User login
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES
Meet the new SHM president: Dr. Danielle Scheurer
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at ideas@hospitalmedicine.org.
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at ideas@hospitalmedicine.org.
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at ideas@hospitalmedicine.org.
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
Rethink urologic cancer treatment in the era of COVID-19
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.
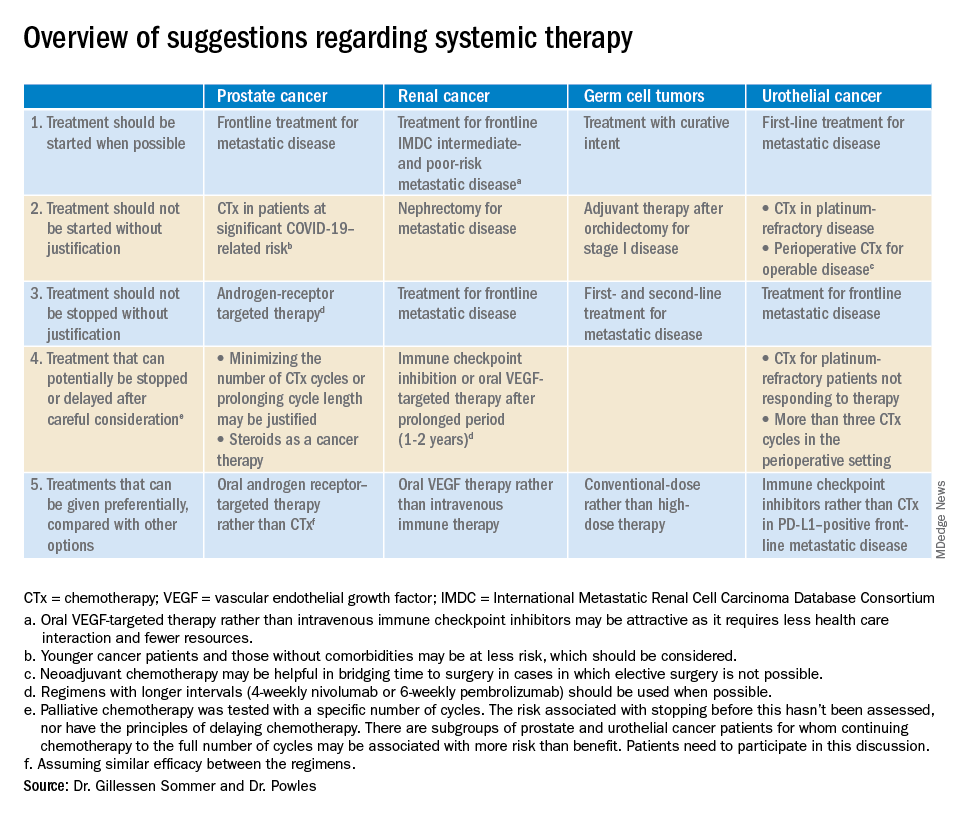
The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.

The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.

The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
FROM EUROPEAN UROLOGY
HM20 canceled: SHM explains why
COVID-19 made holding meeting impossible
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact meetings@hospitalmedicine.org.
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
COVID-19 made holding meeting impossible
COVID-19 made holding meeting impossible
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact meetings@hospitalmedicine.org.
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact meetings@hospitalmedicine.org.
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
High and low trauma yield similar future osteoporotic fracture risk
Average measures of bone mineral density were similar for individuals with high-trauma and low-trauma fractures, and both were significantly distinct from those with no fracture history, based on data from a cohort study of adults aged 40 years and older.
In the past, low-trauma fractures have typically been associated with osteoporosis, wrote William D. Leslie, MD, of the University of Manitoba, Canada, and his colleagues. However, features distinguishing between low- and high-trauma fractures are often arbitrary and “empirical data have questioned whether distinguishing low-trauma from high-trauma fractures is clinically useful for purposes of risk assessment and treatment,” they wrote.
In a study published in Osteoporosis International, the researchers reviewed data from 64,626 individuals with no prior fracture, 858 with high-trauma fractures, and 14,758 with low-trauma fractures. Overall, the average BMD Z-scores for individuals with no previous fracture were slightly positive, while those with either a high-trauma or low-trauma fracture were negative. The scores for individuals with high-trauma fractures or major osteoporotic fractures were similar to those with low-trauma fractures, and significantly lower (P less than .001) than among individuals with no prior fractures.
The study population included adults aged 40 years and older with baseline DXA scans between Jan. 1, 1996, and Mar. 31, 2016. Those with high-trauma fractures were younger than those with low-trauma fractures (65 years vs. 67 years), and fewer individuals with high-trauma fractures were women (77% vs. 87%).
Both high-trauma and low-trauma fractures were similarly and significantly associated with increased risk for incident major osteoporotic fractures (adjusted hazard ratios 1.31 and 1.55, respectively).
The study findings were limited by several factors including incomplete data on external injury codes, the retrospective study design, and the lack of analysis of the time since prior fractures, the researchers noted. However, the results were strengthened by the large sample size, long-term follow-up, and large numbers of incident fractures, they wrote.
The results support data from previous studies and support “the inclusion of high-trauma clinical fractures in clinical assessment for underlying osteoporosis and in the evaluation for intervention to reduce future fracture risk,” they wrote.
In an accompanying editorial, Steven R. Cummings, MD, of California Pacific Medical Center Research Institute, San Francisco, and Richard Eastell, MD, of the University of Sheffield, England, wrote that the practice of rating fractures according to degree of trauma should be eliminated.
“The study adds evidence to the case that it is time to abandon the mistaken beliefs that fractures rated as high trauma are not associated with decreased BMD, indicate no higher risk of subsequent fracture, or are less likely to be prevented by treatments for osteoporosis,” they wrote.
Describing some fractures as due to trauma reinforces the mistaken belief that the fractures are simply due to the trauma, not decreased bone strength, they noted.
“Indeed, we recommend that people stop attempting to rate or record degree of trauma because such ratings are at best inaccurate and would promote the continued neglect of those patients who are misclassified as having fractures that do not warrant evaluation and treatment,” they concluded.
The study received no outside funding. Dr. Leslie, the study’s first author, reported having no financial conflicts to disclose.
Dr. Cummings disclosed consultancy and grant funding from Amgen and Radius. Dr. Eastell disclosed consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, and Haoma Medica and grant funding from Nittobo, IDS, Roche, Amgen, and Alexion.
SOURCE: Leslie WD et al. Osteroporos Int. 2020 Mar 16. doi: 10.1007/s00198-019-05274-2.
Average measures of bone mineral density were similar for individuals with high-trauma and low-trauma fractures, and both were significantly distinct from those with no fracture history, based on data from a cohort study of adults aged 40 years and older.
In the past, low-trauma fractures have typically been associated with osteoporosis, wrote William D. Leslie, MD, of the University of Manitoba, Canada, and his colleagues. However, features distinguishing between low- and high-trauma fractures are often arbitrary and “empirical data have questioned whether distinguishing low-trauma from high-trauma fractures is clinically useful for purposes of risk assessment and treatment,” they wrote.
In a study published in Osteoporosis International, the researchers reviewed data from 64,626 individuals with no prior fracture, 858 with high-trauma fractures, and 14,758 with low-trauma fractures. Overall, the average BMD Z-scores for individuals with no previous fracture were slightly positive, while those with either a high-trauma or low-trauma fracture were negative. The scores for individuals with high-trauma fractures or major osteoporotic fractures were similar to those with low-trauma fractures, and significantly lower (P less than .001) than among individuals with no prior fractures.
The study population included adults aged 40 years and older with baseline DXA scans between Jan. 1, 1996, and Mar. 31, 2016. Those with high-trauma fractures were younger than those with low-trauma fractures (65 years vs. 67 years), and fewer individuals with high-trauma fractures were women (77% vs. 87%).
Both high-trauma and low-trauma fractures were similarly and significantly associated with increased risk for incident major osteoporotic fractures (adjusted hazard ratios 1.31 and 1.55, respectively).
The study findings were limited by several factors including incomplete data on external injury codes, the retrospective study design, and the lack of analysis of the time since prior fractures, the researchers noted. However, the results were strengthened by the large sample size, long-term follow-up, and large numbers of incident fractures, they wrote.
The results support data from previous studies and support “the inclusion of high-trauma clinical fractures in clinical assessment for underlying osteoporosis and in the evaluation for intervention to reduce future fracture risk,” they wrote.
In an accompanying editorial, Steven R. Cummings, MD, of California Pacific Medical Center Research Institute, San Francisco, and Richard Eastell, MD, of the University of Sheffield, England, wrote that the practice of rating fractures according to degree of trauma should be eliminated.
“The study adds evidence to the case that it is time to abandon the mistaken beliefs that fractures rated as high trauma are not associated with decreased BMD, indicate no higher risk of subsequent fracture, or are less likely to be prevented by treatments for osteoporosis,” they wrote.
Describing some fractures as due to trauma reinforces the mistaken belief that the fractures are simply due to the trauma, not decreased bone strength, they noted.
“Indeed, we recommend that people stop attempting to rate or record degree of trauma because such ratings are at best inaccurate and would promote the continued neglect of those patients who are misclassified as having fractures that do not warrant evaluation and treatment,” they concluded.
The study received no outside funding. Dr. Leslie, the study’s first author, reported having no financial conflicts to disclose.
Dr. Cummings disclosed consultancy and grant funding from Amgen and Radius. Dr. Eastell disclosed consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, and Haoma Medica and grant funding from Nittobo, IDS, Roche, Amgen, and Alexion.
SOURCE: Leslie WD et al. Osteroporos Int. 2020 Mar 16. doi: 10.1007/s00198-019-05274-2.
Average measures of bone mineral density were similar for individuals with high-trauma and low-trauma fractures, and both were significantly distinct from those with no fracture history, based on data from a cohort study of adults aged 40 years and older.
In the past, low-trauma fractures have typically been associated with osteoporosis, wrote William D. Leslie, MD, of the University of Manitoba, Canada, and his colleagues. However, features distinguishing between low- and high-trauma fractures are often arbitrary and “empirical data have questioned whether distinguishing low-trauma from high-trauma fractures is clinically useful for purposes of risk assessment and treatment,” they wrote.
In a study published in Osteoporosis International, the researchers reviewed data from 64,626 individuals with no prior fracture, 858 with high-trauma fractures, and 14,758 with low-trauma fractures. Overall, the average BMD Z-scores for individuals with no previous fracture were slightly positive, while those with either a high-trauma or low-trauma fracture were negative. The scores for individuals with high-trauma fractures or major osteoporotic fractures were similar to those with low-trauma fractures, and significantly lower (P less than .001) than among individuals with no prior fractures.
The study population included adults aged 40 years and older with baseline DXA scans between Jan. 1, 1996, and Mar. 31, 2016. Those with high-trauma fractures were younger than those with low-trauma fractures (65 years vs. 67 years), and fewer individuals with high-trauma fractures were women (77% vs. 87%).
Both high-trauma and low-trauma fractures were similarly and significantly associated with increased risk for incident major osteoporotic fractures (adjusted hazard ratios 1.31 and 1.55, respectively).
The study findings were limited by several factors including incomplete data on external injury codes, the retrospective study design, and the lack of analysis of the time since prior fractures, the researchers noted. However, the results were strengthened by the large sample size, long-term follow-up, and large numbers of incident fractures, they wrote.
The results support data from previous studies and support “the inclusion of high-trauma clinical fractures in clinical assessment for underlying osteoporosis and in the evaluation for intervention to reduce future fracture risk,” they wrote.
In an accompanying editorial, Steven R. Cummings, MD, of California Pacific Medical Center Research Institute, San Francisco, and Richard Eastell, MD, of the University of Sheffield, England, wrote that the practice of rating fractures according to degree of trauma should be eliminated.
“The study adds evidence to the case that it is time to abandon the mistaken beliefs that fractures rated as high trauma are not associated with decreased BMD, indicate no higher risk of subsequent fracture, or are less likely to be prevented by treatments for osteoporosis,” they wrote.
Describing some fractures as due to trauma reinforces the mistaken belief that the fractures are simply due to the trauma, not decreased bone strength, they noted.
“Indeed, we recommend that people stop attempting to rate or record degree of trauma because such ratings are at best inaccurate and would promote the continued neglect of those patients who are misclassified as having fractures that do not warrant evaluation and treatment,” they concluded.
The study received no outside funding. Dr. Leslie, the study’s first author, reported having no financial conflicts to disclose.
Dr. Cummings disclosed consultancy and grant funding from Amgen and Radius. Dr. Eastell disclosed consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, and Haoma Medica and grant funding from Nittobo, IDS, Roche, Amgen, and Alexion.
SOURCE: Leslie WD et al. Osteroporos Int. 2020 Mar 16. doi: 10.1007/s00198-019-05274-2.
FROM OSTEOPOROSIS INTERNATIONAL
FDA grants emergency authorization for first rapid antibody test for COVID-19
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
Survey: COVID-19 is getting in our heads
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
Virtual Dermatology: A COVID-19 Update
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.
Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
Translucent Periorbital Papules
The Diagnosis: Apocrine Hidrocystoma
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.
Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
The Diagnosis: Apocrine Hidrocystoma
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.


Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
The Diagnosis: Apocrine Hidrocystoma
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.


Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
A 40-year-old woman was referred to dermatology for evaluation of occasionally pruritic periorbital papules that had gradually increased in size and number over the last 7 to 8 months (top). She had a similar solitary lesion on the left lower eyelid that was removed twice: 10 years and 10 months prior. She was taking an oral contraceptive (desogestrel) but otherwise had no notable medical history or drug allergies. Physical examination revealed individual and clustered translucent papules along the eyelid margins, left medial canthus, and both lateral canthi (bottom).