User login
Emerging Noninvasive Treatments of Nonmelanoma Skin Cancers
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
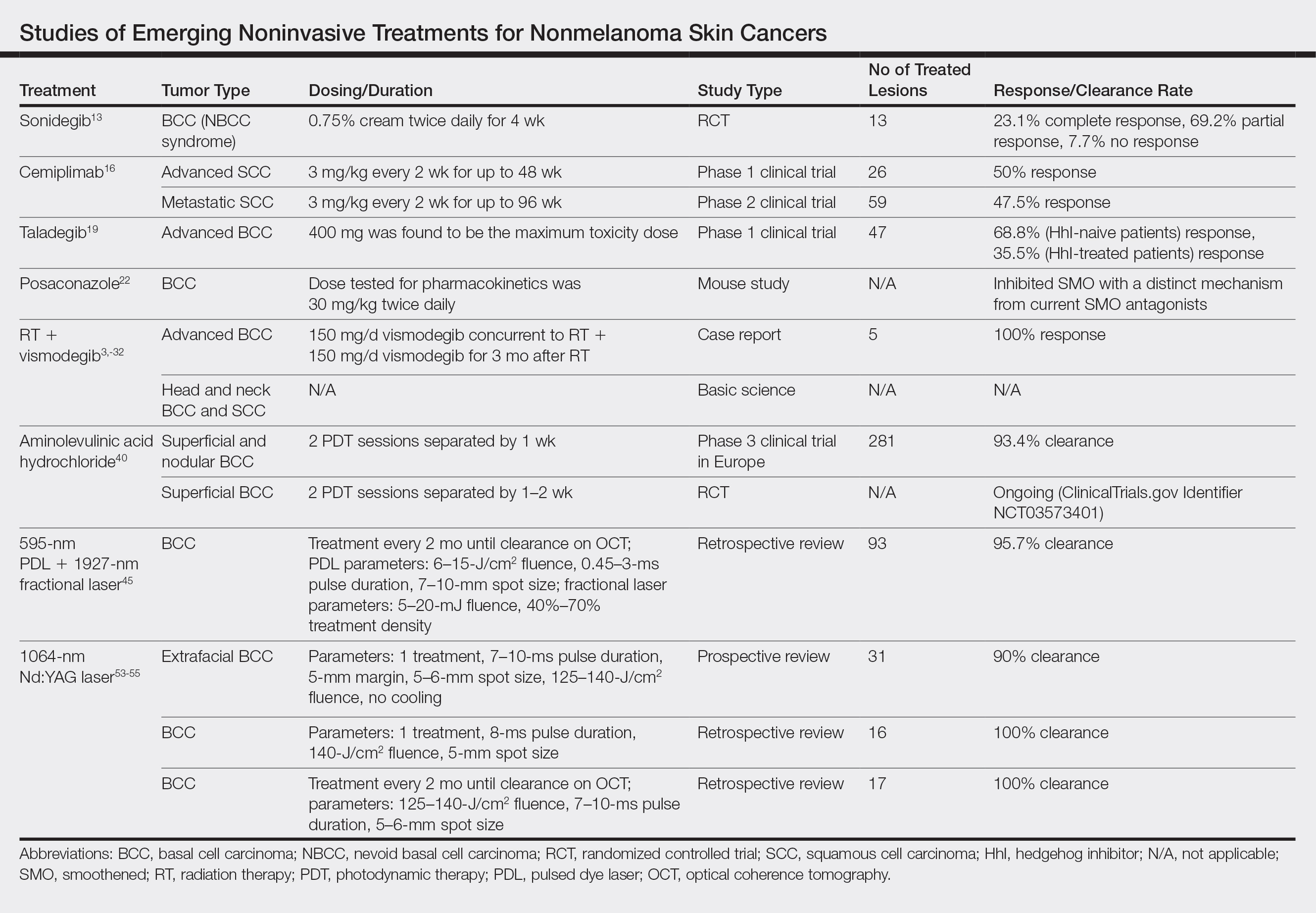
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.
Breach of migrant youths’ confidentiality is unethical, unacceptable
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
Pediatric Dermatology Emergencies
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Practice Points
- Staphylococcal scalded skin syndrome, impetigo, eczema herpeticum, Langerhans cell histiocytosis, infantile hemangiomas, and IgA vasculitis all present potential emergencies in pediatric patients in dermatologic settings.
- Early and accurate identification and management of these entities is critical to avoid short-term and long-term negative sequalae.
What’s Eating You? Human Body Lice (Pediculus humanus corporis)
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Practice Points
- Body lice reside in clothing, particularly folds and seams, and migrate to the host for blood meals. To evaluate for infestation, the clinician should not only look at the skin but also closely examine the patient’s clothing. Clothes also are a target for treatment via washing in hot water.
- Due to observed and theoretical adverse effects of other chemical treatments, benzyl alcohol is the authors’ choice for treatment of head lice.
- Oral ivermectin is a promising future treatment for body lice.
Emollients didn’t prevent atopic dermatitis in high-risk infants
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
FROM THE LANCET
Nail growth
The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.
The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.
Loneliness, social isolation in seniors need urgent attention
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
Combined biopsy method improves diagnostic accuracy in prostate cancer
among men with MRI-visible lesions in a single-center study.
Compared with either method alone, a combination of the two biopsy methods resulted in 9.9% more prostate cancer diagnoses, explained study author Michael Ahdoot, MD, of the National Institutes of Health and colleagues. Their report was published in the New England Journal of Medicine.
“With the addition of MRI-targeted biopsy to systematic biopsy, we may have entered an era of increased diagnostic certainty in prostate cancer,” the researchers wrote.
Their single-center, comparative diagnostic study included 2,103 patients with MRI-visible prostate lesions who underwent both systematic and MRI-targeted biopsy. In cases of multiple biopsies, only the results of the initial biopsies were included.
Each individual specimen was assigned a Gleason score by a genitourinary pathologist and was subsequently categorized into a grade group on a scale of 1-5, with higher scores reflecting greater cancer risk. Grade group 1 was defined as clinically insignificant disease. Grade group 2 was defined as favorable intermediate-risk disease. Grade group 3 or higher was defined as unfavorable intermediate-risk disease or worse.
The primary endpoints were cancer detection rates for each biopsy method, based on grade group. “Among the men who underwent subsequent radical prostatectomy, upgrading and downgrading of grade group from biopsy to whole-mount histopathological analysis of surgical specimens [was also assessed],” the researchers explained.
Among patients who underwent combined biopsy, prostate cancer was identified in 62.4% of patients, and 19.2% underwent radical prostatectomy.
For grade groups 3-5, rates of cancer detection were significantly higher with MRI-targeted biopsy than with systematic biopsy (P less than .01 for all). For grade group 1, detection rates were significantly lower with MRI-targeted biopsy (P less than .01).
“Although many of [the] benefits resulted from MRI-targeted biopsy alone, omission of systematic biopsy would have led to missing the diagnosis of 8.8% of clinically significant cancers,” the researchers reported.
In addition, among patients who underwent radical prostatectomy, the rates of upgrading (grade group 3 or higher) on histopathological analysis were lower for combined biopsy (3.5%) than for MRI-targeted biopsy (8.7%) and systematic biopsy (16.8%).
The researchers acknowledged that a key limitation of this study was the single-center design. As a result, the findings may not be generalizable to other institutions.
However, the researchers concluded that “these findings suggest that combined biopsy provides improved diagnostic accuracy over either systematic or MRI-targeted biopsy alone and better predicts the results of final histopathological analysis.”
The study was funded by the National Institutes of Health, Philips, and the Dr. Mildred Scheel Foundation for Cancer Research. The authors disclosed financial affiliations with Philips, Biocompatibles UK, Boston Scientific, Celsion, and other companies.
SOURCE: Ahdoot M et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1910038.
among men with MRI-visible lesions in a single-center study.
Compared with either method alone, a combination of the two biopsy methods resulted in 9.9% more prostate cancer diagnoses, explained study author Michael Ahdoot, MD, of the National Institutes of Health and colleagues. Their report was published in the New England Journal of Medicine.
“With the addition of MRI-targeted biopsy to systematic biopsy, we may have entered an era of increased diagnostic certainty in prostate cancer,” the researchers wrote.
Their single-center, comparative diagnostic study included 2,103 patients with MRI-visible prostate lesions who underwent both systematic and MRI-targeted biopsy. In cases of multiple biopsies, only the results of the initial biopsies were included.
Each individual specimen was assigned a Gleason score by a genitourinary pathologist and was subsequently categorized into a grade group on a scale of 1-5, with higher scores reflecting greater cancer risk. Grade group 1 was defined as clinically insignificant disease. Grade group 2 was defined as favorable intermediate-risk disease. Grade group 3 or higher was defined as unfavorable intermediate-risk disease or worse.
The primary endpoints were cancer detection rates for each biopsy method, based on grade group. “Among the men who underwent subsequent radical prostatectomy, upgrading and downgrading of grade group from biopsy to whole-mount histopathological analysis of surgical specimens [was also assessed],” the researchers explained.
Among patients who underwent combined biopsy, prostate cancer was identified in 62.4% of patients, and 19.2% underwent radical prostatectomy.
For grade groups 3-5, rates of cancer detection were significantly higher with MRI-targeted biopsy than with systematic biopsy (P less than .01 for all). For grade group 1, detection rates were significantly lower with MRI-targeted biopsy (P less than .01).
“Although many of [the] benefits resulted from MRI-targeted biopsy alone, omission of systematic biopsy would have led to missing the diagnosis of 8.8% of clinically significant cancers,” the researchers reported.
In addition, among patients who underwent radical prostatectomy, the rates of upgrading (grade group 3 or higher) on histopathological analysis were lower for combined biopsy (3.5%) than for MRI-targeted biopsy (8.7%) and systematic biopsy (16.8%).
The researchers acknowledged that a key limitation of this study was the single-center design. As a result, the findings may not be generalizable to other institutions.
However, the researchers concluded that “these findings suggest that combined biopsy provides improved diagnostic accuracy over either systematic or MRI-targeted biopsy alone and better predicts the results of final histopathological analysis.”
The study was funded by the National Institutes of Health, Philips, and the Dr. Mildred Scheel Foundation for Cancer Research. The authors disclosed financial affiliations with Philips, Biocompatibles UK, Boston Scientific, Celsion, and other companies.
SOURCE: Ahdoot M et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1910038.
among men with MRI-visible lesions in a single-center study.
Compared with either method alone, a combination of the two biopsy methods resulted in 9.9% more prostate cancer diagnoses, explained study author Michael Ahdoot, MD, of the National Institutes of Health and colleagues. Their report was published in the New England Journal of Medicine.
“With the addition of MRI-targeted biopsy to systematic biopsy, we may have entered an era of increased diagnostic certainty in prostate cancer,” the researchers wrote.
Their single-center, comparative diagnostic study included 2,103 patients with MRI-visible prostate lesions who underwent both systematic and MRI-targeted biopsy. In cases of multiple biopsies, only the results of the initial biopsies were included.
Each individual specimen was assigned a Gleason score by a genitourinary pathologist and was subsequently categorized into a grade group on a scale of 1-5, with higher scores reflecting greater cancer risk. Grade group 1 was defined as clinically insignificant disease. Grade group 2 was defined as favorable intermediate-risk disease. Grade group 3 or higher was defined as unfavorable intermediate-risk disease or worse.
The primary endpoints were cancer detection rates for each biopsy method, based on grade group. “Among the men who underwent subsequent radical prostatectomy, upgrading and downgrading of grade group from biopsy to whole-mount histopathological analysis of surgical specimens [was also assessed],” the researchers explained.
Among patients who underwent combined biopsy, prostate cancer was identified in 62.4% of patients, and 19.2% underwent radical prostatectomy.
For grade groups 3-5, rates of cancer detection were significantly higher with MRI-targeted biopsy than with systematic biopsy (P less than .01 for all). For grade group 1, detection rates were significantly lower with MRI-targeted biopsy (P less than .01).
“Although many of [the] benefits resulted from MRI-targeted biopsy alone, omission of systematic biopsy would have led to missing the diagnosis of 8.8% of clinically significant cancers,” the researchers reported.
In addition, among patients who underwent radical prostatectomy, the rates of upgrading (grade group 3 or higher) on histopathological analysis were lower for combined biopsy (3.5%) than for MRI-targeted biopsy (8.7%) and systematic biopsy (16.8%).
The researchers acknowledged that a key limitation of this study was the single-center design. As a result, the findings may not be generalizable to other institutions.
However, the researchers concluded that “these findings suggest that combined biopsy provides improved diagnostic accuracy over either systematic or MRI-targeted biopsy alone and better predicts the results of final histopathological analysis.”
The study was funded by the National Institutes of Health, Philips, and the Dr. Mildred Scheel Foundation for Cancer Research. The authors disclosed financial affiliations with Philips, Biocompatibles UK, Boston Scientific, Celsion, and other companies.
SOURCE: Ahdoot M et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1910038.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Combining magnetic resonance imaging (MRI)–targeted and systematic biopsy improved detection of prostate cancer in patients with MRI-visible lesions.
Major finding: When compared with either method alone, combining the methods resulted in 9.9% more prostate cancer diagnoses.
Study details: A comparative diagnostic study of 2,103 men with MRI-visible prostate lesions.
Disclosures: The study was funded by the National Institutes of Health, Philips, and the Dr. Mildred Scheel Foundation for Cancer Research. The authors disclosed financial affiliations with Philips, Biocompatibles UK, Boston Scientific, Celsion, and other companies.
Source: Ahdoot M et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1910038.
Survey: 2020 will see more attacks on ACA
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
Is telerheumatology the future of rheumatology?
MAUI, HAWAII – Alvin F. Wells, MD, PhD, believes he’s seen the future of rheumatology. So he’s taken a deep dive into telerheumatology, going all in.
“Whether you’re in academic, private, or hospital-based practice, in 2020 if you are not thinking about telerheumatology, you and your practice will not be able to compete with growing patient demands, expectations, and need for clinical monitoring. If you do not have a digital/virtual strategy, you do not have a health care strategy,” he asserted at the 2020 Rheumatology Winter Clinical Symposium.
“Begin now,” the rheumatologist advised.
In pursuit of his own telerheumatology strategy, he holds licenses to practice medicine in five states and has licensure pending in five others.
“My goal is to cover 20% of the U.S., so if the local guys can’t see the patients, I can see them virtually,” he explained. “The days of waiting 4-6 months to be seen by a rheumatologist are gone.”
Rheumatologists are already in short supply in most of the country, and a major shortage looms ahead as older practitioners retire. Telerheumatology can help fill that unmet need. But the specialty is behind the curve. In a survey that rated the medical specialties most engaged in telemedicine, the top three spots were held by radiology, psychiatry, and internal medicine. Rheumatology didn’t even crack the top 10, noted Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., and a part-time faculty member at Duke University, the Medical College of Wisconsin, and the Karolinska Institute.
Yet telemedicine is primed for rheumatologic takeoff. Notably, the 2019 update of the American College of Rheumatology recommendations on rheumatoid arthritis disease activity measures incorporates the RAPID3 (Routine Assessment of Patient Index Data) as an endorsed three-question clinical assessment that doesn’t involve a physical exam or laboratory work. The ACR update is recognition that, while every rheumatology patient needs an initial physical exam along with follow-up physical exams at various rates, many patients with well-controlled disease don’t need a physical exam at every physician encounter, he said.
Telerheumatology saves time for both patient and physician. The patient saves travel time, doesn’t miss work, avoids having to arrange for child care in order to make a face-to-face clinic visit, and can schedule more frequent virtual follow-up visits. For the practitioner, telerheumatology means additional consults and – here’s the big one – “You never run behind,” according to Dr. Wells. “For a 15-minute appointment, the patient gets a 5-minute warning, then a 2-minute warning, and at 15 minutes the link is cut. If the fibromyalgia patients want 30 minutes, they pay for 30 minutes.”
He sees the strictly enforced, impersonally delivered electronic time limits as key to running an efficient practice.
“The patients with osteoarthritis who hate the nodules, the fibromyalgia patients because they’re hurting all over, the patients with back pain – you’ve really got to limit those patients because otherwise you’ll be running 30-40 minutes behind for a scheduled 15-minute visit,” he explained.
One rheumatologist’s telemedicine practice
Dr. Wells currently utilizes the Epic electronic health record integrated with a Zoom videoconferencing platform for real-time virtual patient encounters. But he noted that other virtual platforms are available, including Health Tap, American Well, MySpecialistMD, MDLIVE, and TelaDoc. The American Telemedicine Association is a valuable resource for state-by-state medicolegal, reimbursement, and how-to-do-it questions.
At present, he reserves two daily time slots for telerheumatology: one at 8:30-9:00 a.m., the other at 4:30-5:00 p.m. These can be filled with four 15-minute live consults or two 30-minute consults. His goal is to eventually make telerheumatology 20% of his patient load of about 100 patients per week.
His typical 15-minute virtual visit proceeds as follows: It begins with a 3-minute subjective patient assessment, followed by a 5-minute objective assessment which includes the RAPID3, a brief Health Assessment Questionnaire (HAQ) addressing the patient’s pain and overall satisfaction, a virtual joint inspection, the use of high-quality teleultrasound and other technology when warranted, and capture of relevant still photos. This is followed by 5 minutes to relay the treatment plan, and finally a 2-minute recap and summary.
“No niceties. We cut right to the chase,” he noted.
He documents the patient encounter as he goes, dictating his notes throughout the visit.
“When I walk out of the room, I’m done. It’s on to the next patient,” Dr. Wells said.
The reimbursement picture is improving, although major hurdles remain. At present, 48 states and the District of Columbia reimburse for live video telemedicine through Medicaid. And in January 2020, Aetna announced it covers reimbursement for telemedicine in all of its fully insured health plans via the Teladoc platform. Dr. Wells’ patients pay for their telerheumatology out of pocket if their insurance doesn’t cover it.
Telemedicine caveats
Dr. Wells shared his telerheumatology experience as the first half of a point/counterpoint session on telemedicine’s future in the specialty. His debate opponent, Orrin M. Troum, MD, announced at the outset that he is quite interested in getting into telerheumatology; however, while looking into it he has come across issues that for now give him pause and that other rheumatologists need to be aware of.
Legal risks. The telemedicine movement has gotten big enough to draw the scrutiny of federal prosecutors and regulatory enforcement officials. In April 2018, the Department of Health & Human Services Office of the Inspector General (OIG) issued a report that concluded that one-third of all examined telemedicine claims were improper.
“Just imagine who might come knocking on your door,” he said.
Among the most common offenses, according to the OIG, were claims for services outside the limited range currently covered; lack of the requisite HIPAA-compliant two-way audio and visual communication technology with fully encrypted data transmission; services billed by institutional providers not defined by Medicare as telemedicine-eligible; and claims for services received by patients who weren’t located in an officially designated Health Professional Shortage Area or in a rural county as determined by the U.S. Census Bureau.
Telemedicine is no panacea for out-of-control health care costs. A RAND study of participants in the California Public Employees’ Retirement System (CalPERS) concluded that only 12% of beneficiaries who used direct-to-consumer telemedicine did so to replace provider visits. The other 88% added on telemedicine as an additional service. So while telemedicine increased patient access to health care, it also increased the overall cost, observed Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, and in private practice in Santa Monica, Calif.
Talk to your attorney and malpractice insurer before embarking on telerheumatology. Physicians could potentially lose their medical malpractice insurance if they use telemedicine to treat patients located in states where they aren’t licensed to practice, even if through inadvertent error.
Telemedicine isn’t appropriate for all patients. Nearly a decade ago, rheumatologists at Dartmouth-Hitchcock Medical Center launched a telerheumatology service in order to bring specialty care to the largely rural populations of New Hampshire and Vermont. In a review of the experience that included interviews with both patients and providers, investigators concluded that telerheumatology successfully increased access to specialty care in underserved locations and got good satisfaction scores from both providers and beneficiaries. However, fully 19% of patients were found to be inappropriate for their telerheumatology visit, mainly because their disease was too complex or the underlying diagnosis was unclear.
“Almost one-fifth of their patients were inappropriate for telerheumatology. The question is, how are you supposed to know that ahead of time?” Dr. Troum asked.
Patient satisfaction. Dr. Troum’s reading of the literature on patient satisfaction with telerheumatology, coupled with his own extensive experience in clinical practice, makes him think that many of his younger patients with less disease activity might welcome a telerheumatology option, even with strict time boundaries. But his older patients with more disease activity are a different story.
“Typically my middle-aged and older patients won’t accept that without a lot of convincing,” he commented.
Dr. Wells and Dr. Troum had no relevant disclosures regarding their presentations.
MAUI, HAWAII – Alvin F. Wells, MD, PhD, believes he’s seen the future of rheumatology. So he’s taken a deep dive into telerheumatology, going all in.
“Whether you’re in academic, private, or hospital-based practice, in 2020 if you are not thinking about telerheumatology, you and your practice will not be able to compete with growing patient demands, expectations, and need for clinical monitoring. If you do not have a digital/virtual strategy, you do not have a health care strategy,” he asserted at the 2020 Rheumatology Winter Clinical Symposium.
“Begin now,” the rheumatologist advised.
In pursuit of his own telerheumatology strategy, he holds licenses to practice medicine in five states and has licensure pending in five others.
“My goal is to cover 20% of the U.S., so if the local guys can’t see the patients, I can see them virtually,” he explained. “The days of waiting 4-6 months to be seen by a rheumatologist are gone.”
Rheumatologists are already in short supply in most of the country, and a major shortage looms ahead as older practitioners retire. Telerheumatology can help fill that unmet need. But the specialty is behind the curve. In a survey that rated the medical specialties most engaged in telemedicine, the top three spots were held by radiology, psychiatry, and internal medicine. Rheumatology didn’t even crack the top 10, noted Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., and a part-time faculty member at Duke University, the Medical College of Wisconsin, and the Karolinska Institute.
Yet telemedicine is primed for rheumatologic takeoff. Notably, the 2019 update of the American College of Rheumatology recommendations on rheumatoid arthritis disease activity measures incorporates the RAPID3 (Routine Assessment of Patient Index Data) as an endorsed three-question clinical assessment that doesn’t involve a physical exam or laboratory work. The ACR update is recognition that, while every rheumatology patient needs an initial physical exam along with follow-up physical exams at various rates, many patients with well-controlled disease don’t need a physical exam at every physician encounter, he said.
Telerheumatology saves time for both patient and physician. The patient saves travel time, doesn’t miss work, avoids having to arrange for child care in order to make a face-to-face clinic visit, and can schedule more frequent virtual follow-up visits. For the practitioner, telerheumatology means additional consults and – here’s the big one – “You never run behind,” according to Dr. Wells. “For a 15-minute appointment, the patient gets a 5-minute warning, then a 2-minute warning, and at 15 minutes the link is cut. If the fibromyalgia patients want 30 minutes, they pay for 30 minutes.”
He sees the strictly enforced, impersonally delivered electronic time limits as key to running an efficient practice.
“The patients with osteoarthritis who hate the nodules, the fibromyalgia patients because they’re hurting all over, the patients with back pain – you’ve really got to limit those patients because otherwise you’ll be running 30-40 minutes behind for a scheduled 15-minute visit,” he explained.
One rheumatologist’s telemedicine practice
Dr. Wells currently utilizes the Epic electronic health record integrated with a Zoom videoconferencing platform for real-time virtual patient encounters. But he noted that other virtual platforms are available, including Health Tap, American Well, MySpecialistMD, MDLIVE, and TelaDoc. The American Telemedicine Association is a valuable resource for state-by-state medicolegal, reimbursement, and how-to-do-it questions.
At present, he reserves two daily time slots for telerheumatology: one at 8:30-9:00 a.m., the other at 4:30-5:00 p.m. These can be filled with four 15-minute live consults or two 30-minute consults. His goal is to eventually make telerheumatology 20% of his patient load of about 100 patients per week.
His typical 15-minute virtual visit proceeds as follows: It begins with a 3-minute subjective patient assessment, followed by a 5-minute objective assessment which includes the RAPID3, a brief Health Assessment Questionnaire (HAQ) addressing the patient’s pain and overall satisfaction, a virtual joint inspection, the use of high-quality teleultrasound and other technology when warranted, and capture of relevant still photos. This is followed by 5 minutes to relay the treatment plan, and finally a 2-minute recap and summary.
“No niceties. We cut right to the chase,” he noted.
He documents the patient encounter as he goes, dictating his notes throughout the visit.
“When I walk out of the room, I’m done. It’s on to the next patient,” Dr. Wells said.
The reimbursement picture is improving, although major hurdles remain. At present, 48 states and the District of Columbia reimburse for live video telemedicine through Medicaid. And in January 2020, Aetna announced it covers reimbursement for telemedicine in all of its fully insured health plans via the Teladoc platform. Dr. Wells’ patients pay for their telerheumatology out of pocket if their insurance doesn’t cover it.
Telemedicine caveats
Dr. Wells shared his telerheumatology experience as the first half of a point/counterpoint session on telemedicine’s future in the specialty. His debate opponent, Orrin M. Troum, MD, announced at the outset that he is quite interested in getting into telerheumatology; however, while looking into it he has come across issues that for now give him pause and that other rheumatologists need to be aware of.
Legal risks. The telemedicine movement has gotten big enough to draw the scrutiny of federal prosecutors and regulatory enforcement officials. In April 2018, the Department of Health & Human Services Office of the Inspector General (OIG) issued a report that concluded that one-third of all examined telemedicine claims were improper.
“Just imagine who might come knocking on your door,” he said.
Among the most common offenses, according to the OIG, were claims for services outside the limited range currently covered; lack of the requisite HIPAA-compliant two-way audio and visual communication technology with fully encrypted data transmission; services billed by institutional providers not defined by Medicare as telemedicine-eligible; and claims for services received by patients who weren’t located in an officially designated Health Professional Shortage Area or in a rural county as determined by the U.S. Census Bureau.
Telemedicine is no panacea for out-of-control health care costs. A RAND study of participants in the California Public Employees’ Retirement System (CalPERS) concluded that only 12% of beneficiaries who used direct-to-consumer telemedicine did so to replace provider visits. The other 88% added on telemedicine as an additional service. So while telemedicine increased patient access to health care, it also increased the overall cost, observed Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, and in private practice in Santa Monica, Calif.
Talk to your attorney and malpractice insurer before embarking on telerheumatology. Physicians could potentially lose their medical malpractice insurance if they use telemedicine to treat patients located in states where they aren’t licensed to practice, even if through inadvertent error.
Telemedicine isn’t appropriate for all patients. Nearly a decade ago, rheumatologists at Dartmouth-Hitchcock Medical Center launched a telerheumatology service in order to bring specialty care to the largely rural populations of New Hampshire and Vermont. In a review of the experience that included interviews with both patients and providers, investigators concluded that telerheumatology successfully increased access to specialty care in underserved locations and got good satisfaction scores from both providers and beneficiaries. However, fully 19% of patients were found to be inappropriate for their telerheumatology visit, mainly because their disease was too complex or the underlying diagnosis was unclear.
“Almost one-fifth of their patients were inappropriate for telerheumatology. The question is, how are you supposed to know that ahead of time?” Dr. Troum asked.
Patient satisfaction. Dr. Troum’s reading of the literature on patient satisfaction with telerheumatology, coupled with his own extensive experience in clinical practice, makes him think that many of his younger patients with less disease activity might welcome a telerheumatology option, even with strict time boundaries. But his older patients with more disease activity are a different story.
“Typically my middle-aged and older patients won’t accept that without a lot of convincing,” he commented.
Dr. Wells and Dr. Troum had no relevant disclosures regarding their presentations.
MAUI, HAWAII – Alvin F. Wells, MD, PhD, believes he’s seen the future of rheumatology. So he’s taken a deep dive into telerheumatology, going all in.
“Whether you’re in academic, private, or hospital-based practice, in 2020 if you are not thinking about telerheumatology, you and your practice will not be able to compete with growing patient demands, expectations, and need for clinical monitoring. If you do not have a digital/virtual strategy, you do not have a health care strategy,” he asserted at the 2020 Rheumatology Winter Clinical Symposium.
“Begin now,” the rheumatologist advised.
In pursuit of his own telerheumatology strategy, he holds licenses to practice medicine in five states and has licensure pending in five others.
“My goal is to cover 20% of the U.S., so if the local guys can’t see the patients, I can see them virtually,” he explained. “The days of waiting 4-6 months to be seen by a rheumatologist are gone.”
Rheumatologists are already in short supply in most of the country, and a major shortage looms ahead as older practitioners retire. Telerheumatology can help fill that unmet need. But the specialty is behind the curve. In a survey that rated the medical specialties most engaged in telemedicine, the top three spots were held by radiology, psychiatry, and internal medicine. Rheumatology didn’t even crack the top 10, noted Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc., and a part-time faculty member at Duke University, the Medical College of Wisconsin, and the Karolinska Institute.
Yet telemedicine is primed for rheumatologic takeoff. Notably, the 2019 update of the American College of Rheumatology recommendations on rheumatoid arthritis disease activity measures incorporates the RAPID3 (Routine Assessment of Patient Index Data) as an endorsed three-question clinical assessment that doesn’t involve a physical exam or laboratory work. The ACR update is recognition that, while every rheumatology patient needs an initial physical exam along with follow-up physical exams at various rates, many patients with well-controlled disease don’t need a physical exam at every physician encounter, he said.
Telerheumatology saves time for both patient and physician. The patient saves travel time, doesn’t miss work, avoids having to arrange for child care in order to make a face-to-face clinic visit, and can schedule more frequent virtual follow-up visits. For the practitioner, telerheumatology means additional consults and – here’s the big one – “You never run behind,” according to Dr. Wells. “For a 15-minute appointment, the patient gets a 5-minute warning, then a 2-minute warning, and at 15 minutes the link is cut. If the fibromyalgia patients want 30 minutes, they pay for 30 minutes.”
He sees the strictly enforced, impersonally delivered electronic time limits as key to running an efficient practice.
“The patients with osteoarthritis who hate the nodules, the fibromyalgia patients because they’re hurting all over, the patients with back pain – you’ve really got to limit those patients because otherwise you’ll be running 30-40 minutes behind for a scheduled 15-minute visit,” he explained.
One rheumatologist’s telemedicine practice
Dr. Wells currently utilizes the Epic electronic health record integrated with a Zoom videoconferencing platform for real-time virtual patient encounters. But he noted that other virtual platforms are available, including Health Tap, American Well, MySpecialistMD, MDLIVE, and TelaDoc. The American Telemedicine Association is a valuable resource for state-by-state medicolegal, reimbursement, and how-to-do-it questions.
At present, he reserves two daily time slots for telerheumatology: one at 8:30-9:00 a.m., the other at 4:30-5:00 p.m. These can be filled with four 15-minute live consults or two 30-minute consults. His goal is to eventually make telerheumatology 20% of his patient load of about 100 patients per week.
His typical 15-minute virtual visit proceeds as follows: It begins with a 3-minute subjective patient assessment, followed by a 5-minute objective assessment which includes the RAPID3, a brief Health Assessment Questionnaire (HAQ) addressing the patient’s pain and overall satisfaction, a virtual joint inspection, the use of high-quality teleultrasound and other technology when warranted, and capture of relevant still photos. This is followed by 5 minutes to relay the treatment plan, and finally a 2-minute recap and summary.
“No niceties. We cut right to the chase,” he noted.
He documents the patient encounter as he goes, dictating his notes throughout the visit.
“When I walk out of the room, I’m done. It’s on to the next patient,” Dr. Wells said.
The reimbursement picture is improving, although major hurdles remain. At present, 48 states and the District of Columbia reimburse for live video telemedicine through Medicaid. And in January 2020, Aetna announced it covers reimbursement for telemedicine in all of its fully insured health plans via the Teladoc platform. Dr. Wells’ patients pay for their telerheumatology out of pocket if their insurance doesn’t cover it.
Telemedicine caveats
Dr. Wells shared his telerheumatology experience as the first half of a point/counterpoint session on telemedicine’s future in the specialty. His debate opponent, Orrin M. Troum, MD, announced at the outset that he is quite interested in getting into telerheumatology; however, while looking into it he has come across issues that for now give him pause and that other rheumatologists need to be aware of.
Legal risks. The telemedicine movement has gotten big enough to draw the scrutiny of federal prosecutors and regulatory enforcement officials. In April 2018, the Department of Health & Human Services Office of the Inspector General (OIG) issued a report that concluded that one-third of all examined telemedicine claims were improper.
“Just imagine who might come knocking on your door,” he said.
Among the most common offenses, according to the OIG, were claims for services outside the limited range currently covered; lack of the requisite HIPAA-compliant two-way audio and visual communication technology with fully encrypted data transmission; services billed by institutional providers not defined by Medicare as telemedicine-eligible; and claims for services received by patients who weren’t located in an officially designated Health Professional Shortage Area or in a rural county as determined by the U.S. Census Bureau.
Telemedicine is no panacea for out-of-control health care costs. A RAND study of participants in the California Public Employees’ Retirement System (CalPERS) concluded that only 12% of beneficiaries who used direct-to-consumer telemedicine did so to replace provider visits. The other 88% added on telemedicine as an additional service. So while telemedicine increased patient access to health care, it also increased the overall cost, observed Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, and in private practice in Santa Monica, Calif.
Talk to your attorney and malpractice insurer before embarking on telerheumatology. Physicians could potentially lose their medical malpractice insurance if they use telemedicine to treat patients located in states where they aren’t licensed to practice, even if through inadvertent error.
Telemedicine isn’t appropriate for all patients. Nearly a decade ago, rheumatologists at Dartmouth-Hitchcock Medical Center launched a telerheumatology service in order to bring specialty care to the largely rural populations of New Hampshire and Vermont. In a review of the experience that included interviews with both patients and providers, investigators concluded that telerheumatology successfully increased access to specialty care in underserved locations and got good satisfaction scores from both providers and beneficiaries. However, fully 19% of patients were found to be inappropriate for their telerheumatology visit, mainly because their disease was too complex or the underlying diagnosis was unclear.
“Almost one-fifth of their patients were inappropriate for telerheumatology. The question is, how are you supposed to know that ahead of time?” Dr. Troum asked.
Patient satisfaction. Dr. Troum’s reading of the literature on patient satisfaction with telerheumatology, coupled with his own extensive experience in clinical practice, makes him think that many of his younger patients with less disease activity might welcome a telerheumatology option, even with strict time boundaries. But his older patients with more disease activity are a different story.
“Typically my middle-aged and older patients won’t accept that without a lot of convincing,” he commented.
Dr. Wells and Dr. Troum had no relevant disclosures regarding their presentations.
REPORTING FROM RWCS 2020