell scanning may be useful, but lack specificity. No controlled trials to date have evaluated the optimal therapy for lupus enteritis, but pulsed methylprednisolone is often recommended.4 Cyclophosphamide, azathioprine, methotrexate, and cyclosporine have also been used as adjunctive agents. Patients may progress to intestinal infarction and perforation, which augurs a poor prognosis, and early surgical exploration should be considered in severely ill patients.10 Death may occur in more than two‐thirds of patients whose disease progresses to intestinal perforation.1
In summary, a multisystem disease such as SLE requires a comprehensive history, physical exam, and review of systems to establish a correct diagnosis. In our case, an extensive evaluation was necessary to exclude other etiologies of abdominal pain and systemic illness, particularly as infectious and neoplastic conditions occur far more often than lupus enteritis in the general population. However, profound laboratory abnormalities may have preoccupied the attention of treating physicians, leading them to overlook less obvious but important historical and physical findings suggestive of SLE. The cohesively abnormal forest may thus have been obscured by erratically abnormal individual trees. Gastrointestinal symptoms may be underrecognized in SLE. When these result for lupus enteritis, timely recognition may be lifesaving.
- ,.The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature.Semin Arthritis Rheum.1980;9:237.
- ,,.Acute abdominal complications of systemic lupus erythematosus and polyarteritis nodosa.Am J Med.1982;73:525–531.
- ,.Acute gastrointestinal manifestations of systemic lupus erythematosus.Can J Surg.1987;30:185–188.
- ,,.A review of gastrointestinal manifestations of systemic lupus erythematosus.Rheumatology.1999;38:917–932.
- ,.Connective tissue disease and the liver.J Clin Gastroenterol.2002;35:345–349.
- ,,.The spectrum of liver disease in systemic lupus erythematosus: report of 33 histologically‐proved cases and review of the literature.Am J Med.1980;69:187–194.
- ,.Hematologic aspects of systemic lupus erythematosus: current concepts.Ann Intern Med.1977;86:220–229.
- ,.Prevalence and significance of hematological abnormalities in patients with systemic lupus erythematosus.Q J Med1991;80:605–12.
- ,,, et al.Moderate and severe neutropenia in patients with systemic lupus erythematosus.Rheumatology.2006;45:994–998.
- ,,, et al.Acute abdomen in systemic lupus erythematosus: the importance of early laparotomy.Am J Med.1997;103:100–105.
- ,,, et al.Acute abdominal pain in systemic lupus erythematosus: focus on lupus enteritis (gastrointestinal vasculitis).Ann Rheum Dis,2002;61:547–550.
- ,,, et al.Vasculitis in systemic lupus erythematosus.Lupus.1997;6:235–242.
- ,,, et al.CT features of systemic lupus erythematosus in patients with acute abdominal pain: emphasis on ischemic bowel disease.Radiology.1999;211:203–209.
- ,.The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature.Semin Arthritis Rheum.1980;9:237.
- ,,.Acute abdominal complications of systemic lupus erythematosus and polyarteritis nodosa.Am J Med.1982;73:525–531.
- ,.Acute gastrointestinal manifestations of systemic lupus erythematosus.Can J Surg.1987;30:185–188.
- ,,.A review of gastrointestinal manifestations of systemic lupus erythematosus.Rheumatology.1999;38:917–932.
- ,.Connective tissue disease and the liver.J Clin Gastroenterol.2002;35:345–349.
- ,,.The spectrum of liver disease in systemic lupus erythematosus: report of 33 histologically‐proved cases and review of the literature.Am J Med.1980;69:187–194.
- ,.Hematologic aspects of systemic lupus erythematosus: current concepts.Ann Intern Med.1977;86:220–229.
- ,.Prevalence and significance of hematological abnormalities in patients with systemic lupus erythematosus.Q J Med1991;80:605–12.
- ,,, et al.Moderate and severe neutropenia in patients with systemic lupus erythematosus.Rheumatology.2006;45:994–998.
- ,,, et al.Acute abdomen in systemic lupus erythematosus: the importance of early laparotomy.Am J Med.1997;103:100–105.
- ,,, et al.Acute abdominal pain in systemic lupus erythematosus: focus on lupus enteritis (gastrointestinal vasculitis).Ann Rheum Dis,2002;61:547–550.
- ,,, et al.Vasculitis in systemic lupus erythematosus.Lupus.1997;6:235–242.
- ,,, et al.CT features of systemic lupus erythematosus in patients with acute abdominal pain: emphasis on ischemic bowel disease.Radiology.1999;211:203–209.
Bony Metastatic Disease
A60‐year‐old man presented with agitation and a forehead mass (Figs. 1 and 2). His wife had noticed its rapid growth over several weeks and said he had recently become extremely confused and hostile. He had a history of smoking and exposures to silica and Agent Orange. His vitals and oxygen saturation were normal. He was cachectic but in no distress, and although he was uncooperative, his examination did not demonstrate an obvious neurological deficit. Routine electrolytes were within normal limits.
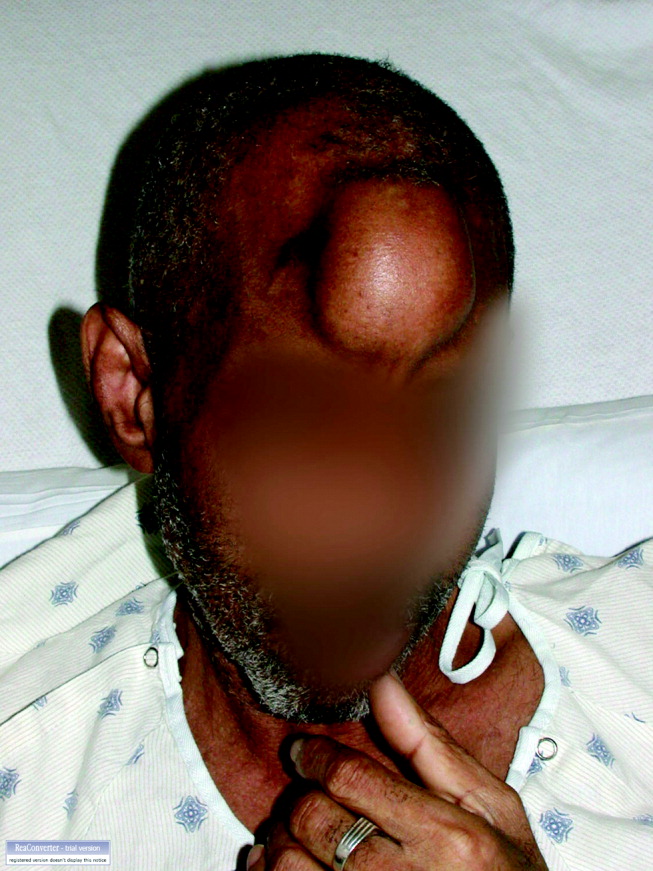
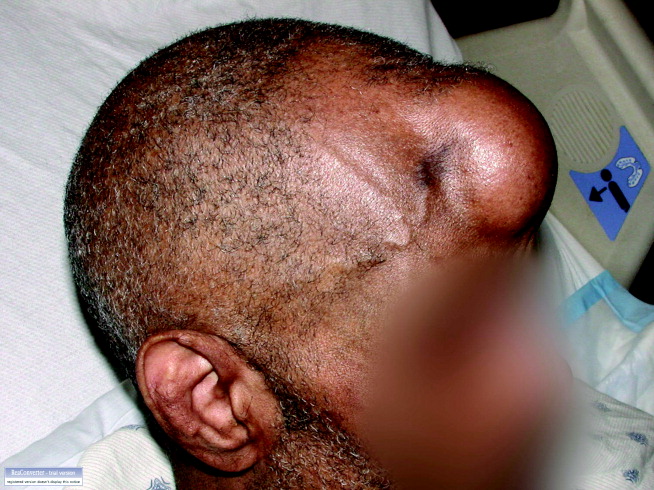
Noncontrast computed tomography of the skull revealed a 5.5 5.4 7.7cm frontal soft‐tissue density eroding his frontal bone and extending intracranially, with extensive displacement of both frontal lobes and invasion into his superior sagittal sinus (Fig. 3) and possible involvement of the brain parenchyma. A subsequent CT scan of the chest demonstrated a 7.8 7.9cm right mass in the lower lobe of the lung encasing the right pulmonary artery and 2 left renal masses.
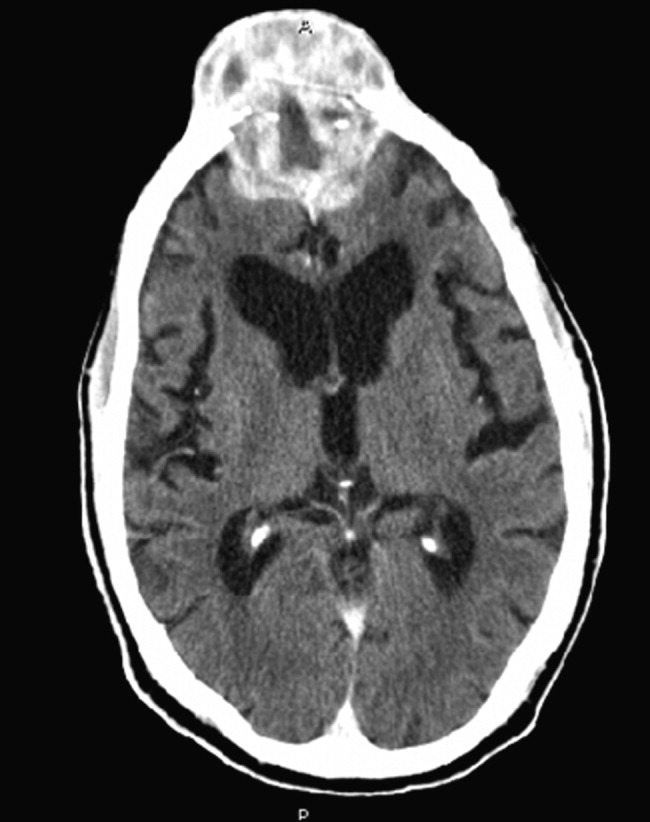
Biopsy of the forehead mass demonstrated a necrotic, poorly differentiated carcinoma with focal clear‐cell features. Immunohistochemical staining was consistent with squamous cell carcinoma.
The forehead mass was painless, and the lung mass unresectable. His behavioral changes persisted and were attributed to the tumor compressing and possibly invading both frontal lobes. At his wife's request, he was discharged on dexamethasone with home hospice and died 2 weeks later.
Although there is little literature on the epidemiology of skull metastases, the most common malignancies to metastasize to the skull are breast and lung carcinomas. Others include prostate, thyroid, myeloma, and melanoma. Symptomatic metastases, including neurological findings, are unusual, except for tumors that metastasize to the skull base and cause cranial nerve deficits. Skeletal metastasis as the first manifestation of lung cancer occurs in about 2% of lung cancer patients and is a marker of poor prognosis. Received 2 November 2006; revision received 15 December 2006; accepted 16 January 2007.
A60‐year‐old man presented with agitation and a forehead mass (Figs. 1 and 2). His wife had noticed its rapid growth over several weeks and said he had recently become extremely confused and hostile. He had a history of smoking and exposures to silica and Agent Orange. His vitals and oxygen saturation were normal. He was cachectic but in no distress, and although he was uncooperative, his examination did not demonstrate an obvious neurological deficit. Routine electrolytes were within normal limits.


Noncontrast computed tomography of the skull revealed a 5.5 5.4 7.7cm frontal soft‐tissue density eroding his frontal bone and extending intracranially, with extensive displacement of both frontal lobes and invasion into his superior sagittal sinus (Fig. 3) and possible involvement of the brain parenchyma. A subsequent CT scan of the chest demonstrated a 7.8 7.9cm right mass in the lower lobe of the lung encasing the right pulmonary artery and 2 left renal masses.

Biopsy of the forehead mass demonstrated a necrotic, poorly differentiated carcinoma with focal clear‐cell features. Immunohistochemical staining was consistent with squamous cell carcinoma.
The forehead mass was painless, and the lung mass unresectable. His behavioral changes persisted and were attributed to the tumor compressing and possibly invading both frontal lobes. At his wife's request, he was discharged on dexamethasone with home hospice and died 2 weeks later.
Although there is little literature on the epidemiology of skull metastases, the most common malignancies to metastasize to the skull are breast and lung carcinomas. Others include prostate, thyroid, myeloma, and melanoma. Symptomatic metastases, including neurological findings, are unusual, except for tumors that metastasize to the skull base and cause cranial nerve deficits. Skeletal metastasis as the first manifestation of lung cancer occurs in about 2% of lung cancer patients and is a marker of poor prognosis. Received 2 November 2006; revision received 15 December 2006; accepted 16 January 2007.
A60‐year‐old man presented with agitation and a forehead mass (Figs. 1 and 2). His wife had noticed its rapid growth over several weeks and said he had recently become extremely confused and hostile. He had a history of smoking and exposures to silica and Agent Orange. His vitals and oxygen saturation were normal. He was cachectic but in no distress, and although he was uncooperative, his examination did not demonstrate an obvious neurological deficit. Routine electrolytes were within normal limits.


Noncontrast computed tomography of the skull revealed a 5.5 5.4 7.7cm frontal soft‐tissue density eroding his frontal bone and extending intracranially, with extensive displacement of both frontal lobes and invasion into his superior sagittal sinus (Fig. 3) and possible involvement of the brain parenchyma. A subsequent CT scan of the chest demonstrated a 7.8 7.9cm right mass in the lower lobe of the lung encasing the right pulmonary artery and 2 left renal masses.

Biopsy of the forehead mass demonstrated a necrotic, poorly differentiated carcinoma with focal clear‐cell features. Immunohistochemical staining was consistent with squamous cell carcinoma.
The forehead mass was painless, and the lung mass unresectable. His behavioral changes persisted and were attributed to the tumor compressing and possibly invading both frontal lobes. At his wife's request, he was discharged on dexamethasone with home hospice and died 2 weeks later.
Although there is little literature on the epidemiology of skull metastases, the most common malignancies to metastasize to the skull are breast and lung carcinomas. Others include prostate, thyroid, myeloma, and melanoma. Symptomatic metastases, including neurological findings, are unusual, except for tumors that metastasize to the skull base and cause cranial nerve deficits. Skeletal metastasis as the first manifestation of lung cancer occurs in about 2% of lung cancer patients and is a marker of poor prognosis. Received 2 November 2006; revision received 15 December 2006; accepted 16 January 2007.
Variation in Ordering CBCs for Bronchiolitis
Bronchiolitis was the most common primary diagnosis of infants hospitalized in the United States from 2000 to 2001.1 Consequently, much research has focused on the effectiveness of management24 and variation in care, especially the use of unproven diagnostic tests such as chest x‐rays.5 Such variation may have substantial financial and medical impact and has been shown to correlate significantly with hospital costs and length of stay.6
Because bronchiolitis is primarily a clinical diagnosis,7 there is no strong evidence to support the role of diagnostic testing, particularly that of complete blood counts (CBCs).8 Moreover, given the limited diagnostic utility of a single CBC, the benefit of obtaining a second CBC, especially with its associated physical discomfort and additional financial costs, is questionable. Yet despite the lack of evidence and rationale to support initial and repeated ordering of CBCs, we suspect that this practice may be more widespread and variable than currently appreciated.
Using a national database of children's hospitals, we sought to determine the frequency with which CBCs are ordered and repeated during hospitalizations for bronchiolitis, the extent to which these practices vary across institutions, and the relationship of these practices to average charges for a hospital stay.
METHODS
Data Source
We analyzed cases of children with bronchiolitis from the Pediatric Health Information System (PHIS) database of the Child Health Corporation of America.9 This database contains inpatient demographic, administrative, and diagnostic data from 36 freestanding, noncompeting children's hospitals in the United States. However, only 30 of the hospitals provided information on diagnostic testing during the period of our study. To protect the participating hospitals, hospitals were deidentified in this analysis. Diagnoses in the database are provided in the International Classification of Disease, 9th revision (ICD‐9), and the All‐Patient Refined Diagnostic Related Groups (APR‐DRGs), version 15 format.
Cases
We included in our sample children who had a primary ICD‐9 discharge code for bronchiolitis (469.11 or 469.19), an APR‐DRG for bronchiolitis/asthma (141), and a discharge date between October 2001 and September 2003.10 We further restricted cases to children less than 12 months of age because this is the age group most frequently hospitalized for bronchiolitis. Only the first admission per child was included in the analysis.
Outcome and Covariates
We identified the number of CBCs ordered using charge codes in the PHIS data. To avoid double counting, we required that the CBCs be charged on different dates of service, and we counted a maximum of 1 CBC per day per patient. We defined a child as having a repeated CBC if more than 1 CBC was charged during the child's hospital stay. Our outcome variable of repeat CBCs was measured dichotomously. We included age, male sex, Medicaid status, season of admission, intensive care unit (ICU) admission, APR‐DRG‐calculated severity scores for bronchiolitis/asthma (to adjust for disease severity), and length of stay as covariates in the regression and ANOVA analyses. All covariates were measured dichotomously, except for mean age and LOS, which were measured continuously.
Statistical Analyses
Bivariate analysis of baseline characteristics were compared across age groups using 2 tests to compare differences between categorical variables and the Student t test to compare differences between continuous variables.
To examine variability across hospitals in the initial and repeat ordering of CBCs, we performed multivariate ANOVA (MANOVA) controlling for age, sex, Medicaid status, illness severity, season of admission, ICU admission, and length of stay (LOS). Because the factors associated with repeat CBCs are not readily apparent, we performed logistic regression to determine which of these factors were significantly associated (P < .05) with having repeat CBCs performed. To account for the influence of age on the management and epidemiology of children with respiratory distress, we stratified MANOVA and regression analyses by age (< 3 months and 3 months. We clustered our regression analysis by hospital to determine whether there was hospital‐specific variation in repeating CBCs.
We performed post hoc analysis after noting additional variable relationships in our results. To determine whether CBC‐ordering patterns differed by severity, we stratified the analysis of repeat CBCs in both the bivariate and multivariate model by disease severity and ICU admission, respectively.
To determine if the number of CBCs ordered was related to admission charges, we categorized hospitals into tertiles (lowest, intermediate, highest) according to the proportion of admissions in which CBCs were ordered. We then calculated average admission hospital stay charges for each hospital. We used Student t tests to examine the relationship between the charges for admissions in hospitals with the intermediate and highest proportion of admissions with CBCs compared with those hospitals with the lowest proportion of admissions with CBCs.
We used Stata 8.0 to conduct our analyses.11 The Children's Hospital and Regional Medical Center Institutional Review Board (Seattle, WA) approved the analysis of the data for this study.
RESULTS
A total of 17,397 children met the inclusion criteria. Children under 3 months were more likely to be covered by Medicaid, be admitted to the ICU, have a longer length of stay, and have at least 1 CBC (Table 1). Of all children hospitalized, 48.2% had at least 1 CBC, and 7.8% had more than 1 CBC performed during their hospital stay. Notably, the proportion of all admissions with at least 1 CBC varied from 23.2% to 79.2% (Fig. 1), and those with repeat CBCs varied from 0% to 18.6% across hospitals (Fig. 2). This variation was significant when stratified by age and adjusted for covariates, which included length of stay and severity of illness (P < .001). In additional post hoc analyses we found differences in ordering pattern by disease severity that should be noted. The proportion of admissions with repeat CBCs varied significantly across severity groups (mild 3.9%, moderate 10.3%, and severe 21.3%, P < .001) and ICU admission status (ICU admission 5.5%, no ICU admission 23%, P <.001). Stratified analyses indicated an interaction between ICU utilization and disease severity, but neither covariate showed significant interactions with other variables in the model (data not shown).
| < 3 Months of Age | 3‐11 Months of Age | |
|---|---|---|
| ||
| Sample size | 7336 | 10,061 |
| Mean age (months) | 1.4 | 5.8 |
| Male (%) | 58.3 | 59.3 |
| Medicaid (%) | 56.0* | 53.8 |
| Admission Season | ||
| October‐February (%) | 71.0 | 70.4 |
| APG‐DRG severity score | ||
| Mild | 63.0 | 63.4 |
| Moderate | 22.4 | 22.7 |
| Severe | 14.6 | 13.8 |
| ICU admission (%) | 15.7 | 11.2 |
| Mean length of stay (days) | 3.1 | 2.8 |
| Received 1 CBC | 53.8 | 44.1 |
| Received > 1 CBC | 9.2 | 6.8 |
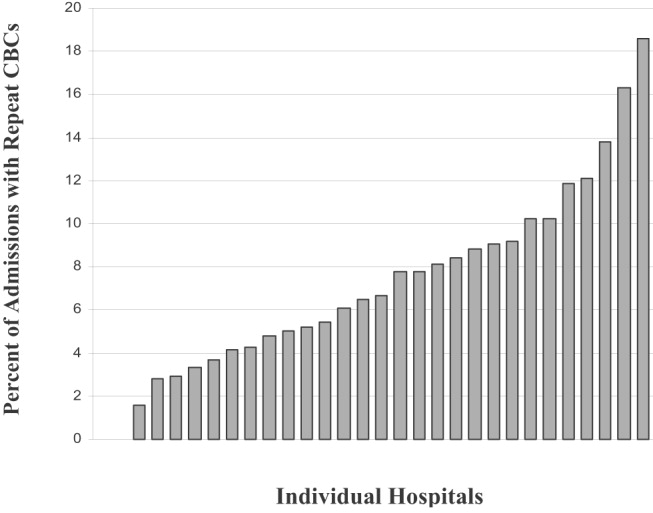
With respect to repeat CBCs, for children at least 3 months old, the strongest predictor was ICU admission (odds ratio [OR] 2.53, 95% CI: 1.69‐3.77), followed by a severe or extreme APR‐DRG severity score (OR 1.75, 95% CI: 1.23‐2.49) and LOS (OR 1.22, 95% CI: 1.15‐1.28). For children less than 3 months old, some of these associations strengthened ICU admission (OR 2.58, 95% CI: 1.84‐3.61), followed by a severe or extreme APR‐DRG severity score (OR 2.31, 95% CI: 1.64‐3.24) and LOS (OR 1.24, 95% CI: 1.16‐1.32). Additional predictors for this age group were a moderate severity score (OR 1.67, 95% CI: 1.29‐2.16) and Medicaid status (OR 1.20, 95% CI: 1.0‐1.43) (Table 2).
| < 3 Months of Age | 311 Months of Age | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR* | 95% CI | Adjusted OR* | 95% CI | |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| Mean Age (months) | 1.04 | 0.841.30 | 0.99 | 0.961.03 | ||||||||||||||||||||||||||||||||||||||||
| Male (%) | 1.01 | 0.851.19 | 0.88 | 0.741.05 | ||||||||||||||||||||||||||||||||||||||||
| Medicaid (%) | 1.20 | 1.001.43 | 0.95 | 0.791.15 | ||||||||||||||||||||||||||||||||||||||||
| Admission Season | ||||||||||||||||||||||||||||||||||||||||||||
| OctoberFebruary | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| MarchSeptember (%) | 1.13 | 0.931.37 | 1.11 | 0.811.53 | ||||||||||||||||||||||||||||||||||||||||
| APGDRG severity score | ||||||||||||||||||||||||||||||||||||||||||||
| Mild | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| Moderate | 1.67 | 1.292.16 | 1.28 | 0.941.76 | ||||||||||||||||||||||||||||||||||||||||
| Severe | 2.31 | 1.643.24 | 1.75 | 1.232.49 | ||||||||||||||||||||||||||||||||||||||||
| ICU admission (%) | 2.58 | 1.843.61 | 2.53 | 1.693.77 | ||||||||||||||||||||||||||||||||||||||||
| Length of stay (days) | 1.24 | 1.161.32 | 1.22 | 1.151.28 | ||||||||||||||||||||||||||||||||||||||||
Compared with hospitals that had the lowest proportion of admissions in which CBCs were ordered, hospitals with higher proportions of CBCs ordered had significantly higher mean charges per hospital stay (Table 3).
| Hospital CBC Levels | Patients | Mean Charge (95% CI) | Mean Difference (95% CI) |
|---|---|---|---|
| |||
| Lowest (23%‐40%) | 5838 | $7293 ($70967489) | Referent |
| Middle (41%59%) | 6673 | $8099 ($78598339) | $807* ($491$1122) |
| Highest (60%79%) | 4886 | $8316 ($80548578) | $1024* ($702$1345) |
DISCUSSION
We found that in a nationwide sample of children hospitalized with bronchiolitis, 48% had at least 1 CBC and nearly 8% had a repeat CBC ordered during their hospital stay. Moreover, even after adjusting for covariates, the proportion of children with initial and repeat CBCs during a single admission varied widely and significantly across a nationwide sample of children's hospitals.
We can only speculate on the reasons for institutional variation. Although it is not unusual for some cases of illness to vary from a standard course and so trigger initial or repeat evaluations with a CBC, we do not have any a priori reason to expect the proportion of unusual cases to vary by institution in a national cohort of children's hospitals. One compelling explanation for this variation is differing institutional patterns of practice. For example, it may be that some institutions have protocols that require the ordering of a CBC on admission. This practice could prompt a costly and unnecessary testing cascade14 generated by an initially abnormal CBC and so could trigger additional testing and/or procedures, such as x‐rays and parental antibiotics. Such a cascade of testing and intervention could conceivably lead to additional, and dependent, costs not captured by a simple tally of the costs of individual CBCs. Indeed, in our analysis we found that those hospitals with higher proportion of admissions in which CBCs were ordered also had significantly higher admission charges that exceeded the cost of a CBC. Previous studies support the finding that institutional variation in care for viral respiratory illness is significantly correlated with hospital costs.6
Limitations of this study should be noted. First, the PHIS database does not provide indications for, results of, or hospital location of tests, so we cannot determine whether clinical condition or results prompted initial and/or repeat testing. However, because children with complicated courses or atypical disease presentations likely have longer hospital stays, severe disease, or additional diagnoses, we attempted to control for these factors in our analysis. Second, although we selected cases based on a discharge diagnosis of bronchiolitis, it is possible that admitting physicians obtained an initial CBC to rule out alternative diagnoses, such as bacteremia, which can occur but is rare in this population.12, 13 It is plausible that bacteremia is most likely in children with other comorbidities or higher disease severity. In additional stratified analyses we did find that the proportion of repeat CBCs increased with higher disease severity and that there was an interaction between severe disease status and ICU admission. However, all participating institutions are children's hospitals and so are likely to treat children with a range of severity of illness and comorbidities. Finally, as with other analyses of the PHIS database, we used charges to identify diagnostic tests.5
Given that more than 120,000 U.S. infants are hospitalized annually with bronchiolitis,15 the cost and discomfort associated with unnecessary testing warrants attention. The issue of cost is particularly relevant in light of recent research findings of increased costs for admissions at freestanding children's hospitals.16 We found that mean charges per hospital stay were significantly higher for hospitals that had a higher proportion of admissions during which multiple CBCs were ordered. Although we cannot exclude illness severity and age as explanations for the higher charges, we have no reason to believe that one freestanding children's hospital would have a sicker and younger population than another. An alternative and compelling explanation is that a variation in the standard of care exists across these hospitals.
The institutional variation in and the limited evidence for the utility of the ordering of CBCs in the evaluation of bronchiolitis call into question the necessity of this testing strategy. Exploration of the reasons for this institutional variation will help to create quality initiatives and directed interventions to improve and standardize care in bronchiolitis.
Acknowledgements
Supported by: Robert Wood Johnson (RWJ) Foundation through the Robert Wood Johnson Clinical Scholars Program. The views expressed do not necessarily represent the views of the Robert Wood Johnson Foundation or the University of Washington. The RWJ Foundation provided salary support for Dr. Tarini. The RWJ Foundation did not have a role in the study's design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication. Dr. Tarini wrote the first draft of the manuscript. All authors have seen and agree with the contents of this manuscript.
- ,,, et al.Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population.Pediatrics. Oct2004;114:e437–e444.
- ,,, et al.Pharmacologic treatment of bronchiolitis in infants and children: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):127–137.
- ,,,.Glucocorticoids for acute viral bronchiolitis in infants and young children.Cochrane Database Syst Rev.2004(3):CD004878.
- ,,.Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old.Cochrane Database Syst Rev.2005;(2):CD004873.
- ,,,,,.Variation in inpatient diagnostic testing and management of bronchiolitis.Pediatrics.2005;115:878–884.
- ,,,,.Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness.Pediatrics.2001;108:851–855.
- .Bronchiolitis. In:Behrman Re KR,Jenson HB, eds.Nelson Textbook of Pediatrics.17th ed.Philadelphia:WB Saunders Co.;2004:1415–1417.
- ,,, et al.Diagnosis and testing in bronchiolitis: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):119–126.
- .Achieving data quality. How data from a pediatric health information system earns the trust of its users.J AHIMA.2004;75(10):22–26.
- ,,,,.A closer look at all‐patient refined DRGs.J AHIMA.2002;73(1):46–50.
- Stata Corp. College Station, TX.
- ,.Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections.Arch Pediatr Adolesc Med.2002;156:322–324.
- ,.Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection.Pediatr Infect Dis J.2004;23:267–269.
- ,.The cascade effect in the clinical care of patients.N Engl J Med.1986;314:512–514.
- ,,,,,.Bronchiolitis‐associated hospitalizations among US children, 1980‐1996.JAMA.1999;282:1440–1446.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
Bronchiolitis was the most common primary diagnosis of infants hospitalized in the United States from 2000 to 2001.1 Consequently, much research has focused on the effectiveness of management24 and variation in care, especially the use of unproven diagnostic tests such as chest x‐rays.5 Such variation may have substantial financial and medical impact and has been shown to correlate significantly with hospital costs and length of stay.6
Because bronchiolitis is primarily a clinical diagnosis,7 there is no strong evidence to support the role of diagnostic testing, particularly that of complete blood counts (CBCs).8 Moreover, given the limited diagnostic utility of a single CBC, the benefit of obtaining a second CBC, especially with its associated physical discomfort and additional financial costs, is questionable. Yet despite the lack of evidence and rationale to support initial and repeated ordering of CBCs, we suspect that this practice may be more widespread and variable than currently appreciated.
Using a national database of children's hospitals, we sought to determine the frequency with which CBCs are ordered and repeated during hospitalizations for bronchiolitis, the extent to which these practices vary across institutions, and the relationship of these practices to average charges for a hospital stay.
METHODS
Data Source
We analyzed cases of children with bronchiolitis from the Pediatric Health Information System (PHIS) database of the Child Health Corporation of America.9 This database contains inpatient demographic, administrative, and diagnostic data from 36 freestanding, noncompeting children's hospitals in the United States. However, only 30 of the hospitals provided information on diagnostic testing during the period of our study. To protect the participating hospitals, hospitals were deidentified in this analysis. Diagnoses in the database are provided in the International Classification of Disease, 9th revision (ICD‐9), and the All‐Patient Refined Diagnostic Related Groups (APR‐DRGs), version 15 format.
Cases
We included in our sample children who had a primary ICD‐9 discharge code for bronchiolitis (469.11 or 469.19), an APR‐DRG for bronchiolitis/asthma (141), and a discharge date between October 2001 and September 2003.10 We further restricted cases to children less than 12 months of age because this is the age group most frequently hospitalized for bronchiolitis. Only the first admission per child was included in the analysis.
Outcome and Covariates
We identified the number of CBCs ordered using charge codes in the PHIS data. To avoid double counting, we required that the CBCs be charged on different dates of service, and we counted a maximum of 1 CBC per day per patient. We defined a child as having a repeated CBC if more than 1 CBC was charged during the child's hospital stay. Our outcome variable of repeat CBCs was measured dichotomously. We included age, male sex, Medicaid status, season of admission, intensive care unit (ICU) admission, APR‐DRG‐calculated severity scores for bronchiolitis/asthma (to adjust for disease severity), and length of stay as covariates in the regression and ANOVA analyses. All covariates were measured dichotomously, except for mean age and LOS, which were measured continuously.
Statistical Analyses
Bivariate analysis of baseline characteristics were compared across age groups using 2 tests to compare differences between categorical variables and the Student t test to compare differences between continuous variables.
To examine variability across hospitals in the initial and repeat ordering of CBCs, we performed multivariate ANOVA (MANOVA) controlling for age, sex, Medicaid status, illness severity, season of admission, ICU admission, and length of stay (LOS). Because the factors associated with repeat CBCs are not readily apparent, we performed logistic regression to determine which of these factors were significantly associated (P < .05) with having repeat CBCs performed. To account for the influence of age on the management and epidemiology of children with respiratory distress, we stratified MANOVA and regression analyses by age (< 3 months and 3 months. We clustered our regression analysis by hospital to determine whether there was hospital‐specific variation in repeating CBCs.
We performed post hoc analysis after noting additional variable relationships in our results. To determine whether CBC‐ordering patterns differed by severity, we stratified the analysis of repeat CBCs in both the bivariate and multivariate model by disease severity and ICU admission, respectively.
To determine if the number of CBCs ordered was related to admission charges, we categorized hospitals into tertiles (lowest, intermediate, highest) according to the proportion of admissions in which CBCs were ordered. We then calculated average admission hospital stay charges for each hospital. We used Student t tests to examine the relationship between the charges for admissions in hospitals with the intermediate and highest proportion of admissions with CBCs compared with those hospitals with the lowest proportion of admissions with CBCs.
We used Stata 8.0 to conduct our analyses.11 The Children's Hospital and Regional Medical Center Institutional Review Board (Seattle, WA) approved the analysis of the data for this study.
RESULTS
A total of 17,397 children met the inclusion criteria. Children under 3 months were more likely to be covered by Medicaid, be admitted to the ICU, have a longer length of stay, and have at least 1 CBC (Table 1). Of all children hospitalized, 48.2% had at least 1 CBC, and 7.8% had more than 1 CBC performed during their hospital stay. Notably, the proportion of all admissions with at least 1 CBC varied from 23.2% to 79.2% (Fig. 1), and those with repeat CBCs varied from 0% to 18.6% across hospitals (Fig. 2). This variation was significant when stratified by age and adjusted for covariates, which included length of stay and severity of illness (P < .001). In additional post hoc analyses we found differences in ordering pattern by disease severity that should be noted. The proportion of admissions with repeat CBCs varied significantly across severity groups (mild 3.9%, moderate 10.3%, and severe 21.3%, P < .001) and ICU admission status (ICU admission 5.5%, no ICU admission 23%, P <.001). Stratified analyses indicated an interaction between ICU utilization and disease severity, but neither covariate showed significant interactions with other variables in the model (data not shown).
| < 3 Months of Age | 3‐11 Months of Age | |
|---|---|---|
| ||
| Sample size | 7336 | 10,061 |
| Mean age (months) | 1.4 | 5.8 |
| Male (%) | 58.3 | 59.3 |
| Medicaid (%) | 56.0* | 53.8 |
| Admission Season | ||
| October‐February (%) | 71.0 | 70.4 |
| APG‐DRG severity score | ||
| Mild | 63.0 | 63.4 |
| Moderate | 22.4 | 22.7 |
| Severe | 14.6 | 13.8 |
| ICU admission (%) | 15.7 | 11.2 |
| Mean length of stay (days) | 3.1 | 2.8 |
| Received 1 CBC | 53.8 | 44.1 |
| Received > 1 CBC | 9.2 | 6.8 |


With respect to repeat CBCs, for children at least 3 months old, the strongest predictor was ICU admission (odds ratio [OR] 2.53, 95% CI: 1.69‐3.77), followed by a severe or extreme APR‐DRG severity score (OR 1.75, 95% CI: 1.23‐2.49) and LOS (OR 1.22, 95% CI: 1.15‐1.28). For children less than 3 months old, some of these associations strengthened ICU admission (OR 2.58, 95% CI: 1.84‐3.61), followed by a severe or extreme APR‐DRG severity score (OR 2.31, 95% CI: 1.64‐3.24) and LOS (OR 1.24, 95% CI: 1.16‐1.32). Additional predictors for this age group were a moderate severity score (OR 1.67, 95% CI: 1.29‐2.16) and Medicaid status (OR 1.20, 95% CI: 1.0‐1.43) (Table 2).
| < 3 Months of Age | 311 Months of Age | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR* | 95% CI | Adjusted OR* | 95% CI | |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| Mean Age (months) | 1.04 | 0.841.30 | 0.99 | 0.961.03 | ||||||||||||||||||||||||||||||||||||||||
| Male (%) | 1.01 | 0.851.19 | 0.88 | 0.741.05 | ||||||||||||||||||||||||||||||||||||||||
| Medicaid (%) | 1.20 | 1.001.43 | 0.95 | 0.791.15 | ||||||||||||||||||||||||||||||||||||||||
| Admission Season | ||||||||||||||||||||||||||||||||||||||||||||
| OctoberFebruary | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| MarchSeptember (%) | 1.13 | 0.931.37 | 1.11 | 0.811.53 | ||||||||||||||||||||||||||||||||||||||||
| APGDRG severity score | ||||||||||||||||||||||||||||||||||||||||||||
| Mild | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| Moderate | 1.67 | 1.292.16 | 1.28 | 0.941.76 | ||||||||||||||||||||||||||||||||||||||||
| Severe | 2.31 | 1.643.24 | 1.75 | 1.232.49 | ||||||||||||||||||||||||||||||||||||||||
| ICU admission (%) | 2.58 | 1.843.61 | 2.53 | 1.693.77 | ||||||||||||||||||||||||||||||||||||||||
| Length of stay (days) | 1.24 | 1.161.32 | 1.22 | 1.151.28 | ||||||||||||||||||||||||||||||||||||||||
Compared with hospitals that had the lowest proportion of admissions in which CBCs were ordered, hospitals with higher proportions of CBCs ordered had significantly higher mean charges per hospital stay (Table 3).
| Hospital CBC Levels | Patients | Mean Charge (95% CI) | Mean Difference (95% CI) |
|---|---|---|---|
| |||
| Lowest (23%‐40%) | 5838 | $7293 ($70967489) | Referent |
| Middle (41%59%) | 6673 | $8099 ($78598339) | $807* ($491$1122) |
| Highest (60%79%) | 4886 | $8316 ($80548578) | $1024* ($702$1345) |
DISCUSSION
We found that in a nationwide sample of children hospitalized with bronchiolitis, 48% had at least 1 CBC and nearly 8% had a repeat CBC ordered during their hospital stay. Moreover, even after adjusting for covariates, the proportion of children with initial and repeat CBCs during a single admission varied widely and significantly across a nationwide sample of children's hospitals.
We can only speculate on the reasons for institutional variation. Although it is not unusual for some cases of illness to vary from a standard course and so trigger initial or repeat evaluations with a CBC, we do not have any a priori reason to expect the proportion of unusual cases to vary by institution in a national cohort of children's hospitals. One compelling explanation for this variation is differing institutional patterns of practice. For example, it may be that some institutions have protocols that require the ordering of a CBC on admission. This practice could prompt a costly and unnecessary testing cascade14 generated by an initially abnormal CBC and so could trigger additional testing and/or procedures, such as x‐rays and parental antibiotics. Such a cascade of testing and intervention could conceivably lead to additional, and dependent, costs not captured by a simple tally of the costs of individual CBCs. Indeed, in our analysis we found that those hospitals with higher proportion of admissions in which CBCs were ordered also had significantly higher admission charges that exceeded the cost of a CBC. Previous studies support the finding that institutional variation in care for viral respiratory illness is significantly correlated with hospital costs.6
Limitations of this study should be noted. First, the PHIS database does not provide indications for, results of, or hospital location of tests, so we cannot determine whether clinical condition or results prompted initial and/or repeat testing. However, because children with complicated courses or atypical disease presentations likely have longer hospital stays, severe disease, or additional diagnoses, we attempted to control for these factors in our analysis. Second, although we selected cases based on a discharge diagnosis of bronchiolitis, it is possible that admitting physicians obtained an initial CBC to rule out alternative diagnoses, such as bacteremia, which can occur but is rare in this population.12, 13 It is plausible that bacteremia is most likely in children with other comorbidities or higher disease severity. In additional stratified analyses we did find that the proportion of repeat CBCs increased with higher disease severity and that there was an interaction between severe disease status and ICU admission. However, all participating institutions are children's hospitals and so are likely to treat children with a range of severity of illness and comorbidities. Finally, as with other analyses of the PHIS database, we used charges to identify diagnostic tests.5
Given that more than 120,000 U.S. infants are hospitalized annually with bronchiolitis,15 the cost and discomfort associated with unnecessary testing warrants attention. The issue of cost is particularly relevant in light of recent research findings of increased costs for admissions at freestanding children's hospitals.16 We found that mean charges per hospital stay were significantly higher for hospitals that had a higher proportion of admissions during which multiple CBCs were ordered. Although we cannot exclude illness severity and age as explanations for the higher charges, we have no reason to believe that one freestanding children's hospital would have a sicker and younger population than another. An alternative and compelling explanation is that a variation in the standard of care exists across these hospitals.
The institutional variation in and the limited evidence for the utility of the ordering of CBCs in the evaluation of bronchiolitis call into question the necessity of this testing strategy. Exploration of the reasons for this institutional variation will help to create quality initiatives and directed interventions to improve and standardize care in bronchiolitis.
Acknowledgements
Supported by: Robert Wood Johnson (RWJ) Foundation through the Robert Wood Johnson Clinical Scholars Program. The views expressed do not necessarily represent the views of the Robert Wood Johnson Foundation or the University of Washington. The RWJ Foundation provided salary support for Dr. Tarini. The RWJ Foundation did not have a role in the study's design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication. Dr. Tarini wrote the first draft of the manuscript. All authors have seen and agree with the contents of this manuscript.
Bronchiolitis was the most common primary diagnosis of infants hospitalized in the United States from 2000 to 2001.1 Consequently, much research has focused on the effectiveness of management24 and variation in care, especially the use of unproven diagnostic tests such as chest x‐rays.5 Such variation may have substantial financial and medical impact and has been shown to correlate significantly with hospital costs and length of stay.6
Because bronchiolitis is primarily a clinical diagnosis,7 there is no strong evidence to support the role of diagnostic testing, particularly that of complete blood counts (CBCs).8 Moreover, given the limited diagnostic utility of a single CBC, the benefit of obtaining a second CBC, especially with its associated physical discomfort and additional financial costs, is questionable. Yet despite the lack of evidence and rationale to support initial and repeated ordering of CBCs, we suspect that this practice may be more widespread and variable than currently appreciated.
Using a national database of children's hospitals, we sought to determine the frequency with which CBCs are ordered and repeated during hospitalizations for bronchiolitis, the extent to which these practices vary across institutions, and the relationship of these practices to average charges for a hospital stay.
METHODS
Data Source
We analyzed cases of children with bronchiolitis from the Pediatric Health Information System (PHIS) database of the Child Health Corporation of America.9 This database contains inpatient demographic, administrative, and diagnostic data from 36 freestanding, noncompeting children's hospitals in the United States. However, only 30 of the hospitals provided information on diagnostic testing during the period of our study. To protect the participating hospitals, hospitals were deidentified in this analysis. Diagnoses in the database are provided in the International Classification of Disease, 9th revision (ICD‐9), and the All‐Patient Refined Diagnostic Related Groups (APR‐DRGs), version 15 format.
Cases
We included in our sample children who had a primary ICD‐9 discharge code for bronchiolitis (469.11 or 469.19), an APR‐DRG for bronchiolitis/asthma (141), and a discharge date between October 2001 and September 2003.10 We further restricted cases to children less than 12 months of age because this is the age group most frequently hospitalized for bronchiolitis. Only the first admission per child was included in the analysis.
Outcome and Covariates
We identified the number of CBCs ordered using charge codes in the PHIS data. To avoid double counting, we required that the CBCs be charged on different dates of service, and we counted a maximum of 1 CBC per day per patient. We defined a child as having a repeated CBC if more than 1 CBC was charged during the child's hospital stay. Our outcome variable of repeat CBCs was measured dichotomously. We included age, male sex, Medicaid status, season of admission, intensive care unit (ICU) admission, APR‐DRG‐calculated severity scores for bronchiolitis/asthma (to adjust for disease severity), and length of stay as covariates in the regression and ANOVA analyses. All covariates were measured dichotomously, except for mean age and LOS, which were measured continuously.
Statistical Analyses
Bivariate analysis of baseline characteristics were compared across age groups using 2 tests to compare differences between categorical variables and the Student t test to compare differences between continuous variables.
To examine variability across hospitals in the initial and repeat ordering of CBCs, we performed multivariate ANOVA (MANOVA) controlling for age, sex, Medicaid status, illness severity, season of admission, ICU admission, and length of stay (LOS). Because the factors associated with repeat CBCs are not readily apparent, we performed logistic regression to determine which of these factors were significantly associated (P < .05) with having repeat CBCs performed. To account for the influence of age on the management and epidemiology of children with respiratory distress, we stratified MANOVA and regression analyses by age (< 3 months and 3 months. We clustered our regression analysis by hospital to determine whether there was hospital‐specific variation in repeating CBCs.
We performed post hoc analysis after noting additional variable relationships in our results. To determine whether CBC‐ordering patterns differed by severity, we stratified the analysis of repeat CBCs in both the bivariate and multivariate model by disease severity and ICU admission, respectively.
To determine if the number of CBCs ordered was related to admission charges, we categorized hospitals into tertiles (lowest, intermediate, highest) according to the proportion of admissions in which CBCs were ordered. We then calculated average admission hospital stay charges for each hospital. We used Student t tests to examine the relationship between the charges for admissions in hospitals with the intermediate and highest proportion of admissions with CBCs compared with those hospitals with the lowest proportion of admissions with CBCs.
We used Stata 8.0 to conduct our analyses.11 The Children's Hospital and Regional Medical Center Institutional Review Board (Seattle, WA) approved the analysis of the data for this study.
RESULTS
A total of 17,397 children met the inclusion criteria. Children under 3 months were more likely to be covered by Medicaid, be admitted to the ICU, have a longer length of stay, and have at least 1 CBC (Table 1). Of all children hospitalized, 48.2% had at least 1 CBC, and 7.8% had more than 1 CBC performed during their hospital stay. Notably, the proportion of all admissions with at least 1 CBC varied from 23.2% to 79.2% (Fig. 1), and those with repeat CBCs varied from 0% to 18.6% across hospitals (Fig. 2). This variation was significant when stratified by age and adjusted for covariates, which included length of stay and severity of illness (P < .001). In additional post hoc analyses we found differences in ordering pattern by disease severity that should be noted. The proportion of admissions with repeat CBCs varied significantly across severity groups (mild 3.9%, moderate 10.3%, and severe 21.3%, P < .001) and ICU admission status (ICU admission 5.5%, no ICU admission 23%, P <.001). Stratified analyses indicated an interaction between ICU utilization and disease severity, but neither covariate showed significant interactions with other variables in the model (data not shown).
| < 3 Months of Age | 3‐11 Months of Age | |
|---|---|---|
| ||
| Sample size | 7336 | 10,061 |
| Mean age (months) | 1.4 | 5.8 |
| Male (%) | 58.3 | 59.3 |
| Medicaid (%) | 56.0* | 53.8 |
| Admission Season | ||
| October‐February (%) | 71.0 | 70.4 |
| APG‐DRG severity score | ||
| Mild | 63.0 | 63.4 |
| Moderate | 22.4 | 22.7 |
| Severe | 14.6 | 13.8 |
| ICU admission (%) | 15.7 | 11.2 |
| Mean length of stay (days) | 3.1 | 2.8 |
| Received 1 CBC | 53.8 | 44.1 |
| Received > 1 CBC | 9.2 | 6.8 |


With respect to repeat CBCs, for children at least 3 months old, the strongest predictor was ICU admission (odds ratio [OR] 2.53, 95% CI: 1.69‐3.77), followed by a severe or extreme APR‐DRG severity score (OR 1.75, 95% CI: 1.23‐2.49) and LOS (OR 1.22, 95% CI: 1.15‐1.28). For children less than 3 months old, some of these associations strengthened ICU admission (OR 2.58, 95% CI: 1.84‐3.61), followed by a severe or extreme APR‐DRG severity score (OR 2.31, 95% CI: 1.64‐3.24) and LOS (OR 1.24, 95% CI: 1.16‐1.32). Additional predictors for this age group were a moderate severity score (OR 1.67, 95% CI: 1.29‐2.16) and Medicaid status (OR 1.20, 95% CI: 1.0‐1.43) (Table 2).
| < 3 Months of Age | 311 Months of Age | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR* | 95% CI | Adjusted OR* | 95% CI | |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| Mean Age (months) | 1.04 | 0.841.30 | 0.99 | 0.961.03 | ||||||||||||||||||||||||||||||||||||||||
| Male (%) | 1.01 | 0.851.19 | 0.88 | 0.741.05 | ||||||||||||||||||||||||||||||||||||||||
| Medicaid (%) | 1.20 | 1.001.43 | 0.95 | 0.791.15 | ||||||||||||||||||||||||||||||||||||||||
| Admission Season | ||||||||||||||||||||||||||||||||||||||||||||
| OctoberFebruary | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| MarchSeptember (%) | 1.13 | 0.931.37 | 1.11 | 0.811.53 | ||||||||||||||||||||||||||||||||||||||||
| APGDRG severity score | ||||||||||||||||||||||||||||||||||||||||||||
| Mild | Referent | Referent | ||||||||||||||||||||||||||||||||||||||||||
| Moderate | 1.67 | 1.292.16 | 1.28 | 0.941.76 | ||||||||||||||||||||||||||||||||||||||||
| Severe | 2.31 | 1.643.24 | 1.75 | 1.232.49 | ||||||||||||||||||||||||||||||||||||||||
| ICU admission (%) | 2.58 | 1.843.61 | 2.53 | 1.693.77 | ||||||||||||||||||||||||||||||||||||||||
| Length of stay (days) | 1.24 | 1.161.32 | 1.22 | 1.151.28 | ||||||||||||||||||||||||||||||||||||||||
Compared with hospitals that had the lowest proportion of admissions in which CBCs were ordered, hospitals with higher proportions of CBCs ordered had significantly higher mean charges per hospital stay (Table 3).
| Hospital CBC Levels | Patients | Mean Charge (95% CI) | Mean Difference (95% CI) |
|---|---|---|---|
| |||
| Lowest (23%‐40%) | 5838 | $7293 ($70967489) | Referent |
| Middle (41%59%) | 6673 | $8099 ($78598339) | $807* ($491$1122) |
| Highest (60%79%) | 4886 | $8316 ($80548578) | $1024* ($702$1345) |
DISCUSSION
We found that in a nationwide sample of children hospitalized with bronchiolitis, 48% had at least 1 CBC and nearly 8% had a repeat CBC ordered during their hospital stay. Moreover, even after adjusting for covariates, the proportion of children with initial and repeat CBCs during a single admission varied widely and significantly across a nationwide sample of children's hospitals.
We can only speculate on the reasons for institutional variation. Although it is not unusual for some cases of illness to vary from a standard course and so trigger initial or repeat evaluations with a CBC, we do not have any a priori reason to expect the proportion of unusual cases to vary by institution in a national cohort of children's hospitals. One compelling explanation for this variation is differing institutional patterns of practice. For example, it may be that some institutions have protocols that require the ordering of a CBC on admission. This practice could prompt a costly and unnecessary testing cascade14 generated by an initially abnormal CBC and so could trigger additional testing and/or procedures, such as x‐rays and parental antibiotics. Such a cascade of testing and intervention could conceivably lead to additional, and dependent, costs not captured by a simple tally of the costs of individual CBCs. Indeed, in our analysis we found that those hospitals with higher proportion of admissions in which CBCs were ordered also had significantly higher admission charges that exceeded the cost of a CBC. Previous studies support the finding that institutional variation in care for viral respiratory illness is significantly correlated with hospital costs.6
Limitations of this study should be noted. First, the PHIS database does not provide indications for, results of, or hospital location of tests, so we cannot determine whether clinical condition or results prompted initial and/or repeat testing. However, because children with complicated courses or atypical disease presentations likely have longer hospital stays, severe disease, or additional diagnoses, we attempted to control for these factors in our analysis. Second, although we selected cases based on a discharge diagnosis of bronchiolitis, it is possible that admitting physicians obtained an initial CBC to rule out alternative diagnoses, such as bacteremia, which can occur but is rare in this population.12, 13 It is plausible that bacteremia is most likely in children with other comorbidities or higher disease severity. In additional stratified analyses we did find that the proportion of repeat CBCs increased with higher disease severity and that there was an interaction between severe disease status and ICU admission. However, all participating institutions are children's hospitals and so are likely to treat children with a range of severity of illness and comorbidities. Finally, as with other analyses of the PHIS database, we used charges to identify diagnostic tests.5
Given that more than 120,000 U.S. infants are hospitalized annually with bronchiolitis,15 the cost and discomfort associated with unnecessary testing warrants attention. The issue of cost is particularly relevant in light of recent research findings of increased costs for admissions at freestanding children's hospitals.16 We found that mean charges per hospital stay were significantly higher for hospitals that had a higher proportion of admissions during which multiple CBCs were ordered. Although we cannot exclude illness severity and age as explanations for the higher charges, we have no reason to believe that one freestanding children's hospital would have a sicker and younger population than another. An alternative and compelling explanation is that a variation in the standard of care exists across these hospitals.
The institutional variation in and the limited evidence for the utility of the ordering of CBCs in the evaluation of bronchiolitis call into question the necessity of this testing strategy. Exploration of the reasons for this institutional variation will help to create quality initiatives and directed interventions to improve and standardize care in bronchiolitis.
Acknowledgements
Supported by: Robert Wood Johnson (RWJ) Foundation through the Robert Wood Johnson Clinical Scholars Program. The views expressed do not necessarily represent the views of the Robert Wood Johnson Foundation or the University of Washington. The RWJ Foundation provided salary support for Dr. Tarini. The RWJ Foundation did not have a role in the study's design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication. Dr. Tarini wrote the first draft of the manuscript. All authors have seen and agree with the contents of this manuscript.
- ,,, et al.Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population.Pediatrics. Oct2004;114:e437–e444.
- ,,, et al.Pharmacologic treatment of bronchiolitis in infants and children: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):127–137.
- ,,,.Glucocorticoids for acute viral bronchiolitis in infants and young children.Cochrane Database Syst Rev.2004(3):CD004878.
- ,,.Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old.Cochrane Database Syst Rev.2005;(2):CD004873.
- ,,,,,.Variation in inpatient diagnostic testing and management of bronchiolitis.Pediatrics.2005;115:878–884.
- ,,,,.Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness.Pediatrics.2001;108:851–855.
- .Bronchiolitis. In:Behrman Re KR,Jenson HB, eds.Nelson Textbook of Pediatrics.17th ed.Philadelphia:WB Saunders Co.;2004:1415–1417.
- ,,, et al.Diagnosis and testing in bronchiolitis: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):119–126.
- .Achieving data quality. How data from a pediatric health information system earns the trust of its users.J AHIMA.2004;75(10):22–26.
- ,,,,.A closer look at all‐patient refined DRGs.J AHIMA.2002;73(1):46–50.
- Stata Corp. College Station, TX.
- ,.Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections.Arch Pediatr Adolesc Med.2002;156:322–324.
- ,.Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection.Pediatr Infect Dis J.2004;23:267–269.
- ,.The cascade effect in the clinical care of patients.N Engl J Med.1986;314:512–514.
- ,,,,,.Bronchiolitis‐associated hospitalizations among US children, 1980‐1996.JAMA.1999;282:1440–1446.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
- ,,, et al.Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population.Pediatrics. Oct2004;114:e437–e444.
- ,,, et al.Pharmacologic treatment of bronchiolitis in infants and children: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):127–137.
- ,,,.Glucocorticoids for acute viral bronchiolitis in infants and young children.Cochrane Database Syst Rev.2004(3):CD004878.
- ,,.Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old.Cochrane Database Syst Rev.2005;(2):CD004873.
- ,,,,,.Variation in inpatient diagnostic testing and management of bronchiolitis.Pediatrics.2005;115:878–884.
- ,,,,.Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness.Pediatrics.2001;108:851–855.
- .Bronchiolitis. In:Behrman Re KR,Jenson HB, eds.Nelson Textbook of Pediatrics.17th ed.Philadelphia:WB Saunders Co.;2004:1415–1417.
- ,,, et al.Diagnosis and testing in bronchiolitis: a systematic review.Arch Pediatr Adolesc Med.2004;158(2):119–126.
- .Achieving data quality. How data from a pediatric health information system earns the trust of its users.J AHIMA.2004;75(10):22–26.
- ,,,,.A closer look at all‐patient refined DRGs.J AHIMA.2002;73(1):46–50.
- Stata Corp. College Station, TX.
- ,.Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections.Arch Pediatr Adolesc Med.2002;156:322–324.
- ,.Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection.Pediatr Infect Dis J.2004;23:267–269.
- ,.The cascade effect in the clinical care of patients.N Engl J Med.1986;314:512–514.
- ,,,,,.Bronchiolitis‐associated hospitalizations among US children, 1980‐1996.JAMA.1999;282:1440–1446.
- ,,.Lengths of stay and costs associated with children's hospitals.Pediatrics.2005;115:839–844.
Copyright © 2007 Society of Hospital Medicine
VTE PX in ED Admissions
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).
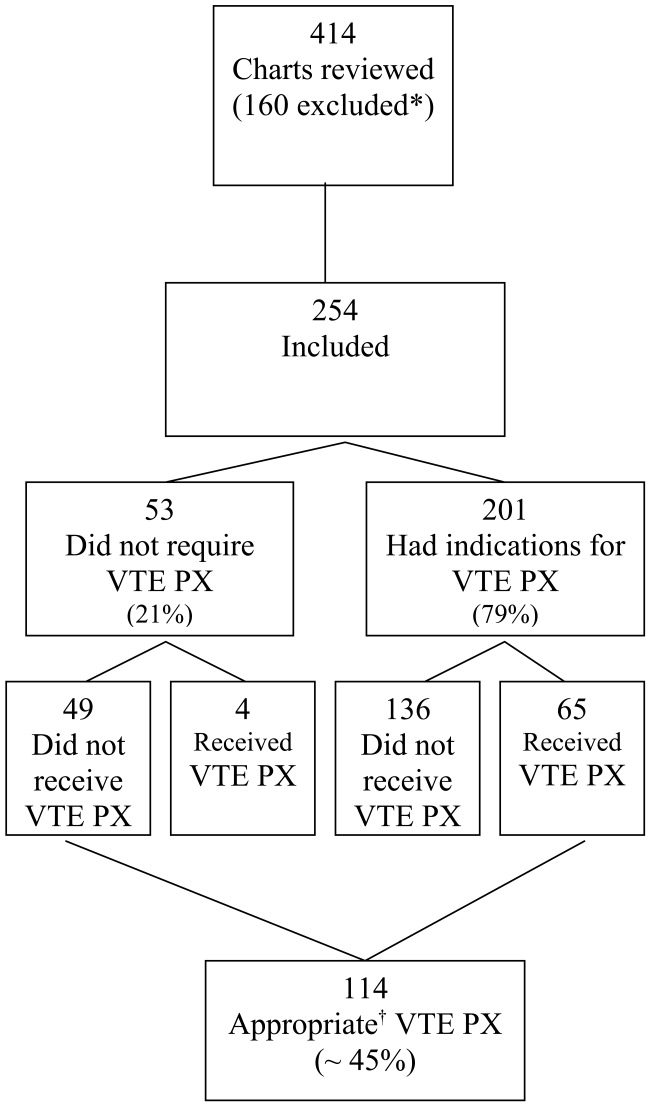
Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).

Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
Venous thromboembolism prophylaxis (VTE PX) has been identified as an area of primary importance to improve patient safety in research and clinical practice.13 Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, often preventable life‐threatening condition for hospitalized patients.4 Up to half of patients admitted to the hospital are admitted from the emergency department (ED). Most of these patients are acutely ill with multiple risk factors for VTE. To reduce the incidence of VTE, these patients require routine evaluation to determine if thromboprophylaxis is needed, and when indicated, therapy should be started promptly on admission. The Seventh American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy outlines recommendations for VTE PX that reduce the development of DVT and PE.3 Despite there being effective VTE PX and the current focus on increasing its utilization to improve patient safety, VTE PX is underutilized. In particular, the subgroup of patients admitted from the ED, a group at high risk for VTE, has been neglected in the literature.
Our hypothesis was that VTE PX is underutilized in patients admitted through the ED. The specific objective of this study was to measure the rate at which hospitalized patients admitted though the ED received VTE PX.
METHODS
The study was conducted with the approval of and in accordance with the ethical standards of the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. Prior to initiating chart review, passive consent was sought from physicians who were identified through the hospital medical records system as having admitted patients to this hospital through the ED in the preceding 6 months. Physicians were contacted twice in writing in a 1‐month period prior to study inception. Those who objected to their charts being reviewed were to notify the investigators. Otherwise, they were assumed to have consented to chart review. Fifteen percent of physicians declined chart review. Physicians were not informed of the particulars of the study, only that medication use in the ED was being evaluated.
This study was conducted at a private 900‐bed urban teaching hospital. The ED evaluates approximately 31,000 patients per year, predominantly a medical population. During the previous year, the ED had admitted roughly 30 patients per day, or 36% of all patients examined. Approximately 29% of admissions to this hospital (800/month) are admitted through the ED.
A convenience sample of every other hospital admission through the ED during 1 month was prospectively identified for inclusion in the study and chart review. Data were abstracted by a single reviewer on admission and at the time of discharge. The following data were collected: demographic characteristics, anticoagulant use or existing IVC filter, diagnoses, indications for full‐dose anticoagulation, indications for VTE PX (ie, immobilization, respiratory failure, congestive heart failure, limb trauma, surgery, or stroke), whether therapeutic anticoagulation or VTE PX was given, and date of initiation of this regimen, contraindications to anticoagulation, primary physician, and use of a standard order set. Patients were excluded if the attending physician declined chart review via the passive consent process. Other exclusion criteria were: receiving full‐dose anticoagulants before presentation to the ED, presence of an inferior vena cava (IVC) filter, indication for full‐dose anticoagulation (presented with DVT, PE, acute coronary syndrome), renal failure requiring hemodialysis (controversial risk for VTE59), length of stay (LOS) less than 2 days, and admission for psychiatric evaluation or treatment.
A modified Caprini's Risk Assessment Model for Surgical and Nonsurgical Patients was used to classify VTE risk.10 This tool assigns points to VTE risk factors so that risk and the need for VTE PX can be determined. For example, major surgery, central venous access, age older than 60 years, and bed rest for more than 72 hours are each assigned 2 points; higher‐risk factors such as hip or leg fractures or stroke are each assigned 5 points. This tool is generally in accord with the ACCP guidelines. Modifications made to this tool were to assign 3 points to patients in respiratory failure on ventilators and 5 points to patients who were critically ill on vasopressor medication. Decreased venous return associated with mechanical ventilation and peripheral vasoconstriction associated with the use of vasopressor medication justified the addition of these risk factors.11, 12 Patients were assigned to one of these risk categories: no risk (0 points), low risk (1 point), moderate risk (2 points), high risk (3‐4 points), or very high risk (5 or more points). As indicated by this risk assessment tool, those with moderate, high, or very high risk were considered in need of VTE PX.
Appropriate VTE PX was defined as any currently accepted medical (unfractionated heparin, low‐molecular‐weight heparin, or warfarin for orthopedic patients) or mechanical methods of VTE PX (sequential compression devices, and graduated compression stockings) for those in need and no VTE PX if none indicated. Aspirin, clopidogrel, or a combination of the 2 was not considered sufficient VTE PX.3 In addition, we established whether VTE PX as determined by the modified Caprini score was in line with ACCP guidelines, taking into account contraindications to anticoagulation. Preprinted order sets were divided into those that included VTE PX and those that did not. Order sets that included options for VTE PX were defined as standard order sets.
The primary objective of this study was to determine how frequently VTE PX was implemented in ED admissions. Secondary objectives were determining factors associated with correct VTE PX decision making and the proximity of orders for VTE PX to the time of admission.
Statistical Methods
The SAS system was used to perform chi‐square analysis of independent predictors of VTE PX. The dependent variable, which was dichotomous, was whether correct VTE PX decision making had occurred. Factors associated with VTE PX were considered significant if the P value was less than .05. Odds ratios were calculated along with 95% confidence intervals for all significant predictors of VTE PX. Multiple logistic regression analysis was performed to provide adjusted odds ratios and to arrive at a summary risk measure. Candidate independent variables for the multiple logistic regression analysis included all variables screened in the univariate analyses. A first‐pass stepwise model was developed, followed by a best‐subsets run with manual stepping. Although bed rest was on the margin of statistical significance (P = .059), we retained it in the model because it was is a well‐recognized risk factor for which the other model terms needed to be adjusted, and it was nine‐tenths of 1% above the critical value.
RESULTS
Four hundred and fourteen charts of patient admissions were reviewed, of which 254 met the inclusion criteria. One‐hundred and sixty patients were excluded because they received full‐dose anticoagulation or had an existing IVC filter prior to admission (49 patients), received treatment with full‐dose anticoagulation in the ED (42 patients), had a LOS of less than 2 days (39 patients), or had end‐stage renal disease requiring hemodialysis (30 patients; Fig. 1).

Eighty percent of patients were admitted for medical problems, and 20% were admitted for surgery (Table 1). The most frequent admitting diagnoses were abdominal pain, congestive heart failure, chronic obstructive pulmonary disease, altered mental status, cerebral vascular accident, and pneumonia. The average patient had 5 comorbid conditions, the most frequently noted were hypertension, diabetes mellitus, anemia, urinary tract infections, and coronary artery disease. The principal admitting services were general medicine, pulmonary, cardiology, hematology‐oncology, neurology, surgery, and gastroenterology. Six patients died (2.4%), and 2 patients were diagnosed with pulmonary emboli (0.8%). The study group's average length of stay was 6.7 days (range 2‐52 days), 48.8% were male, and average age was 61 19.7 years. Overall, the correct VTE PX decision making occurred in 44.9% of patients admitted, including the 49 of 254 patients who did not require and did not receive VTE PX. Of the 254 patients, 201 (79%) had indications for VTE PX, 65 of whom (32.3%) received it (Table 2). For those receiving VTE PX, 78% of orders were written within the first day of hospitalization.
| Category | Primary Diagnosis | Number of Patients | Percent |
|---|---|---|---|
| Medical (80%) | Neurological | 47 | 19% |
| Cardiovascular | 39 | 15% | |
| Pulmonary | 35 | 14% | |
| Gastrointestinal | 27 | 11% | |
| Other medical | 22 | 9% | |
| Renal | 9 | 4% | |
| Cancer | 7 | 3% | |
| Hematological | 7 | 3% | |
| Musculoskeletal | 6 | 2% | |
| Endocrine | 3 | 1% | |
| Total Medical | 202 | ||
| Surgical (20%) | Gastrointestinal | 28 | 11% |
| Orthopedic/spine | 11 | 4% | |
| Other surgical | 8 | 3% | |
| Neurosurgical | 3 | 1% | |
| Cancer | 1 | 0% | |
| Genitourinary | 1 | 0% | |
| Total Surgical | 52 | ||
| Total (100%) | 254 | 100% |
| Patients | Percent | |
|---|---|---|
| ||
| Appropriate decisions made regarding VTE PX* | 114/254 | 44.9% |
| Indications for VTE PX | 201/254 | 79% |
| Required active VTE PX and received it | 65/201 | 32% |
| Utilized SOS and ordered VTE PX | 18/26 | 69% |
When the data were reanalyzed per ACCP guidelines using the modified Caprini's risk assessment tool, the results were consistent with the initial findings. Overall, 46% of all patients (116 of 254) received prophylaxis in compliance with ACCP guidelines. In this group, 52 of 116 patients (44.8%) did not require and did not receive VTE PX. Sixty‐four patients (32% of those with indications for prophylaxis) had indications for VTE PX, were in compliance with ACCP guidelines, and received the indicated prophylaxis (30 patients received mechanical prophylaxis, 19 patients received medical prophylaxis, and 15 patients received both medical and mechanical prophylaxis). The difference between the assessments was explained by high‐risk patients with no contraindications to medical prophylaxis who received only mechanical prophylaxis but required medical prophylaxis through ACCP guidelines. Note, the Caprini tool recommended medical prophylaxis for these high‐risk patients; however, our original application was simply to assess if prophylaxis was employed. In addition, several patients with a prolonged INR suggestive of bleeding risk or autoprophylaxis were reclassified as compliant and not needing prophylaxis.
Fifty‐five patients with indications for VTE prophylaxis had contraindications to medical prophylaxis: 44 had bleeding risk, 8 had spine injury or surgery, and 3 had brain metastases and thrombocytopenia. Twenty of the 55 patients (36%) received mechanical prophylaxis; they were considered in compliance with ACCP guidelines and were included in the appropriate decisions regarding VTE PX count. Prophylaxed patients at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of those prophylaxed patients who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
Standard order sets increased the likelihood of appropriate VTE PX. Increasing age and a primary cardiovascular diagnosis (chest pain, congestive heart failure, syncope/near‐syncope, chronic ischemic heart disease, sinus tachycardia) decreased the likelihood of VTE PX (Table 3). VTE PX was not significantly related to bed rest (OR = 1.46, P = .14). In 26 of the 254 patient admissions, standard order sets that included VTE PX were utilized. Of these 26 patients, 69.2% (18; P = .01) received appropriate VTE PX compared with the overall rate of 44.9% receiving appropriate VTE PX. The use of VTE PX was significantly associated with level of risk: from 0% in patients at no or low risk of VTE to 47% in patients at very high risk (P = .0001). This significance persisted when controlling for age greater than 60 years (Table 4).
| Patients | Received Appropriate PX | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | Odds Ratio* | 95% CI | P |
| |||||||
| Overall | 254 | (100.0) | 114 | (44.9) | |||
| Age (years) | |||||||
| 16‐47 | 59 | (23.2) | 37 | (62.7) | 0.97 | 0.96‐0.98 | .0001 |
| 48‐64 | 68 | (26.8) | 38 | (55.9) | (.0001) | ||
| 65‐78 | 61 | (24.0) | 17 | (27.9) | |||
| 79‐95 | 66 | (25.0) | 22 | (33.3) | |||
| CV diagnosis | |||||||
| Yes | 39 | (15.4) | 6 | (15.4) | 0.18 | 0.07‐0.45 | .0001 |
| No | 215 | (84.6) | 108 | (50.2) | 1 | ||
| Bedrest | |||||||
| Yes | 125 | (49.2) | 62 | (49.6) | 1.46 | 0.89‐2.40 | .14 |
| No | 129 | (50.8) | 52 | (40.3) | 1 | ||
| Standardized orders | |||||||
| Yes | 26 | (10.2) | 18 | (69.2) | 3.09 | 1.29‐7.41 | .009 |
| No | 228 | (89.8) | 96 | (42.1) | 1 | ||
| Risk Level | Age < 60 Years | Age > 60 Years | |||
|---|---|---|---|---|---|
| Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Number Prophylaxed/Number at Risk Level | Percent Prophylaxed | Total Percent VTE PX | |
| |||||
| Very high (93) | 10/20 | 50% | 34/73 | 47% | 47% |
| High (71) | 10/35 | 29% | 6/36 | 17% | 23% |
| Moderate (53) | 4/25 | 16% | 1/28 | 4% | 9% |
| Low (29) | 0/29 | 0% | 0 | 0% | 0% |
| None (8) | 0/8 | 0% | 0 | 0% | 0% |
| Total (254) | 24/117 | 41/137 | 65/254* | ||
Aspirin and other antiplatelet medications (clopidogrel, dipyridamole, and cilostazol) were ordered for 22 and 5 patients, respectively, of the 39 patients with primary cardiovascular diagnosis who had indications for VTE PX but did not receive it. Forty‐seven percent (17 of 36 with activity orders) of those in our cardiovascular at‐risk but not prophylaxed group had activity orders of ambulatory ad lib or had physical therapy ordered.
DISCUSSION
An estimated 200,000‐300,000 cases of VTE with 60,000‐200,000 fatal pulmonary emboli occur annually.1316 The inpatient fatality rate due to PE is estimated to be 12%.13 The frequency of VTE varies with risk that relates to the population studied and the diagnosis. VTE rates range from 3%‐55% for medical patients to 80% for patients who receive total hip replacement or have multiple trauma, though the higher numbers cited are based on studies using fibrinogen uptake scanning or venography, with the true rates probably between the extremes noted.3, 4, 17, 18 Many of these acutely ill patients are admitted through the ED. Though VTE is common in patients admitted through the ED, with respect to VTE PX, this population is understudied.
In this study, the first to our knowledge to focus on VTE PX in an unselected cohort of ED admissions, the most significant findings were: 79% of ED admissions had indications for VTE PX, yet only 32% of those received it, and 78% of these orders were written within the first day of hospitalization. We also noted a direct association of the use of VTE PX with the level of risk, which increased from 9% in the moderate‐risk group to 23% for high‐risk patients and 47% for very‐high‐risk patients (P < .0001; Table 4.). Thus, most of our patients, including those at highest risk for VTE never received prophylaxis at any time during their hospitalization. Also explored in this study was the relationship of risk factors for VTE with the use of prophylaxis. These risk factors were age, cardiovascular diagnosis, and use of standard order sets. Increasing age and having a primary cardiovascular diagnosis (ie, congestive heart failure, atrial fibrillation) were the risk factors that increased the likelihood of receiving VTE. Therefore, it was expected that the rate of VTE PX would be higher for patients who were older or had these diagnoses. However, in the current study, increasing age alone did not influence the likelihood of physicians ordering VTE PX. In addition, we found markedly decreased rates of VTE PX in cardiac patients.
Other investigators have reported similar findings in selected groups of hospitalized patients.1922 A retrospective chart review of internal medicine discharges from 2 Italian hospitals determined that VTE PX was prescribed in 46.4% and 58.3% of at‐risk patients in nonteaching and teaching hospitals, respectively.20 In a retrospective study of surgical patients in 20 hospitals, 38% of patients received VTE PX.21 Similar results were found in a registry of hospitalized patients who developed VTE, in which only 42% of patients who developed VTE received VTE PX within 30 days prior to diagnosis.23
Bosson et al. reported no increased use of VTE PX in patients with myocardial infarction, similar to that in the current study, though they did find VTE PX administered more frequently to patients with congestive heart failure.22 Antiplatelet medications and activity orders are commonly prescribed for cardiac patients. According to reports that indicated a degree of protection from antiplatelet agents,24, 25 frequent use of activity orders, and the belief that ambulation eliminates the risk of VTE, it is possible physicians believed patients were sufficiently prophylaxed. However, although early ambulation and antiplatelet medications decrease risk of VTE, neither is sufficient to prevent it.3 The administration of aspirin and other antiplatelet medications implies that in our study group bleeding risk was not the primary deterrent to ordering VTE PX. Furthermore, bleeding risk would not be a deterrent to mechanical VTE PX.
In the current study, use of standard order sets was associated with correct decision making and increased use of VTE PX. Risk of VTE might be decreased through the use of standard order sets that result in increased utilization of VTE PX. However, despite evidence that standard order sets can successfully modify prescribing patterns,2629 Cook et al. found that only 5 of 29 Canadian ICU directors surveyed for their approach to VTE prevention and diagnosis in critically ill patients used preprinted orders.30
The present study had several limitations. First, determination of VTE was not an end point. As a single‐center study of prospectively selected subjects, this would have required too large a sample to be feasible. Our data may be biased by not including patients admitted by physicians who declined to allow their charts to be reviewed. However, although physicians were informed that we were examining drug use of patients admitted through the ED, they were not aware that the study focused on VTE PX. Our results are consistent with results of inpatient studies citing inadequate VTE PX.19, 21, 31, 32 Using the modified Caprini Scoring System, we found that only 32% of patients with indications for VTE PX received it. This result was unchanged when stratifying using ACCP guidelines. Finally, we found that prophylaxed patients who were at moderate to high risk were more likely to receive mechanical prophylaxis, whereas two‐thirds of patients who received prophylaxis who were at very high risk received medical prophylaxis or a combination of medical and mechanical prophylaxis.
CONCLUSIONS
Most patients needing VTE PX did not receive it, and those who did receive VTE PX usually had it prescribed in the first 24 hours. As risk factors increased, patients were more often prophylaxed, though fewer than 50% of those in the very‐high‐risk group received VTE PX. This study suggests that in hospital systems similar to ours with 30% or more of hospital admissions coming from the ED implementing a standard order set for patients admitted through the ED may increase VTE PX, which, in turn, could have a major impact on their course. Future studies need to determine the best way to implement these changes.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 (prepared by the University of California at San Francisco–Stanford Evidence‐Based Practice Center under Contract No. 290‐97‐0013), AHRQ Publication No. 01‐E058,Rockville, MD:Agency for Healthcare Research and Quality; July2001.
- ,,, et al.Prevention of venous thromboembolism.Chest.2004;126:338S–400S.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for pulmonary embolism in chronic dialysis patients.J Nephrol.2002;15:241–247.
- ,.Thrombosis in end‐stage renal disease.Sem Dialysis.2003;16:245–256.
- ,,.Venous thromboembolism in end‐stage renal disease.Am J Kidney Dis.2000;36:405–411.
- ,,.Pulmonary embolism in end stage renal disease.Intensive Care Med.1990;16:405–407.
- ,,,.Deep vein thrombosis in end‐stage renal disease.ASAIO J.1994;40:103–105.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Sem Hematol.2001;38(suppl 5):12–19.
- ,,,,,.Influence of positive airway pressure on the pressure gradient for venous return in humans.J Appl Physiol.2000;88:926–932.
- ,,,,,.Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis.Crit Care Med.2002;30:771–774.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,,.Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study.Arch Intern Med.1998;158:585–593.
- .Venous Thromboembolism epidemiology: implications for prevention and management.Semin Thromb Hemost.2002;28(suppl 2):3–13.
- .Major Pulmonary embolism.Chest.2002;121:877–905.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341:793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- ,,.New onset venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment.Chest.2000;118:1680–1684.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Thrombosis.Haematologica.2002;87:746–750.
- ,,,,.Underuse of venous thromboembolism prophylaxis for general surgery patients.Arch Intern Med.1998;158:1909–1912.
- ,,, et al.Deep vein thrombosis in elderly patients hospitalized in subacute care facilities.Arch Intern Med.2003;163:2613–2618.
- ,.A prospective registry of 5451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- ,,.Acute pulmonary embolism: don't ignore the platelet.Circulation.2002;106:1748–1749.
- Collaborative overview of randomized trials of antiplatelet therapy—III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients.BMJ.1994;308:235–246.
- ,,,,,.Changing clinical practice. Prospective study of the impact of the continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,, et al.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,,.Reduction of incorrect antibiotic dosing through a structured educational order form.Arch Intern Med.1988;148:1720–1724.
- ,.The use of an antibiotic order form for antibiotic utilization review: influence on physicians' prescribing patterns.J Infect Dis.1984;150:803–807.
- ,,, et al.Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey.Crit Care.2001;5:336–342.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
Copyright © 2007 Society of Hospital Medicine
Stress‐Ulcer Prophylaxis for General Medical Patients
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).
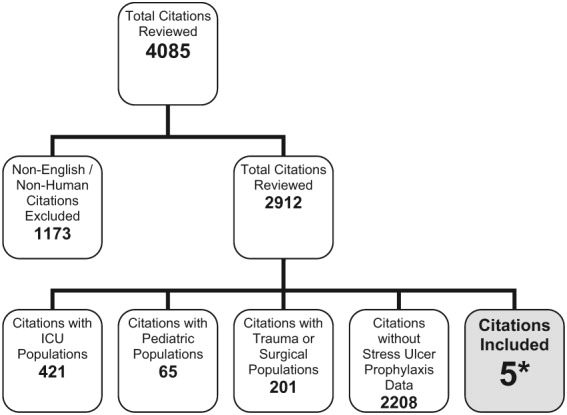
Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).

Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).

Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.
Editorial
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.