User login
Over-the-scope hemoclip prevails for upper GI bleeding
SAN ANTONIO – Use of a large over-the-scope hemoclip for initial endoscopic treatment of severe nonvariceal upper gastrointestinal bleeding resulted in a markedly lower 30-day rebleeding rate compared with standard endoscopic hemostasis in the first-ever randomized prospective clinical trial addressing the issue, Dennis M. Jensen, MD, reported at the annual meeting of the American College of Gastroenterology.
The over-the-scope clip also resulted in significantly fewer complications and cut the red blood cell transfusion rate in half, added Dr. Jensen, professor of medicine at the University of California, Los Angeles.
“The results appear to relate to the clip’s superior ability to obliterate critical arterial blood flow underneath the stigmata of hemorrhage and thereby reduce lesion rebleeding,” according to Dr. Jensen.
However, he emphasized a couple of caveats regarding this highly effective intervention.
First, it’s best reserved for patients with major stigmata of hemorrhage: that is, active arterial bleeding, a nonbleeding visible vessel, and/or adherent clot. That’s where all the benefit lies. Study participants with minor stigmata of hemorrhage – mere oozing bleeding or flat spots with arterial flow by Doppler – did just fine with standard endoscopic hemostasis, and in that setting the over-the-scope clip offered no additional benefit.
Second, there’s a significant learning curve involved in successful use of the clip.
“If someone’s going to be using this they have to get additional training. There’s a lot of tricks to using this,” the gastroenterologist cautioned.
The two-center prospective trial included 49 patients with severe nonvariceal upper GI bleeding who were randomized double-blind to the over-the-scope clip or standard hemostasis with hemoclips and/or application of a multipolar probe with epinephrine pre-injection as initial therapy. The severe bleeding was due to peptic ulcers in 40 patients and Dieulafoy’s lesions in the rest. All participants received high-dose proton pump inhibitor therapy after randomization.
The primary endpoint was clinically significant rebleeding within 30 days following initial therapy. This occurred in 7 of 25 patients (28%) on standard treatment and in 1 of 24 (4%) treated with the over-the-scope clip. This translated to an 85% relative risk reduction, with an impressive low number-needed-to-treat of 4.2.
Among the 35 patients with major stigmata of hemorrhage, the rebleeding rate was 35% in the over-the-scope clip group, compared with 6.3% with standard therapy, with a number-needed-to-treat of 3.5.
All four severe complications – a stroke, aspiration pneumonia, a case of severe heart failure, and a bleeding ischemic ulcer secondary to angiographic embolization – occurred in the standard therapy group. Patients in that group also averaged a 1.3-day-longer hospital length of stay and 2.8 more days in the ICU; however, those trends didn’t achieve statistical significance because of the small study size.
One audience member leapt to his feet to declare: “This is the study we’ve all been waiting for.” He pressed Dr. Jensen for technical details about the procedure.
Dr. Jensen explained that the large clip goes over an 11-mm-diameter endoscope with a 3-mm hood and no teeth. But he cautioned that some gastroenterologists in a busy community practice may find the procedure too time- and labor-intensive for their liking.
“It really takes two people to treat a duodenal ulcer. Somebody has to push quite firmly and suction very hard as you try to deploy this. By suctioning hard, the clip will burrow in so long as it’s centered on the stigmata of hemorrhage; that’s really key,” according to Dr. Jensen.
The procedure takes longer than standard endoscopic hemostasis because the over-the-scope clip limits visualization. So the patient must be scoped twice: the first time with a clipless diagnostic endoscope, so the operator can get his or her bearings; then that scope needs to be taken out, the patient is reintubated, and the over-the-scope clip is brought to bear.
“You shouldn’t just grab this off the shelf and try to use it in an emergency. You’ll really have problems. People have to be taught with porcine models and they need to review the stigmata,” Dr. Jensen said.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
SOURCE: Jensen DM. ACG 2019, Abstract 8.
SAN ANTONIO – Use of a large over-the-scope hemoclip for initial endoscopic treatment of severe nonvariceal upper gastrointestinal bleeding resulted in a markedly lower 30-day rebleeding rate compared with standard endoscopic hemostasis in the first-ever randomized prospective clinical trial addressing the issue, Dennis M. Jensen, MD, reported at the annual meeting of the American College of Gastroenterology.
The over-the-scope clip also resulted in significantly fewer complications and cut the red blood cell transfusion rate in half, added Dr. Jensen, professor of medicine at the University of California, Los Angeles.
“The results appear to relate to the clip’s superior ability to obliterate critical arterial blood flow underneath the stigmata of hemorrhage and thereby reduce lesion rebleeding,” according to Dr. Jensen.
However, he emphasized a couple of caveats regarding this highly effective intervention.
First, it’s best reserved for patients with major stigmata of hemorrhage: that is, active arterial bleeding, a nonbleeding visible vessel, and/or adherent clot. That’s where all the benefit lies. Study participants with minor stigmata of hemorrhage – mere oozing bleeding or flat spots with arterial flow by Doppler – did just fine with standard endoscopic hemostasis, and in that setting the over-the-scope clip offered no additional benefit.
Second, there’s a significant learning curve involved in successful use of the clip.
“If someone’s going to be using this they have to get additional training. There’s a lot of tricks to using this,” the gastroenterologist cautioned.
The two-center prospective trial included 49 patients with severe nonvariceal upper GI bleeding who were randomized double-blind to the over-the-scope clip or standard hemostasis with hemoclips and/or application of a multipolar probe with epinephrine pre-injection as initial therapy. The severe bleeding was due to peptic ulcers in 40 patients and Dieulafoy’s lesions in the rest. All participants received high-dose proton pump inhibitor therapy after randomization.
The primary endpoint was clinically significant rebleeding within 30 days following initial therapy. This occurred in 7 of 25 patients (28%) on standard treatment and in 1 of 24 (4%) treated with the over-the-scope clip. This translated to an 85% relative risk reduction, with an impressive low number-needed-to-treat of 4.2.
Among the 35 patients with major stigmata of hemorrhage, the rebleeding rate was 35% in the over-the-scope clip group, compared with 6.3% with standard therapy, with a number-needed-to-treat of 3.5.
All four severe complications – a stroke, aspiration pneumonia, a case of severe heart failure, and a bleeding ischemic ulcer secondary to angiographic embolization – occurred in the standard therapy group. Patients in that group also averaged a 1.3-day-longer hospital length of stay and 2.8 more days in the ICU; however, those trends didn’t achieve statistical significance because of the small study size.
One audience member leapt to his feet to declare: “This is the study we’ve all been waiting for.” He pressed Dr. Jensen for technical details about the procedure.
Dr. Jensen explained that the large clip goes over an 11-mm-diameter endoscope with a 3-mm hood and no teeth. But he cautioned that some gastroenterologists in a busy community practice may find the procedure too time- and labor-intensive for their liking.
“It really takes two people to treat a duodenal ulcer. Somebody has to push quite firmly and suction very hard as you try to deploy this. By suctioning hard, the clip will burrow in so long as it’s centered on the stigmata of hemorrhage; that’s really key,” according to Dr. Jensen.
The procedure takes longer than standard endoscopic hemostasis because the over-the-scope clip limits visualization. So the patient must be scoped twice: the first time with a clipless diagnostic endoscope, so the operator can get his or her bearings; then that scope needs to be taken out, the patient is reintubated, and the over-the-scope clip is brought to bear.
“You shouldn’t just grab this off the shelf and try to use it in an emergency. You’ll really have problems. People have to be taught with porcine models and they need to review the stigmata,” Dr. Jensen said.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
SOURCE: Jensen DM. ACG 2019, Abstract 8.
SAN ANTONIO – Use of a large over-the-scope hemoclip for initial endoscopic treatment of severe nonvariceal upper gastrointestinal bleeding resulted in a markedly lower 30-day rebleeding rate compared with standard endoscopic hemostasis in the first-ever randomized prospective clinical trial addressing the issue, Dennis M. Jensen, MD, reported at the annual meeting of the American College of Gastroenterology.
The over-the-scope clip also resulted in significantly fewer complications and cut the red blood cell transfusion rate in half, added Dr. Jensen, professor of medicine at the University of California, Los Angeles.
“The results appear to relate to the clip’s superior ability to obliterate critical arterial blood flow underneath the stigmata of hemorrhage and thereby reduce lesion rebleeding,” according to Dr. Jensen.
However, he emphasized a couple of caveats regarding this highly effective intervention.
First, it’s best reserved for patients with major stigmata of hemorrhage: that is, active arterial bleeding, a nonbleeding visible vessel, and/or adherent clot. That’s where all the benefit lies. Study participants with minor stigmata of hemorrhage – mere oozing bleeding or flat spots with arterial flow by Doppler – did just fine with standard endoscopic hemostasis, and in that setting the over-the-scope clip offered no additional benefit.
Second, there’s a significant learning curve involved in successful use of the clip.
“If someone’s going to be using this they have to get additional training. There’s a lot of tricks to using this,” the gastroenterologist cautioned.
The two-center prospective trial included 49 patients with severe nonvariceal upper GI bleeding who were randomized double-blind to the over-the-scope clip or standard hemostasis with hemoclips and/or application of a multipolar probe with epinephrine pre-injection as initial therapy. The severe bleeding was due to peptic ulcers in 40 patients and Dieulafoy’s lesions in the rest. All participants received high-dose proton pump inhibitor therapy after randomization.
The primary endpoint was clinically significant rebleeding within 30 days following initial therapy. This occurred in 7 of 25 patients (28%) on standard treatment and in 1 of 24 (4%) treated with the over-the-scope clip. This translated to an 85% relative risk reduction, with an impressive low number-needed-to-treat of 4.2.
Among the 35 patients with major stigmata of hemorrhage, the rebleeding rate was 35% in the over-the-scope clip group, compared with 6.3% with standard therapy, with a number-needed-to-treat of 3.5.
All four severe complications – a stroke, aspiration pneumonia, a case of severe heart failure, and a bleeding ischemic ulcer secondary to angiographic embolization – occurred in the standard therapy group. Patients in that group also averaged a 1.3-day-longer hospital length of stay and 2.8 more days in the ICU; however, those trends didn’t achieve statistical significance because of the small study size.
One audience member leapt to his feet to declare: “This is the study we’ve all been waiting for.” He pressed Dr. Jensen for technical details about the procedure.
Dr. Jensen explained that the large clip goes over an 11-mm-diameter endoscope with a 3-mm hood and no teeth. But he cautioned that some gastroenterologists in a busy community practice may find the procedure too time- and labor-intensive for their liking.
“It really takes two people to treat a duodenal ulcer. Somebody has to push quite firmly and suction very hard as you try to deploy this. By suctioning hard, the clip will burrow in so long as it’s centered on the stigmata of hemorrhage; that’s really key,” according to Dr. Jensen.
The procedure takes longer than standard endoscopic hemostasis because the over-the-scope clip limits visualization. So the patient must be scoped twice: the first time with a clipless diagnostic endoscope, so the operator can get his or her bearings; then that scope needs to be taken out, the patient is reintubated, and the over-the-scope clip is brought to bear.
“You shouldn’t just grab this off the shelf and try to use it in an emergency. You’ll really have problems. People have to be taught with porcine models and they need to review the stigmata,” Dr. Jensen said.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
SOURCE: Jensen DM. ACG 2019, Abstract 8.
REPORTING FROM ACG 2019
Do Probiotics Reduce C diff Risk in Hospitalized Patients?
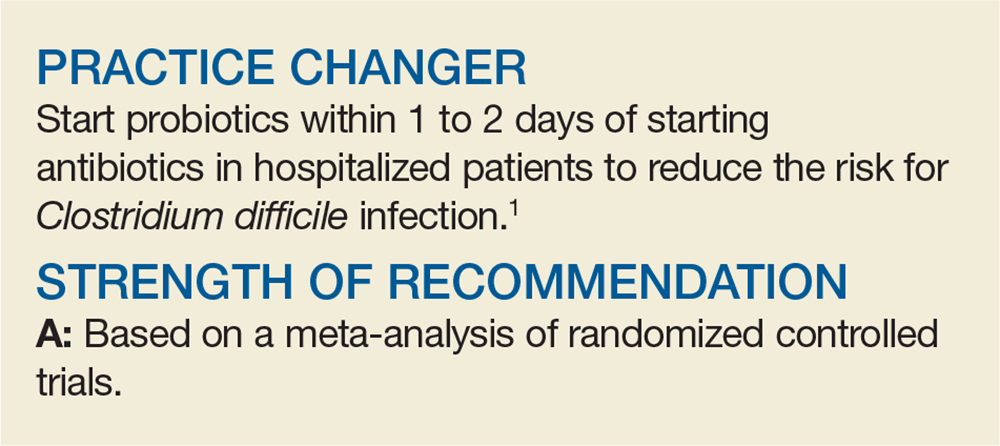
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
Training, alerts up the odds of discussions about genomic testing costs
Training and alerts increase the likelihood that oncologists will discuss the costs of genomic testing and related treatments with their patients, suggests a nationally representative survey of oncologists.
“Testing can be expensive, and not all tests and related treatments are covered by health insurance,” note the investigators, who were led by K. Robin Yabroff, PhD, an epidemiologist and senior scientific director of the Surveillance and Health Services Research Program at the American Cancer Society in Atlanta.
Using data from the 2017 National Survey of Precision Medicine in Cancer Treatment, the investigators analyzed factors associated with cost discussions among 1,220 oncologists who had discussed genomic testing with their patients in the past year.
Results reported in the Journal of the National Cancer Institute showed that 50.0% of the oncologists often discussed the likely costs of genomic testing and related treatments with patients and 26.3% sometimes did, while 23.7% never or rarely did.
In adjusted analyses, oncologists were more likely to often discuss costs, versus rarely or never, if they had formal training in genomic testing (odds ratio, 1.74). And they were more likely to sometimes or often have these discussions if their practice had electronic medical record alerts for genomic testing (odds ratios, 2.09 and 2.22).
Additional physician factors positively associated with cost discussions were treating only solid cancers or both solid and hematologic cancers versus only hematologic cancers, and using next-generation sequencing gene panel tests. Additional practice factors showing positive associations included seeing a volume of 100 or more patients per month; having 10% or more of patients who were insured by Medicaid or were self-paying or uninsured; and being located in the West as compared with the Northeast.
When the survey was conducted, the cost of available genomic tests to inform treatment was $300 to more than $10,000, and molecularly targeted therapies commonly had a price tag exceeding $100,000 per year, Dr. Yabroff and coinvestigators note. Moreover, insurance coverage of this testing was in limbo.
“With rapid growth in the availability of genomic tests and targeted treatments for cancer and a large pipeline of treatments in development, improving provider discussions about expected out-of-pocket costs will be critical for ensuring informed patient treatment decision making and the opportunity to plan for treatment expenses and help address out-of-pocket costs by linking patients with available resources, and ensuring high-quality cancer care,” they maintain.
“Interventions targeting modifiable oncologist and practice factors, such as training in genomic testing and use of EMR alerts, may help improve cost discussions about genomic testing and related treatments,” the investigators conclude.
Dr. Yabroff did not disclose any relevant conflicts of interest. The study did not receive any specific funding; the survey was funded by the National Institutes of Health.
SOURCE: Yabroff KR et al. J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz173.
Many oncology patients experience financial toxicity, whereby the high cost of care not covered by insurance takes a personal toll that can include bankruptcy, reduced treatment adherence, and ongoing stress, Richard L. Schilsky, MD, notes in an editorial.
Professional associations have developed frameworks to capture an intervention’s magnitude of clinical benefit and impact on the disease and patient – and sometimes the related cost. “However, the extent to which any of these frameworks is useful to guide decision making is hard to determine, perhaps because the perceived value of an intervention often depends on the lens through which it is viewed,” he comments.
Discussions about costs are only the first step in informed decision making, as the investigators point out. “In any context, the value of the test depends on its impact on clinical decision making and patient outcome, that is, its clinical utility,” Dr. Schilsky maintains.
Key challenges oncologists face in discussing these issues with patients, as also outlined by the investigators, include limited time, lack of training materials and discussion guides, and poor price transparency, he notes.
“But the biggest challenge may be explaining to a patient the nuances of context of use and clinical utility that define the true value of a tumor biomarker test,” Dr. Schilsky concludes. “Patients need to know not just what the test will cost but how it will inform their care, impact their options, affect their outcomes and whether, in the long run, it might even guide them to better treatments and/or lower their overall costs of care. Further research on how best to convey these complex issues in the course of a clinical encounter is desperately needed before we can effectively ‘talk the talk’ about tumor genomic testing.”
Richard L. Schilsky, MD, is senior vice president and chief medical officer of the American Society of Clinical Oncology, Alexandria, Va. These comments are taken from the editorial accompanying the study by Yabroff et al (J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz175).
Many oncology patients experience financial toxicity, whereby the high cost of care not covered by insurance takes a personal toll that can include bankruptcy, reduced treatment adherence, and ongoing stress, Richard L. Schilsky, MD, notes in an editorial.
Professional associations have developed frameworks to capture an intervention’s magnitude of clinical benefit and impact on the disease and patient – and sometimes the related cost. “However, the extent to which any of these frameworks is useful to guide decision making is hard to determine, perhaps because the perceived value of an intervention often depends on the lens through which it is viewed,” he comments.
Discussions about costs are only the first step in informed decision making, as the investigators point out. “In any context, the value of the test depends on its impact on clinical decision making and patient outcome, that is, its clinical utility,” Dr. Schilsky maintains.
Key challenges oncologists face in discussing these issues with patients, as also outlined by the investigators, include limited time, lack of training materials and discussion guides, and poor price transparency, he notes.
“But the biggest challenge may be explaining to a patient the nuances of context of use and clinical utility that define the true value of a tumor biomarker test,” Dr. Schilsky concludes. “Patients need to know not just what the test will cost but how it will inform their care, impact their options, affect their outcomes and whether, in the long run, it might even guide them to better treatments and/or lower their overall costs of care. Further research on how best to convey these complex issues in the course of a clinical encounter is desperately needed before we can effectively ‘talk the talk’ about tumor genomic testing.”
Richard L. Schilsky, MD, is senior vice president and chief medical officer of the American Society of Clinical Oncology, Alexandria, Va. These comments are taken from the editorial accompanying the study by Yabroff et al (J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz175).
Many oncology patients experience financial toxicity, whereby the high cost of care not covered by insurance takes a personal toll that can include bankruptcy, reduced treatment adherence, and ongoing stress, Richard L. Schilsky, MD, notes in an editorial.
Professional associations have developed frameworks to capture an intervention’s magnitude of clinical benefit and impact on the disease and patient – and sometimes the related cost. “However, the extent to which any of these frameworks is useful to guide decision making is hard to determine, perhaps because the perceived value of an intervention often depends on the lens through which it is viewed,” he comments.
Discussions about costs are only the first step in informed decision making, as the investigators point out. “In any context, the value of the test depends on its impact on clinical decision making and patient outcome, that is, its clinical utility,” Dr. Schilsky maintains.
Key challenges oncologists face in discussing these issues with patients, as also outlined by the investigators, include limited time, lack of training materials and discussion guides, and poor price transparency, he notes.
“But the biggest challenge may be explaining to a patient the nuances of context of use and clinical utility that define the true value of a tumor biomarker test,” Dr. Schilsky concludes. “Patients need to know not just what the test will cost but how it will inform their care, impact their options, affect their outcomes and whether, in the long run, it might even guide them to better treatments and/or lower their overall costs of care. Further research on how best to convey these complex issues in the course of a clinical encounter is desperately needed before we can effectively ‘talk the talk’ about tumor genomic testing.”
Richard L. Schilsky, MD, is senior vice president and chief medical officer of the American Society of Clinical Oncology, Alexandria, Va. These comments are taken from the editorial accompanying the study by Yabroff et al (J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz175).
Training and alerts increase the likelihood that oncologists will discuss the costs of genomic testing and related treatments with their patients, suggests a nationally representative survey of oncologists.
“Testing can be expensive, and not all tests and related treatments are covered by health insurance,” note the investigators, who were led by K. Robin Yabroff, PhD, an epidemiologist and senior scientific director of the Surveillance and Health Services Research Program at the American Cancer Society in Atlanta.
Using data from the 2017 National Survey of Precision Medicine in Cancer Treatment, the investigators analyzed factors associated with cost discussions among 1,220 oncologists who had discussed genomic testing with their patients in the past year.
Results reported in the Journal of the National Cancer Institute showed that 50.0% of the oncologists often discussed the likely costs of genomic testing and related treatments with patients and 26.3% sometimes did, while 23.7% never or rarely did.
In adjusted analyses, oncologists were more likely to often discuss costs, versus rarely or never, if they had formal training in genomic testing (odds ratio, 1.74). And they were more likely to sometimes or often have these discussions if their practice had electronic medical record alerts for genomic testing (odds ratios, 2.09 and 2.22).
Additional physician factors positively associated with cost discussions were treating only solid cancers or both solid and hematologic cancers versus only hematologic cancers, and using next-generation sequencing gene panel tests. Additional practice factors showing positive associations included seeing a volume of 100 or more patients per month; having 10% or more of patients who were insured by Medicaid or were self-paying or uninsured; and being located in the West as compared with the Northeast.
When the survey was conducted, the cost of available genomic tests to inform treatment was $300 to more than $10,000, and molecularly targeted therapies commonly had a price tag exceeding $100,000 per year, Dr. Yabroff and coinvestigators note. Moreover, insurance coverage of this testing was in limbo.
“With rapid growth in the availability of genomic tests and targeted treatments for cancer and a large pipeline of treatments in development, improving provider discussions about expected out-of-pocket costs will be critical for ensuring informed patient treatment decision making and the opportunity to plan for treatment expenses and help address out-of-pocket costs by linking patients with available resources, and ensuring high-quality cancer care,” they maintain.
“Interventions targeting modifiable oncologist and practice factors, such as training in genomic testing and use of EMR alerts, may help improve cost discussions about genomic testing and related treatments,” the investigators conclude.
Dr. Yabroff did not disclose any relevant conflicts of interest. The study did not receive any specific funding; the survey was funded by the National Institutes of Health.
SOURCE: Yabroff KR et al. J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz173.
Training and alerts increase the likelihood that oncologists will discuss the costs of genomic testing and related treatments with their patients, suggests a nationally representative survey of oncologists.
“Testing can be expensive, and not all tests and related treatments are covered by health insurance,” note the investigators, who were led by K. Robin Yabroff, PhD, an epidemiologist and senior scientific director of the Surveillance and Health Services Research Program at the American Cancer Society in Atlanta.
Using data from the 2017 National Survey of Precision Medicine in Cancer Treatment, the investigators analyzed factors associated with cost discussions among 1,220 oncologists who had discussed genomic testing with their patients in the past year.
Results reported in the Journal of the National Cancer Institute showed that 50.0% of the oncologists often discussed the likely costs of genomic testing and related treatments with patients and 26.3% sometimes did, while 23.7% never or rarely did.
In adjusted analyses, oncologists were more likely to often discuss costs, versus rarely or never, if they had formal training in genomic testing (odds ratio, 1.74). And they were more likely to sometimes or often have these discussions if their practice had electronic medical record alerts for genomic testing (odds ratios, 2.09 and 2.22).
Additional physician factors positively associated with cost discussions were treating only solid cancers or both solid and hematologic cancers versus only hematologic cancers, and using next-generation sequencing gene panel tests. Additional practice factors showing positive associations included seeing a volume of 100 or more patients per month; having 10% or more of patients who were insured by Medicaid or were self-paying or uninsured; and being located in the West as compared with the Northeast.
When the survey was conducted, the cost of available genomic tests to inform treatment was $300 to more than $10,000, and molecularly targeted therapies commonly had a price tag exceeding $100,000 per year, Dr. Yabroff and coinvestigators note. Moreover, insurance coverage of this testing was in limbo.
“With rapid growth in the availability of genomic tests and targeted treatments for cancer and a large pipeline of treatments in development, improving provider discussions about expected out-of-pocket costs will be critical for ensuring informed patient treatment decision making and the opportunity to plan for treatment expenses and help address out-of-pocket costs by linking patients with available resources, and ensuring high-quality cancer care,” they maintain.
“Interventions targeting modifiable oncologist and practice factors, such as training in genomic testing and use of EMR alerts, may help improve cost discussions about genomic testing and related treatments,” the investigators conclude.
Dr. Yabroff did not disclose any relevant conflicts of interest. The study did not receive any specific funding; the survey was funded by the National Institutes of Health.
SOURCE: Yabroff KR et al. J Natl Cancer Inst. 2019 Nov 1. doi: 10.1093/jnci/djz173.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
MIPS, E/M changes highlight 2020 Medicare fee schedule
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
Serum urate level governs management of difficult-to-treat gout
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Robotic bronchoscopy beat standard techniques for targeting lung nodules
NEW ORLEANS –
A prospective study in a cadaver model showed that robotic bronchoscopy targeted nodules more effectively than electromagnetic navigation or an ultrathin bronchoscope with radial endobronchial ultrasound (UTB-rEBUS).
“This is really the first study to randomize, blind, and compare procedural outcomes between existing technologies in advanced bronchoscopy,” said Lonny Yarmus, DO, of Johns Hopkins Medicine in Baltimore.
Dr. Yarmus described this study, PRECISION-1, at the annual meeting of the American Society of Chest Physicians. The study was designed to compare the following:
- UTB-rEBUS (3.0 mm outer diameter and 1.7 mm working channel).
- Electromagnetic navigation bronchoscopy (Superdimension version 7.1).
- Robotic bronchoscopy (3.5 mm outer diameter and 2.0-mm working channel).
With all methods, a 21-gauge needle was used. For each nodule, UTB-rEBUS was done first to eliminate potential localization bias. The subsequent order of electromagnetic navigation and robotic bronchoscopy was determined based on block randomization.
Eight bronchoscopists performed a total of 60 procedures using each of the methods to target 20 nodules implanted in cadavers. The nodules were distributed across all lobes, 80% were in the outer third of the lung, and 50% had a positive bronchus sign on computed tomography (CT). The mean nodule size was 16.5 plus or minus 1.5 mm.
The study’s primary endpoint was the ability to localize and puncture target nodules within a maximum of three attempts per method. This includes center, peripheral, and distal punctures of nodules. Cone-beam CT was used to confirm that needles punctured the target lesions. The bronchoscopists were blinded to cone-beam CT results, and a blinded, independent investigator assessed whether nodule punctures were successful. The primary endpoint was met in 25% of UTB-rEBUS procedures, 45% of electromagnetic navigation procedures, and 80% of robotic bronchoscopy procedures.
The study’s secondary endpoint was localization success, which was defined as navigation to within needle biopsy distance of the nodule. This includes center, peripheral, and distal punctures of nodules, as well as adjacent punctures (touching the nodule but not within it). The secondary endpoint was met in 35% of UTB-rEBUS procedures, 65% of electromagnetic navigation procedures, and 90% of robotic bronchoscopy procedures.
The researchers also assessed successful navigation, which was defined as the provider localizing with software or radial ultrasound and passing the needle to make a biopsy attempt. Navigation was successful in 65% of UTB-rEBUS procedures, 85% of electromagnetic navigation procedures, and 100% of robotic bronchoscopy procedures.
“Utilization of robotic bronchoscopy with shape-sensing technology can significantly increase the ability to localize and puncture lesions when compared with standard existing technologies,” Dr. Yarmus said in closing.
He did note that this research was done in a cadaveric model, so “prospective, randomized, and comparative in vivo studies are needed.”
This study was funded by the Association of Interventional Pulmonary Program Directors. Dr. Yarmus disclosed government and societal funding and relationships with Boston Scientific, Veran, Medtronic, Intuitive, Auris, Erbe, Olympus, BD, Rocket, Ambu, Inspire Medical, and AstraZeneca.
SOURCE: Yarmus L et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.311.
NEW ORLEANS –
A prospective study in a cadaver model showed that robotic bronchoscopy targeted nodules more effectively than electromagnetic navigation or an ultrathin bronchoscope with radial endobronchial ultrasound (UTB-rEBUS).
“This is really the first study to randomize, blind, and compare procedural outcomes between existing technologies in advanced bronchoscopy,” said Lonny Yarmus, DO, of Johns Hopkins Medicine in Baltimore.
Dr. Yarmus described this study, PRECISION-1, at the annual meeting of the American Society of Chest Physicians. The study was designed to compare the following:
- UTB-rEBUS (3.0 mm outer diameter and 1.7 mm working channel).
- Electromagnetic navigation bronchoscopy (Superdimension version 7.1).
- Robotic bronchoscopy (3.5 mm outer diameter and 2.0-mm working channel).
With all methods, a 21-gauge needle was used. For each nodule, UTB-rEBUS was done first to eliminate potential localization bias. The subsequent order of electromagnetic navigation and robotic bronchoscopy was determined based on block randomization.
Eight bronchoscopists performed a total of 60 procedures using each of the methods to target 20 nodules implanted in cadavers. The nodules were distributed across all lobes, 80% were in the outer third of the lung, and 50% had a positive bronchus sign on computed tomography (CT). The mean nodule size was 16.5 plus or minus 1.5 mm.
The study’s primary endpoint was the ability to localize and puncture target nodules within a maximum of three attempts per method. This includes center, peripheral, and distal punctures of nodules. Cone-beam CT was used to confirm that needles punctured the target lesions. The bronchoscopists were blinded to cone-beam CT results, and a blinded, independent investigator assessed whether nodule punctures were successful. The primary endpoint was met in 25% of UTB-rEBUS procedures, 45% of electromagnetic navigation procedures, and 80% of robotic bronchoscopy procedures.
The study’s secondary endpoint was localization success, which was defined as navigation to within needle biopsy distance of the nodule. This includes center, peripheral, and distal punctures of nodules, as well as adjacent punctures (touching the nodule but not within it). The secondary endpoint was met in 35% of UTB-rEBUS procedures, 65% of electromagnetic navigation procedures, and 90% of robotic bronchoscopy procedures.
The researchers also assessed successful navigation, which was defined as the provider localizing with software or radial ultrasound and passing the needle to make a biopsy attempt. Navigation was successful in 65% of UTB-rEBUS procedures, 85% of electromagnetic navigation procedures, and 100% of robotic bronchoscopy procedures.
“Utilization of robotic bronchoscopy with shape-sensing technology can significantly increase the ability to localize and puncture lesions when compared with standard existing technologies,” Dr. Yarmus said in closing.
He did note that this research was done in a cadaveric model, so “prospective, randomized, and comparative in vivo studies are needed.”
This study was funded by the Association of Interventional Pulmonary Program Directors. Dr. Yarmus disclosed government and societal funding and relationships with Boston Scientific, Veran, Medtronic, Intuitive, Auris, Erbe, Olympus, BD, Rocket, Ambu, Inspire Medical, and AstraZeneca.
SOURCE: Yarmus L et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.311.
NEW ORLEANS –
A prospective study in a cadaver model showed that robotic bronchoscopy targeted nodules more effectively than electromagnetic navigation or an ultrathin bronchoscope with radial endobronchial ultrasound (UTB-rEBUS).
“This is really the first study to randomize, blind, and compare procedural outcomes between existing technologies in advanced bronchoscopy,” said Lonny Yarmus, DO, of Johns Hopkins Medicine in Baltimore.
Dr. Yarmus described this study, PRECISION-1, at the annual meeting of the American Society of Chest Physicians. The study was designed to compare the following:
- UTB-rEBUS (3.0 mm outer diameter and 1.7 mm working channel).
- Electromagnetic navigation bronchoscopy (Superdimension version 7.1).
- Robotic bronchoscopy (3.5 mm outer diameter and 2.0-mm working channel).
With all methods, a 21-gauge needle was used. For each nodule, UTB-rEBUS was done first to eliminate potential localization bias. The subsequent order of electromagnetic navigation and robotic bronchoscopy was determined based on block randomization.
Eight bronchoscopists performed a total of 60 procedures using each of the methods to target 20 nodules implanted in cadavers. The nodules were distributed across all lobes, 80% were in the outer third of the lung, and 50% had a positive bronchus sign on computed tomography (CT). The mean nodule size was 16.5 plus or minus 1.5 mm.
The study’s primary endpoint was the ability to localize and puncture target nodules within a maximum of three attempts per method. This includes center, peripheral, and distal punctures of nodules. Cone-beam CT was used to confirm that needles punctured the target lesions. The bronchoscopists were blinded to cone-beam CT results, and a blinded, independent investigator assessed whether nodule punctures were successful. The primary endpoint was met in 25% of UTB-rEBUS procedures, 45% of electromagnetic navigation procedures, and 80% of robotic bronchoscopy procedures.
The study’s secondary endpoint was localization success, which was defined as navigation to within needle biopsy distance of the nodule. This includes center, peripheral, and distal punctures of nodules, as well as adjacent punctures (touching the nodule but not within it). The secondary endpoint was met in 35% of UTB-rEBUS procedures, 65% of electromagnetic navigation procedures, and 90% of robotic bronchoscopy procedures.
The researchers also assessed successful navigation, which was defined as the provider localizing with software or radial ultrasound and passing the needle to make a biopsy attempt. Navigation was successful in 65% of UTB-rEBUS procedures, 85% of electromagnetic navigation procedures, and 100% of robotic bronchoscopy procedures.
“Utilization of robotic bronchoscopy with shape-sensing technology can significantly increase the ability to localize and puncture lesions when compared with standard existing technologies,” Dr. Yarmus said in closing.
He did note that this research was done in a cadaveric model, so “prospective, randomized, and comparative in vivo studies are needed.”
This study was funded by the Association of Interventional Pulmonary Program Directors. Dr. Yarmus disclosed government and societal funding and relationships with Boston Scientific, Veran, Medtronic, Intuitive, Auris, Erbe, Olympus, BD, Rocket, Ambu, Inspire Medical, and AstraZeneca.
SOURCE: Yarmus L et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.311.
REPORTING FROM CHEST 2019
First NCCN guideline on hematopoietic cell transplantation focuses on GVHD
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
New screening test validated for cognitive impairment in lupus
new research suggests.
In a paper published in Arthritis Care & Research, researchers assessed the validity of the Automated Neuropsychological Assessment Metrics (ANAM) test in 211 adult patients with systemic lupus erythematosus (SLE).
First author Oshrat E. Tayer-Shifman, MD, of the University of Toronto Lupus Clinic and coauthors wrote that current assessment of cognitive impairment in adults with SLE is done using the American College of Rheumatology neuropsychological battery (ACR-NB). However, this approach involves protected tests that require specialized personnel and takes around 1 hour to administer, as well as time for scoring and interpretation.
“For many clinics, these are notable barriers to accessing CI [cognitive impairment] assessment, as health care payers do not cover these costs,” the investigators wrote. And although briefer cognitive screening tools, such as the Montreal Cognitive Assessment, the Controlled Oral Word Association Test, and the Hopkins Verbal Learning Test–Revised, have been examined in studies of patients with SLE, “they also require specialized personnel for administration and interpretation and cannot be self-administered. In addition, their validity for the screening for CI in SLE has not been well established. Thus, there is an unmet need for a screening assessment for CI that is validated for SLE and that can be applied in an ambulatory clinic setting without specialized personnel.”
The full ANAM battery requires about 40 minutes and has been used to screen cognitive performance in a range of clinical contexts.
A total of 96 patients (46%) had CI and 52 (25%) did not, according to the ACR-NB, while the results were indeterminate in the remaining 63 (30%).
The study showed that patients without CI performed significantly better on the majority of the ANAM subtests in comparison with patients who have cognitive impairment. This was particularly evident on mean reaction time and the number of correct responses per minute (a measure of cognitive efficiency), but less so for percentage of correct responses and consistency of response speed.
“Three of the most affected cognitive domains in the CI patients in this cohort, as well as in previous studies, were learning and memory, visual spatial construction, and simple attention and speed of processing,” the researchers wrote.
The investigators created testing models using the subtests that were most discriminative for CI. The two best models included one encompassing the percentage of correct responses, consistency of response speed, and mean reaction time, as well as one encompassing these three factors as well as the number of correct responses per minute. The investigators then derived candidate ANAM composite indices from these two models. For one composite index that used 8 of the 15 ANAM subtests and included five of the six cognitive domains tested on the ACR-NB, a high and a low cutoff value gave an area under the curve of 79%, sensitivity of 80%-89%, specificity of 54%-70%, positive predictive value of 78%-83%, and negative predictive value of 65%-74%.
The composite index performed similarly well among patients with or without neuropsychiatric lupus.
“This approach not only enables us to use a cost-effective screening approach without specialized personnel, but we have reduced the duration of the ANAM battery itself. The ANAM [version 4 General Neuropsychological Screening] full battery requires approximately 40 minutes to administer, while our analyses enable us to limit the number of ANAM subtests used, shortening the testing duration to 20 minutes,” they wrote.
The investigators noted that the study included only individuals with sufficient English language ability to complete the tests, and they also excluded patients with indeterminate cognitive status. “We reasoned that they represented a nonhomogeneous group, and without a clear consensus on the definition of CI in SLE patients, we chose to concentrate on the more clearly defined CI SLE patients.”
The study was supported by grants from the Arthritis Society of Canada, Physician’s Services, the Kathi and Peter Kaiser Family, and the Lou and Marissa Rocca Family. One author was supported by the Arthritis Society and the Canadian Rheumatology Association. No conflicts of interest were declared.
SOURCE: Tayer-Shifman OE et al. Arthritis Care Res. 2019 Oct 18. doi: 10.1002/acr.24096.
new research suggests.
In a paper published in Arthritis Care & Research, researchers assessed the validity of the Automated Neuropsychological Assessment Metrics (ANAM) test in 211 adult patients with systemic lupus erythematosus (SLE).
First author Oshrat E. Tayer-Shifman, MD, of the University of Toronto Lupus Clinic and coauthors wrote that current assessment of cognitive impairment in adults with SLE is done using the American College of Rheumatology neuropsychological battery (ACR-NB). However, this approach involves protected tests that require specialized personnel and takes around 1 hour to administer, as well as time for scoring and interpretation.
“For many clinics, these are notable barriers to accessing CI [cognitive impairment] assessment, as health care payers do not cover these costs,” the investigators wrote. And although briefer cognitive screening tools, such as the Montreal Cognitive Assessment, the Controlled Oral Word Association Test, and the Hopkins Verbal Learning Test–Revised, have been examined in studies of patients with SLE, “they also require specialized personnel for administration and interpretation and cannot be self-administered. In addition, their validity for the screening for CI in SLE has not been well established. Thus, there is an unmet need for a screening assessment for CI that is validated for SLE and that can be applied in an ambulatory clinic setting without specialized personnel.”
The full ANAM battery requires about 40 minutes and has been used to screen cognitive performance in a range of clinical contexts.
A total of 96 patients (46%) had CI and 52 (25%) did not, according to the ACR-NB, while the results were indeterminate in the remaining 63 (30%).
The study showed that patients without CI performed significantly better on the majority of the ANAM subtests in comparison with patients who have cognitive impairment. This was particularly evident on mean reaction time and the number of correct responses per minute (a measure of cognitive efficiency), but less so for percentage of correct responses and consistency of response speed.
“Three of the most affected cognitive domains in the CI patients in this cohort, as well as in previous studies, were learning and memory, visual spatial construction, and simple attention and speed of processing,” the researchers wrote.
The investigators created testing models using the subtests that were most discriminative for CI. The two best models included one encompassing the percentage of correct responses, consistency of response speed, and mean reaction time, as well as one encompassing these three factors as well as the number of correct responses per minute. The investigators then derived candidate ANAM composite indices from these two models. For one composite index that used 8 of the 15 ANAM subtests and included five of the six cognitive domains tested on the ACR-NB, a high and a low cutoff value gave an area under the curve of 79%, sensitivity of 80%-89%, specificity of 54%-70%, positive predictive value of 78%-83%, and negative predictive value of 65%-74%.
The composite index performed similarly well among patients with or without neuropsychiatric lupus.
“This approach not only enables us to use a cost-effective screening approach without specialized personnel, but we have reduced the duration of the ANAM battery itself. The ANAM [version 4 General Neuropsychological Screening] full battery requires approximately 40 minutes to administer, while our analyses enable us to limit the number of ANAM subtests used, shortening the testing duration to 20 minutes,” they wrote.
The investigators noted that the study included only individuals with sufficient English language ability to complete the tests, and they also excluded patients with indeterminate cognitive status. “We reasoned that they represented a nonhomogeneous group, and without a clear consensus on the definition of CI in SLE patients, we chose to concentrate on the more clearly defined CI SLE patients.”
The study was supported by grants from the Arthritis Society of Canada, Physician’s Services, the Kathi and Peter Kaiser Family, and the Lou and Marissa Rocca Family. One author was supported by the Arthritis Society and the Canadian Rheumatology Association. No conflicts of interest were declared.
SOURCE: Tayer-Shifman OE et al. Arthritis Care Res. 2019 Oct 18. doi: 10.1002/acr.24096.
new research suggests.
In a paper published in Arthritis Care & Research, researchers assessed the validity of the Automated Neuropsychological Assessment Metrics (ANAM) test in 211 adult patients with systemic lupus erythematosus (SLE).
First author Oshrat E. Tayer-Shifman, MD, of the University of Toronto Lupus Clinic and coauthors wrote that current assessment of cognitive impairment in adults with SLE is done using the American College of Rheumatology neuropsychological battery (ACR-NB). However, this approach involves protected tests that require specialized personnel and takes around 1 hour to administer, as well as time for scoring and interpretation.
“For many clinics, these are notable barriers to accessing CI [cognitive impairment] assessment, as health care payers do not cover these costs,” the investigators wrote. And although briefer cognitive screening tools, such as the Montreal Cognitive Assessment, the Controlled Oral Word Association Test, and the Hopkins Verbal Learning Test–Revised, have been examined in studies of patients with SLE, “they also require specialized personnel for administration and interpretation and cannot be self-administered. In addition, their validity for the screening for CI in SLE has not been well established. Thus, there is an unmet need for a screening assessment for CI that is validated for SLE and that can be applied in an ambulatory clinic setting without specialized personnel.”
The full ANAM battery requires about 40 minutes and has been used to screen cognitive performance in a range of clinical contexts.
A total of 96 patients (46%) had CI and 52 (25%) did not, according to the ACR-NB, while the results were indeterminate in the remaining 63 (30%).
The study showed that patients without CI performed significantly better on the majority of the ANAM subtests in comparison with patients who have cognitive impairment. This was particularly evident on mean reaction time and the number of correct responses per minute (a measure of cognitive efficiency), but less so for percentage of correct responses and consistency of response speed.
“Three of the most affected cognitive domains in the CI patients in this cohort, as well as in previous studies, were learning and memory, visual spatial construction, and simple attention and speed of processing,” the researchers wrote.
The investigators created testing models using the subtests that were most discriminative for CI. The two best models included one encompassing the percentage of correct responses, consistency of response speed, and mean reaction time, as well as one encompassing these three factors as well as the number of correct responses per minute. The investigators then derived candidate ANAM composite indices from these two models. For one composite index that used 8 of the 15 ANAM subtests and included five of the six cognitive domains tested on the ACR-NB, a high and a low cutoff value gave an area under the curve of 79%, sensitivity of 80%-89%, specificity of 54%-70%, positive predictive value of 78%-83%, and negative predictive value of 65%-74%.
The composite index performed similarly well among patients with or without neuropsychiatric lupus.
“This approach not only enables us to use a cost-effective screening approach without specialized personnel, but we have reduced the duration of the ANAM battery itself. The ANAM [version 4 General Neuropsychological Screening] full battery requires approximately 40 minutes to administer, while our analyses enable us to limit the number of ANAM subtests used, shortening the testing duration to 20 minutes,” they wrote.
The investigators noted that the study included only individuals with sufficient English language ability to complete the tests, and they also excluded patients with indeterminate cognitive status. “We reasoned that they represented a nonhomogeneous group, and without a clear consensus on the definition of CI in SLE patients, we chose to concentrate on the more clearly defined CI SLE patients.”
The study was supported by grants from the Arthritis Society of Canada, Physician’s Services, the Kathi and Peter Kaiser Family, and the Lou and Marissa Rocca Family. One author was supported by the Arthritis Society and the Canadian Rheumatology Association. No conflicts of interest were declared.
SOURCE: Tayer-Shifman OE et al. Arthritis Care Res. 2019 Oct 18. doi: 10.1002/acr.24096.
FROM ARTHRITIS CARE & RESEARCH
Adiposis Dolorosa Pain Management
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Evaluating a Veterans Affairs Home-Based Primary Care Population for Patients at High Risk of Osteoporosis
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.