User login
Productivity-based salary structure not associated with value-based culture
Background: Although new payment models have been implemented by the Centers for Medicare & Medicaid Services (CMS) for hospital reimbursement, little is known about the effects of reimbursement models on the culture of providing value-based care among individual hospitalists. The concern is that productivity-based models increase pressure on hospitalists to maximize volume and billing, as opposed to focusing on value.
Study design: Observational, cross-sectional, survey-based study.
Setting: A total of 12 hospitals in California, which represented university, community, and safety-net settings.
Synopsis: Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCS), a validated tool that assesses value-based decision making. Components of the survey assessed leadership and health system messaging, data transparency and access, comfort with cost conversations, and blame-free environments. Hospitalists were also asked to self-report their reimbursement structure: salary alone, salary plus productivity, or salary plus value-based adjustments.
A total of 255 hospitalists completed the survey. The mean HVCCS score was 50.2 on a 0-100 scale. Hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (beta = –6.2; 95% confidence interval, –9.9 to –2.5) when compared with hospitalists paid with salary alone. An association was not found between HVCCS score and reimbursement with salary plus value-based adjustments when compared with salary alone, though this finding may have been limited by sample size.
Bottom line: A hospitalist reimbursement model of salary plus productivity was associated with lower measures of value-based care culture.
Citation: Gupta R et al. Association between hospitalist productivity payments and high-value care culture. J Hosp Med. 2019;14(1):16-21.
Dr. Huang is a physician adviser and associate clinical professor in the division of hospital medicine at the University of California, San Diego.
Background: Although new payment models have been implemented by the Centers for Medicare & Medicaid Services (CMS) for hospital reimbursement, little is known about the effects of reimbursement models on the culture of providing value-based care among individual hospitalists. The concern is that productivity-based models increase pressure on hospitalists to maximize volume and billing, as opposed to focusing on value.
Study design: Observational, cross-sectional, survey-based study.
Setting: A total of 12 hospitals in California, which represented university, community, and safety-net settings.
Synopsis: Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCS), a validated tool that assesses value-based decision making. Components of the survey assessed leadership and health system messaging, data transparency and access, comfort with cost conversations, and blame-free environments. Hospitalists were also asked to self-report their reimbursement structure: salary alone, salary plus productivity, or salary plus value-based adjustments.
A total of 255 hospitalists completed the survey. The mean HVCCS score was 50.2 on a 0-100 scale. Hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (beta = –6.2; 95% confidence interval, –9.9 to –2.5) when compared with hospitalists paid with salary alone. An association was not found between HVCCS score and reimbursement with salary plus value-based adjustments when compared with salary alone, though this finding may have been limited by sample size.
Bottom line: A hospitalist reimbursement model of salary plus productivity was associated with lower measures of value-based care culture.
Citation: Gupta R et al. Association between hospitalist productivity payments and high-value care culture. J Hosp Med. 2019;14(1):16-21.
Dr. Huang is a physician adviser and associate clinical professor in the division of hospital medicine at the University of California, San Diego.
Background: Although new payment models have been implemented by the Centers for Medicare & Medicaid Services (CMS) for hospital reimbursement, little is known about the effects of reimbursement models on the culture of providing value-based care among individual hospitalists. The concern is that productivity-based models increase pressure on hospitalists to maximize volume and billing, as opposed to focusing on value.
Study design: Observational, cross-sectional, survey-based study.
Setting: A total of 12 hospitals in California, which represented university, community, and safety-net settings.
Synopsis: Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCS), a validated tool that assesses value-based decision making. Components of the survey assessed leadership and health system messaging, data transparency and access, comfort with cost conversations, and blame-free environments. Hospitalists were also asked to self-report their reimbursement structure: salary alone, salary plus productivity, or salary plus value-based adjustments.
A total of 255 hospitalists completed the survey. The mean HVCCS score was 50.2 on a 0-100 scale. Hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (beta = –6.2; 95% confidence interval, –9.9 to –2.5) when compared with hospitalists paid with salary alone. An association was not found between HVCCS score and reimbursement with salary plus value-based adjustments when compared with salary alone, though this finding may have been limited by sample size.
Bottom line: A hospitalist reimbursement model of salary plus productivity was associated with lower measures of value-based care culture.
Citation: Gupta R et al. Association between hospitalist productivity payments and high-value care culture. J Hosp Med. 2019;14(1):16-21.
Dr. Huang is a physician adviser and associate clinical professor in the division of hospital medicine at the University of California, San Diego.
Endoscopic therapy decreases recurrence of intestinal metaplasia, dysplasia in patients with Barrett’s esophagus
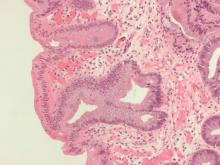
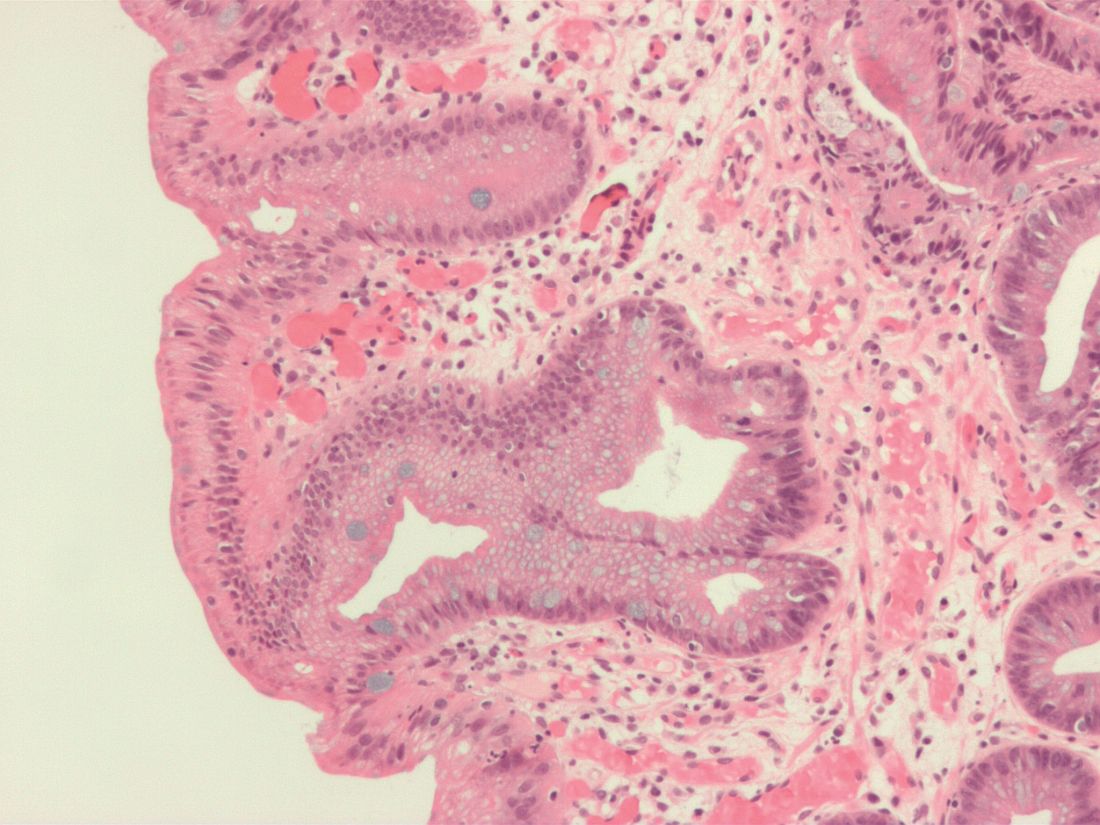
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Studies reinforce clinical experience and intuition
In this edition of “How I will treat my next patient,” I examine two recently published efforts to enlighten our sensitivity to the seriousness of immune-related adverse events (IrAEs) in patients on immune checkpoint inhibitors (ICIs) and the effect of delays in initiating systemic adjuvant therapy on the long-term outcomes of patients with resected pancreatic cancer.
IrAEs requiring hospitalization
Investigators led by Aanika Balaji of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, reviewed a 6-month audit of inpatient oncology admissions of solid-tumor patients who had ever received ICIs and ascertained the prevalence of hospitalization for management of IrAEs (J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703). To determine that an IrAE had occurred they required: consensus among two oncologists, clinical improvement with immune-directed therapy, exclusion of alternative diagnoses or pathologic confirmation of an IrAE, or chronic management of an IrAE for more than 6 months.
The bottom line: They found a cumulative incidence of a confirmed IrAEs among hospitalized ICI-treated solid tumor patients of 23%. As expected, the majority (65%) were grade 3-4 in severity. In total, 91% required multidisciplinary management, and 65% improved or resolved. But 87% of patients never received an ICI again.
Patients with preexisting autoimmune disease (25% of patients, although they included hypothyroidism in that group) were not more vulnerable to an IrAE with ICI therapy (odds ratio, 1.0; 95% confidence interval, 0.3-4.0). Not unsurprisingly, the median age was higher for ICI-treated patients who were admitted for IrAEs than for those not admitted (68 years vs. 59 years; OR, 5.4; 95% CI, 1.6-17.8), and more admitted patients had received combination ICIs than single agents (OR, 6.8; 95% CI, 2.0-23.2).
The median time from beginning ICIs to an IrAE-related hospitalization was 64 days, and the median number of ICI doses was one, with a wide range for both days and doses. The authors were quick to comment that this is a small, academic, single-institution survey over a brief period of time and that the generalizability of the results is uncertain.
What this means in practice
This publication changes very little for most practicing oncologists, but it does reinforce that ICI therapy can cause unpredictable, severe IrAEs. Clinical markers for selecting patients at highest risk are imperfect. As with chemotherapy, the patients we worry about the most – older individuals and patients treated with drug combinations – are, in fact, the ones we should be worrying about the most.
In view of the potential severity and impact of IrAEs, research efforts should place equal priority on identifying biomarkers of toxicity, such as tumor mutation burden, and biomarkers of efficacy (JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.3221). The potential financial and societal effects, as well as lost opportunity costs in the form of alternative therapies and early referral to hospice, demand no less, particularly in an era of value-based health care reimbursement.
Timing of adjuvant treatment
Sung Jun Ma, MD, department of radiation medicine at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues analyzed data from the more than 7,500 stage I-II resected pancreatic cancer patients in the National Cancer Database, of whom more than 5,400 ultimately received adjuvant therapy (chemotherapy with or without radiation). The patients were treated during 2004-2015. Appropriately, the investigators focused on correlating survival duration with the interval between surgery and initiation of adjuvant therapy. Other endpoints would be hard to accurately measure and verify without detailed clinical information (JAMA Network Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126).
They found that the best overall survival was associated with starting adjuvant treatment 28-59 days after surgery – not earlier (17% higher mortality) and not later (9% higher). Patients who did not start adjuvant treatment until more than 90 days post operatively still had an overall survival benefit (hazard ratio, 0.75; 95% confidence interval, 0.66-0.85; P less than .001), a more impressive hazard ratio than that seen for any particular interval between surgery and adjuvant treatment. Overall survival at 2 years was 47.2% versus 38% for the adjuvant therapy and surgery alone cohorts, respectively, with no overlap in the 95% confidence intervals.
As expected, longer delays to receive adjuvant treatment were associated with longer inpatient surgical stays, advanced age, black race, lower income, and a readmission for a postoperative complication within 30 days.
What this means in practice
This is another study that verifies that the patients we worry about most – older patients, those with a complicated recovery from surgery, and those with fewer supportive resources – are exactly the patients we should worry about most. It changes very little for most practicing oncologists. The analysis validates the importance of adjuvant therapy for patients who are able to receive it – whenever that is.
The data collection in this publication precedes recent improvements in adjuvant chemotherapy for resected pancreatic cancer, such as FOLFIRINOX or gemcitabine plus capecitabine. In an era of improved treatment, delays in initiating therapy may be less important since better treatment overcomes many prognostic variables that are significant for less effective therapy.
In my opinion, this large-data analysis is not really hypothesis-generating or practice-changing, but it does compel us to continue research efforts to improve surgical morbidity, identify better adjuvant and advanced disease regimens, and consider neoadjuvant treatment so that more than 72% of patients can receive all components of the multimodality treatment they need.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine two recently published efforts to enlighten our sensitivity to the seriousness of immune-related adverse events (IrAEs) in patients on immune checkpoint inhibitors (ICIs) and the effect of delays in initiating systemic adjuvant therapy on the long-term outcomes of patients with resected pancreatic cancer.
IrAEs requiring hospitalization
Investigators led by Aanika Balaji of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, reviewed a 6-month audit of inpatient oncology admissions of solid-tumor patients who had ever received ICIs and ascertained the prevalence of hospitalization for management of IrAEs (J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703). To determine that an IrAE had occurred they required: consensus among two oncologists, clinical improvement with immune-directed therapy, exclusion of alternative diagnoses or pathologic confirmation of an IrAE, or chronic management of an IrAE for more than 6 months.
The bottom line: They found a cumulative incidence of a confirmed IrAEs among hospitalized ICI-treated solid tumor patients of 23%. As expected, the majority (65%) were grade 3-4 in severity. In total, 91% required multidisciplinary management, and 65% improved or resolved. But 87% of patients never received an ICI again.
Patients with preexisting autoimmune disease (25% of patients, although they included hypothyroidism in that group) were not more vulnerable to an IrAE with ICI therapy (odds ratio, 1.0; 95% confidence interval, 0.3-4.0). Not unsurprisingly, the median age was higher for ICI-treated patients who were admitted for IrAEs than for those not admitted (68 years vs. 59 years; OR, 5.4; 95% CI, 1.6-17.8), and more admitted patients had received combination ICIs than single agents (OR, 6.8; 95% CI, 2.0-23.2).
The median time from beginning ICIs to an IrAE-related hospitalization was 64 days, and the median number of ICI doses was one, with a wide range for both days and doses. The authors were quick to comment that this is a small, academic, single-institution survey over a brief period of time and that the generalizability of the results is uncertain.
What this means in practice
This publication changes very little for most practicing oncologists, but it does reinforce that ICI therapy can cause unpredictable, severe IrAEs. Clinical markers for selecting patients at highest risk are imperfect. As with chemotherapy, the patients we worry about the most – older individuals and patients treated with drug combinations – are, in fact, the ones we should be worrying about the most.
In view of the potential severity and impact of IrAEs, research efforts should place equal priority on identifying biomarkers of toxicity, such as tumor mutation burden, and biomarkers of efficacy (JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.3221). The potential financial and societal effects, as well as lost opportunity costs in the form of alternative therapies and early referral to hospice, demand no less, particularly in an era of value-based health care reimbursement.
Timing of adjuvant treatment
Sung Jun Ma, MD, department of radiation medicine at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues analyzed data from the more than 7,500 stage I-II resected pancreatic cancer patients in the National Cancer Database, of whom more than 5,400 ultimately received adjuvant therapy (chemotherapy with or without radiation). The patients were treated during 2004-2015. Appropriately, the investigators focused on correlating survival duration with the interval between surgery and initiation of adjuvant therapy. Other endpoints would be hard to accurately measure and verify without detailed clinical information (JAMA Network Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126).
They found that the best overall survival was associated with starting adjuvant treatment 28-59 days after surgery – not earlier (17% higher mortality) and not later (9% higher). Patients who did not start adjuvant treatment until more than 90 days post operatively still had an overall survival benefit (hazard ratio, 0.75; 95% confidence interval, 0.66-0.85; P less than .001), a more impressive hazard ratio than that seen for any particular interval between surgery and adjuvant treatment. Overall survival at 2 years was 47.2% versus 38% for the adjuvant therapy and surgery alone cohorts, respectively, with no overlap in the 95% confidence intervals.
As expected, longer delays to receive adjuvant treatment were associated with longer inpatient surgical stays, advanced age, black race, lower income, and a readmission for a postoperative complication within 30 days.
What this means in practice
This is another study that verifies that the patients we worry about most – older patients, those with a complicated recovery from surgery, and those with fewer supportive resources – are exactly the patients we should worry about most. It changes very little for most practicing oncologists. The analysis validates the importance of adjuvant therapy for patients who are able to receive it – whenever that is.
The data collection in this publication precedes recent improvements in adjuvant chemotherapy for resected pancreatic cancer, such as FOLFIRINOX or gemcitabine plus capecitabine. In an era of improved treatment, delays in initiating therapy may be less important since better treatment overcomes many prognostic variables that are significant for less effective therapy.
In my opinion, this large-data analysis is not really hypothesis-generating or practice-changing, but it does compel us to continue research efforts to improve surgical morbidity, identify better adjuvant and advanced disease regimens, and consider neoadjuvant treatment so that more than 72% of patients can receive all components of the multimodality treatment they need.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine two recently published efforts to enlighten our sensitivity to the seriousness of immune-related adverse events (IrAEs) in patients on immune checkpoint inhibitors (ICIs) and the effect of delays in initiating systemic adjuvant therapy on the long-term outcomes of patients with resected pancreatic cancer.
IrAEs requiring hospitalization
Investigators led by Aanika Balaji of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, reviewed a 6-month audit of inpatient oncology admissions of solid-tumor patients who had ever received ICIs and ascertained the prevalence of hospitalization for management of IrAEs (J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703). To determine that an IrAE had occurred they required: consensus among two oncologists, clinical improvement with immune-directed therapy, exclusion of alternative diagnoses or pathologic confirmation of an IrAE, or chronic management of an IrAE for more than 6 months.
The bottom line: They found a cumulative incidence of a confirmed IrAEs among hospitalized ICI-treated solid tumor patients of 23%. As expected, the majority (65%) were grade 3-4 in severity. In total, 91% required multidisciplinary management, and 65% improved or resolved. But 87% of patients never received an ICI again.
Patients with preexisting autoimmune disease (25% of patients, although they included hypothyroidism in that group) were not more vulnerable to an IrAE with ICI therapy (odds ratio, 1.0; 95% confidence interval, 0.3-4.0). Not unsurprisingly, the median age was higher for ICI-treated patients who were admitted for IrAEs than for those not admitted (68 years vs. 59 years; OR, 5.4; 95% CI, 1.6-17.8), and more admitted patients had received combination ICIs than single agents (OR, 6.8; 95% CI, 2.0-23.2).
The median time from beginning ICIs to an IrAE-related hospitalization was 64 days, and the median number of ICI doses was one, with a wide range for both days and doses. The authors were quick to comment that this is a small, academic, single-institution survey over a brief period of time and that the generalizability of the results is uncertain.
What this means in practice
This publication changes very little for most practicing oncologists, but it does reinforce that ICI therapy can cause unpredictable, severe IrAEs. Clinical markers for selecting patients at highest risk are imperfect. As with chemotherapy, the patients we worry about the most – older individuals and patients treated with drug combinations – are, in fact, the ones we should be worrying about the most.
In view of the potential severity and impact of IrAEs, research efforts should place equal priority on identifying biomarkers of toxicity, such as tumor mutation burden, and biomarkers of efficacy (JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.3221). The potential financial and societal effects, as well as lost opportunity costs in the form of alternative therapies and early referral to hospice, demand no less, particularly in an era of value-based health care reimbursement.
Timing of adjuvant treatment
Sung Jun Ma, MD, department of radiation medicine at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues analyzed data from the more than 7,500 stage I-II resected pancreatic cancer patients in the National Cancer Database, of whom more than 5,400 ultimately received adjuvant therapy (chemotherapy with or without radiation). The patients were treated during 2004-2015. Appropriately, the investigators focused on correlating survival duration with the interval between surgery and initiation of adjuvant therapy. Other endpoints would be hard to accurately measure and verify without detailed clinical information (JAMA Network Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126).
They found that the best overall survival was associated with starting adjuvant treatment 28-59 days after surgery – not earlier (17% higher mortality) and not later (9% higher). Patients who did not start adjuvant treatment until more than 90 days post operatively still had an overall survival benefit (hazard ratio, 0.75; 95% confidence interval, 0.66-0.85; P less than .001), a more impressive hazard ratio than that seen for any particular interval between surgery and adjuvant treatment. Overall survival at 2 years was 47.2% versus 38% for the adjuvant therapy and surgery alone cohorts, respectively, with no overlap in the 95% confidence intervals.
As expected, longer delays to receive adjuvant treatment were associated with longer inpatient surgical stays, advanced age, black race, lower income, and a readmission for a postoperative complication within 30 days.
What this means in practice
This is another study that verifies that the patients we worry about most – older patients, those with a complicated recovery from surgery, and those with fewer supportive resources – are exactly the patients we should worry about most. It changes very little for most practicing oncologists. The analysis validates the importance of adjuvant therapy for patients who are able to receive it – whenever that is.
The data collection in this publication precedes recent improvements in adjuvant chemotherapy for resected pancreatic cancer, such as FOLFIRINOX or gemcitabine plus capecitabine. In an era of improved treatment, delays in initiating therapy may be less important since better treatment overcomes many prognostic variables that are significant for less effective therapy.
In my opinion, this large-data analysis is not really hypothesis-generating or practice-changing, but it does compel us to continue research efforts to improve surgical morbidity, identify better adjuvant and advanced disease regimens, and consider neoadjuvant treatment so that more than 72% of patients can receive all components of the multimodality treatment they need.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Prior DMARD use in RA may limit adalimumab treatment response
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
A history of using multiple conventional synthetic disease-modifying antirheumatic drugs (DMARDs) is a key predictor for poorer response to adalimumab therapy in rheumatoid arthritis patients, according to data from a pair of studies with a total of 274 patients.
Although patients with RA who have failed methotrexate or tumor necrosis factor inhibitor therapy respond less than methotrexate-naive patients, “it remains unknown if response to the first biologic DMARD, in particular a [tumor necrosis factor inhibitor], depends on disease duration or prior numbers of failed [conventional synthetic] DMARDs,” wrote Daniel Aletaha, MD, of the Medical University of Vienna and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from two randomized, controlled trials of patients with RA. In the larger trial of 207 adults (known as DE019), past use of two or more conventional synthetic DMARDs was associated with less improvement in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) after 24 weeks of adalimumab (Humira), compared with use of one or no DMARDs (–1.8 vs. –2.2, respectively). Similarly, disease activity and disability scores improved significantly less in patients who had used two or more DMARDs, compared with those who used one or no DMARDs, according to the Simplified Disease Activity Index (SDAI; –22.1 vs. –26.9) and the Health Assessment Questionnaire Disability Index (HAQ-DI; –0.43 vs. –0.64).
The researchers also examined the role of disease duration on treatment response. Overall, patients with disease duration greater than 10 years showed more improvement at 24 weeks than did those with disease duration less than 1 year, based on HAQ-DI scores (1.1 vs. 0.7), but final scores on the SDAI and DAS28-CRP were not significantly different between those with disease duration greater than 10 years and those with duration of less than 1 year. These results suggest that the impact of DMARDs holds true regardless of disease duration, the researchers noted.
The findings were similar with regard to number of prior conventional synthetic DMARDs and the effects of disease duration in the second trial of 67 patients, known as the ARMADA study.
The study findings were limited by several factors, including the post hoc analysis design, use of only adalimumab data, and the small number of patients in several subgroups, the researchers noted. However, the results support the need for more standardized treatment guidelines and suggest that RA patients who fail to respond to methotrexate soon after RA diagnosis may benefit most from adding adalimumab, they said.
“Furthermore, these findings should be considered in future trials when defining inclusion criteria not only by duration of disease but also by number of prior DMARDs,” they concluded.
The study was sponsored by AbbVie, which markets adalimumab. Dr. Aletaha disclosed grants and consulting fees from AbbVie, as well as other pharmaceutical companies. Four of the authors were current or former employees of AbbVie, and some other authors also reported financial relationships with the company.
SOURCE: Aletaha D et al. Ann Rheum Dis. 2019 Aug 21. doi: 10.1136/annrheumdis-2018-214918.
FROM ANNALS OF THE RHEUMATIC DISEASES
Cerliponase alfa continues to impress for CLN2 disease
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
REPORTING FROM IEC 2019
Combo therapy outcomes for West syndrome prove no better than monotherapy
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM IEC 2019
Time or money?
The authors of a recent study published in the Annals of Internal Medicine estimate that physician burnout is costing this country’s health care system $4.6 billion annually, using a conservative base-case model (Ann Intern Med. 2019;170[11]:784-90). I guess we shouldn’t be surprised at the magnitude of the drain on our economy caused by unhappy physicians. We all know colleagues who are showing signs of burnout. And, you may be feeling yourself that the challenges of work are taking too great a toll on your physical and mental health? Would you be happier if you had more time?
A study reported in Harvard Business Review has looked at recent college graduates to determine if how they prioritize time and money can predict their future happiness (“Are New Graduates Happier Making More Money or Having More Time?” July 25, 2019). The researchers at the Harvard Business School surveyed 1,000 college students in the 2015 and 2016 classes of the University of British Columbia, Vancouver. The students were asked to match themselves with descriptions of fictitious individuals to determine whether in general they prioritized time or money. The researchers then assessed the students’ level of happiness by asking them, “How satisfied are you with life overall?”
At a 2-year follow-up, the researchers found that, even taking into account the students’ level of happiness at the beginning of the study, “those who prioritized time were happier.” The authors also found that time-oriented people don’t necessarily work less or even earn more money, prompting their conclusion there is “strong evidence that valuing time puts people on a trajectory toward job satisfaction and well-being.”
Do the results of this study of Canadian college students provide any answers for our epidemic of physician burnout? One could argue that, if we wanted to minimize burnout, medical schools should include an assessment of each applicant’s level of happiness and how she or he prioritizes time and money using methods similar those used in this study? The problem is that some students are so heavily committed to becoming physicians that they would game the system and provide answers that will project the image that they are happy and prioritize time over money, when in reality they are ticking time bombs of discontent.
The bigger problem with interpreting the results of this study is that the subjects were Canadians who have significantly less educational debt than the medical students in this country. And as the authors observe, “people with objective financial constraints ... are more likely to focus on having more money.” Until we solve the problem of the high cost of medical education the system will continue to select for physicians whose decisions are too heavily influenced by their educational debt.
Finally, it is important to consider that time-oriented individuals don’t always work less, rather they make decisions that make it more likely that they will pursue activities they find enjoyable. For example, accepting a higher-paying job that requires an additional 3 hours of commute each day lays the foundation for a life in which a large portion of one’s day is expended in an activity that few of us find enjoyable. Choosing a long commute is a personal decision. Spending nearly 2 hours each day tethered to an EHR system was not something most physicians anticipated when they were choosing a career.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The authors of a recent study published in the Annals of Internal Medicine estimate that physician burnout is costing this country’s health care system $4.6 billion annually, using a conservative base-case model (Ann Intern Med. 2019;170[11]:784-90). I guess we shouldn’t be surprised at the magnitude of the drain on our economy caused by unhappy physicians. We all know colleagues who are showing signs of burnout. And, you may be feeling yourself that the challenges of work are taking too great a toll on your physical and mental health? Would you be happier if you had more time?
A study reported in Harvard Business Review has looked at recent college graduates to determine if how they prioritize time and money can predict their future happiness (“Are New Graduates Happier Making More Money or Having More Time?” July 25, 2019). The researchers at the Harvard Business School surveyed 1,000 college students in the 2015 and 2016 classes of the University of British Columbia, Vancouver. The students were asked to match themselves with descriptions of fictitious individuals to determine whether in general they prioritized time or money. The researchers then assessed the students’ level of happiness by asking them, “How satisfied are you with life overall?”
At a 2-year follow-up, the researchers found that, even taking into account the students’ level of happiness at the beginning of the study, “those who prioritized time were happier.” The authors also found that time-oriented people don’t necessarily work less or even earn more money, prompting their conclusion there is “strong evidence that valuing time puts people on a trajectory toward job satisfaction and well-being.”
Do the results of this study of Canadian college students provide any answers for our epidemic of physician burnout? One could argue that, if we wanted to minimize burnout, medical schools should include an assessment of each applicant’s level of happiness and how she or he prioritizes time and money using methods similar those used in this study? The problem is that some students are so heavily committed to becoming physicians that they would game the system and provide answers that will project the image that they are happy and prioritize time over money, when in reality they are ticking time bombs of discontent.
The bigger problem with interpreting the results of this study is that the subjects were Canadians who have significantly less educational debt than the medical students in this country. And as the authors observe, “people with objective financial constraints ... are more likely to focus on having more money.” Until we solve the problem of the high cost of medical education the system will continue to select for physicians whose decisions are too heavily influenced by their educational debt.
Finally, it is important to consider that time-oriented individuals don’t always work less, rather they make decisions that make it more likely that they will pursue activities they find enjoyable. For example, accepting a higher-paying job that requires an additional 3 hours of commute each day lays the foundation for a life in which a large portion of one’s day is expended in an activity that few of us find enjoyable. Choosing a long commute is a personal decision. Spending nearly 2 hours each day tethered to an EHR system was not something most physicians anticipated when they were choosing a career.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The authors of a recent study published in the Annals of Internal Medicine estimate that physician burnout is costing this country’s health care system $4.6 billion annually, using a conservative base-case model (Ann Intern Med. 2019;170[11]:784-90). I guess we shouldn’t be surprised at the magnitude of the drain on our economy caused by unhappy physicians. We all know colleagues who are showing signs of burnout. And, you may be feeling yourself that the challenges of work are taking too great a toll on your physical and mental health? Would you be happier if you had more time?
A study reported in Harvard Business Review has looked at recent college graduates to determine if how they prioritize time and money can predict their future happiness (“Are New Graduates Happier Making More Money or Having More Time?” July 25, 2019). The researchers at the Harvard Business School surveyed 1,000 college students in the 2015 and 2016 classes of the University of British Columbia, Vancouver. The students were asked to match themselves with descriptions of fictitious individuals to determine whether in general they prioritized time or money. The researchers then assessed the students’ level of happiness by asking them, “How satisfied are you with life overall?”
At a 2-year follow-up, the researchers found that, even taking into account the students’ level of happiness at the beginning of the study, “those who prioritized time were happier.” The authors also found that time-oriented people don’t necessarily work less or even earn more money, prompting their conclusion there is “strong evidence that valuing time puts people on a trajectory toward job satisfaction and well-being.”
Do the results of this study of Canadian college students provide any answers for our epidemic of physician burnout? One could argue that, if we wanted to minimize burnout, medical schools should include an assessment of each applicant’s level of happiness and how she or he prioritizes time and money using methods similar those used in this study? The problem is that some students are so heavily committed to becoming physicians that they would game the system and provide answers that will project the image that they are happy and prioritize time over money, when in reality they are ticking time bombs of discontent.
The bigger problem with interpreting the results of this study is that the subjects were Canadians who have significantly less educational debt than the medical students in this country. And as the authors observe, “people with objective financial constraints ... are more likely to focus on having more money.” Until we solve the problem of the high cost of medical education the system will continue to select for physicians whose decisions are too heavily influenced by their educational debt.
Finally, it is important to consider that time-oriented individuals don’t always work less, rather they make decisions that make it more likely that they will pursue activities they find enjoyable. For example, accepting a higher-paying job that requires an additional 3 hours of commute each day lays the foundation for a life in which a large portion of one’s day is expended in an activity that few of us find enjoyable. Choosing a long commute is a personal decision. Spending nearly 2 hours each day tethered to an EHR system was not something most physicians anticipated when they were choosing a career.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Open Clinical Trials for Patients With Lung Cancers (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California
IHS Launches Pilot to Redress Racial Misclassification in Records
American Indians and Alaska Natives (AI/AN) are often misidentified as other races in public health administrative records. In the Northwest, for instance, the Northwest Tribal Epidemiology Center (NTEC) has found that about 10% of AI/AN birth and death records and up to 60% of hospitalization records are misclassified.
Racial misclassification makes it difficult to accurately assess the health of Native people: The numbers affected by a disease may appear lower or higher than they actually are. It can muddle and misrepresent information in birth certificates, cancer registries, death certificates, emergency department records, hospitalization records, injury reports. Without accurate health data, says Lisa Neel, director of the Indian Health Service (IHS) Tribal Epidemiology Center Program, tribes cannot make informed decisions about how best to serve their people.
That is why the IHS and the Center recently signed an agreement supporting a new information-sharing project. The agreement will allow the IHS to provide the NTEC with a list of people who have received health services at IHS, tribal, and urban Indian health programs in the Portland Area. The list will include no information about patients’ medical histories and will not be shared outside the NTEC. The center will then compare the list with outside information sources, such as state cancer registries, to check for racial misclassification.
The NTEC is 1 of 13 national “EpiCenters” charged with collecting tribal health status data, evaluating data monitoring and delivery systems, and helping tribes identify local priorities for health care delivery and health education. NTEC serves the 43 federally recognized tribes in Idaho, Oregon, and Washington. The center is housed in the Northwest Portland Area Indian Health Board (NPAIHB), whose delegates, representing the member tribes, direct and oversee activities, including health promotion, disease prevention, training and technical assistance.
The IHS plans for this to be a pilot project, possibly pointing the way for other tribal EpiCenters to launch similar projects.
American Indians and Alaska Natives (AI/AN) are often misidentified as other races in public health administrative records. In the Northwest, for instance, the Northwest Tribal Epidemiology Center (NTEC) has found that about 10% of AI/AN birth and death records and up to 60% of hospitalization records are misclassified.
Racial misclassification makes it difficult to accurately assess the health of Native people: The numbers affected by a disease may appear lower or higher than they actually are. It can muddle and misrepresent information in birth certificates, cancer registries, death certificates, emergency department records, hospitalization records, injury reports. Without accurate health data, says Lisa Neel, director of the Indian Health Service (IHS) Tribal Epidemiology Center Program, tribes cannot make informed decisions about how best to serve their people.
That is why the IHS and the Center recently signed an agreement supporting a new information-sharing project. The agreement will allow the IHS to provide the NTEC with a list of people who have received health services at IHS, tribal, and urban Indian health programs in the Portland Area. The list will include no information about patients’ medical histories and will not be shared outside the NTEC. The center will then compare the list with outside information sources, such as state cancer registries, to check for racial misclassification.
The NTEC is 1 of 13 national “EpiCenters” charged with collecting tribal health status data, evaluating data monitoring and delivery systems, and helping tribes identify local priorities for health care delivery and health education. NTEC serves the 43 federally recognized tribes in Idaho, Oregon, and Washington. The center is housed in the Northwest Portland Area Indian Health Board (NPAIHB), whose delegates, representing the member tribes, direct and oversee activities, including health promotion, disease prevention, training and technical assistance.
The IHS plans for this to be a pilot project, possibly pointing the way for other tribal EpiCenters to launch similar projects.
American Indians and Alaska Natives (AI/AN) are often misidentified as other races in public health administrative records. In the Northwest, for instance, the Northwest Tribal Epidemiology Center (NTEC) has found that about 10% of AI/AN birth and death records and up to 60% of hospitalization records are misclassified.
Racial misclassification makes it difficult to accurately assess the health of Native people: The numbers affected by a disease may appear lower or higher than they actually are. It can muddle and misrepresent information in birth certificates, cancer registries, death certificates, emergency department records, hospitalization records, injury reports. Without accurate health data, says Lisa Neel, director of the Indian Health Service (IHS) Tribal Epidemiology Center Program, tribes cannot make informed decisions about how best to serve their people.
That is why the IHS and the Center recently signed an agreement supporting a new information-sharing project. The agreement will allow the IHS to provide the NTEC with a list of people who have received health services at IHS, tribal, and urban Indian health programs in the Portland Area. The list will include no information about patients’ medical histories and will not be shared outside the NTEC. The center will then compare the list with outside information sources, such as state cancer registries, to check for racial misclassification.
The NTEC is 1 of 13 national “EpiCenters” charged with collecting tribal health status data, evaluating data monitoring and delivery systems, and helping tribes identify local priorities for health care delivery and health education. NTEC serves the 43 federally recognized tribes in Idaho, Oregon, and Washington. The center is housed in the Northwest Portland Area Indian Health Board (NPAIHB), whose delegates, representing the member tribes, direct and oversee activities, including health promotion, disease prevention, training and technical assistance.
The IHS plans for this to be a pilot project, possibly pointing the way for other tribal EpiCenters to launch similar projects.
ACIP issues 2 new recs on HPV vaccination
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.