User login
2019 Update on cervical disease
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
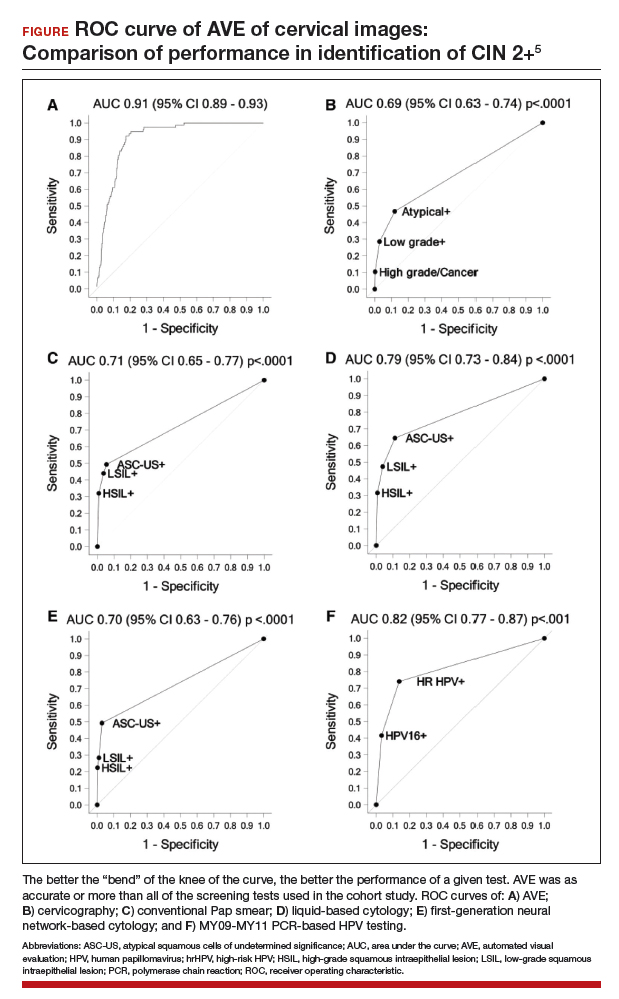
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).
Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
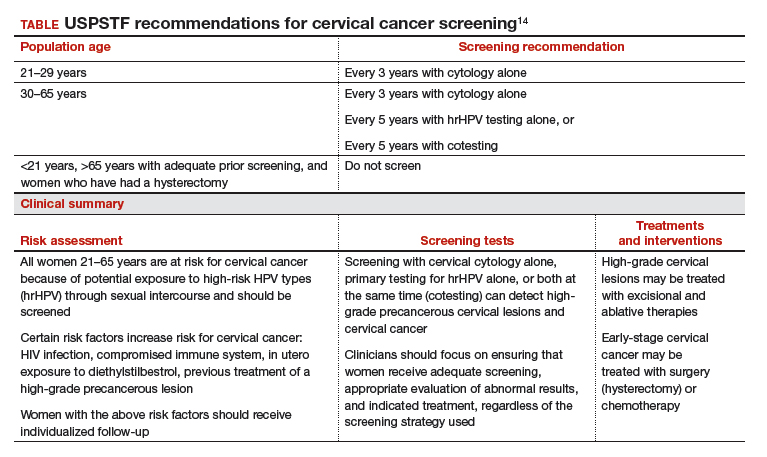
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.
Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Cluster headache is associated with increased suicidality
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
FROM CEPHALAGIA
Key clinical point: Cluster headache is associated with increased suicidality during attacks and within the active period.
Major finding: Cluster headache attacks increased the risk of active suicidal ideation (odds ratio, 15.55).
Study details: A prospective, multicenter study of 175 patients with cluster headache.
Disclosures: The study was supported by a grant from the Korean Neurological Association.
Source: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
ASCO: Deintensified treatment in p16+ oropharyngeal cancer needs evaluation
Treatment deintensification for patients with p16+ oropharyngeal cancer (OPC) should occur only in the context of a clinical trial, according to a provisional clinical opinion released by the American Society of Clinical Oncology (ASCO).
“The hypothesis that deescalation of treatment intensity for patients with p16+ OPC can reduce long-term toxicity without compromising survival is compelling and necessitates careful study and the analysis of well-designed clinical trials before changing current treatment standards, wrote David J. Adelstein, MD, of Case Western Reserve University, Cleveland, along with his associates on the expert panel. Their report is in the Journal of Clinical Oncology.
The panel undertook a review of the literature for evidence pertaining to the treatment of patients with HPV-mediated p16+ OPC with radiation, transoral surgery, concomitant chemoradiotherapy, and chemotherapy, in addition to immunotherapy and targeted therapy. Both randomized and nonrandomized studies were included in the review, and expert consensus opinion was taken into consideration.
After the review, the panelists concluded that the presumption that deintensified treatment in patients with p16+ OPC can lower long-term adverse effects without impacting survival is still a hypothesis that warrants further testing. While early findings of deintensified treatment techniques show promise, current treatment recommendations have not changed, they said.
“The standard of care for the definitive nonoperative management of cisplatin-eligible patients with advanced disease is concurrent chemoradiation with high-dose cisplatin given every 3 weeks,” the panel wrote. “For patients undergoing initial surgical resection, adjuvant chemoradiation with concurrent high-dose cisplatin given every 3 weeks is recommended for patients with positive margins and/or extranodal tumor extension,” they added.
At present, they recommend that deintensified treatment for patients with p16+ OPC should occur only in the context of a clinical trial.
The panel acknowledged that establishing definitive recommendations for all possible clinical scenarios is challenging because of restrictive exclusion criteria in key clinical trials. As a result, the accuracy of outcome data may be limited to specific patient populations.
More information on the provisional clinical opinion is available on the ASCO website.
ASCO funded the study. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Genentech, PDS Biotechnology, and several others.
SOURCE: Adelstein DJ et al. J Clin Oncol. 2019 Apr 25. doi: 10.1200/JCO.19.00441.
Treatment deintensification for patients with p16+ oropharyngeal cancer (OPC) should occur only in the context of a clinical trial, according to a provisional clinical opinion released by the American Society of Clinical Oncology (ASCO).
“The hypothesis that deescalation of treatment intensity for patients with p16+ OPC can reduce long-term toxicity without compromising survival is compelling and necessitates careful study and the analysis of well-designed clinical trials before changing current treatment standards, wrote David J. Adelstein, MD, of Case Western Reserve University, Cleveland, along with his associates on the expert panel. Their report is in the Journal of Clinical Oncology.
The panel undertook a review of the literature for evidence pertaining to the treatment of patients with HPV-mediated p16+ OPC with radiation, transoral surgery, concomitant chemoradiotherapy, and chemotherapy, in addition to immunotherapy and targeted therapy. Both randomized and nonrandomized studies were included in the review, and expert consensus opinion was taken into consideration.
After the review, the panelists concluded that the presumption that deintensified treatment in patients with p16+ OPC can lower long-term adverse effects without impacting survival is still a hypothesis that warrants further testing. While early findings of deintensified treatment techniques show promise, current treatment recommendations have not changed, they said.
“The standard of care for the definitive nonoperative management of cisplatin-eligible patients with advanced disease is concurrent chemoradiation with high-dose cisplatin given every 3 weeks,” the panel wrote. “For patients undergoing initial surgical resection, adjuvant chemoradiation with concurrent high-dose cisplatin given every 3 weeks is recommended for patients with positive margins and/or extranodal tumor extension,” they added.
At present, they recommend that deintensified treatment for patients with p16+ OPC should occur only in the context of a clinical trial.
The panel acknowledged that establishing definitive recommendations for all possible clinical scenarios is challenging because of restrictive exclusion criteria in key clinical trials. As a result, the accuracy of outcome data may be limited to specific patient populations.
More information on the provisional clinical opinion is available on the ASCO website.
ASCO funded the study. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Genentech, PDS Biotechnology, and several others.
SOURCE: Adelstein DJ et al. J Clin Oncol. 2019 Apr 25. doi: 10.1200/JCO.19.00441.
Treatment deintensification for patients with p16+ oropharyngeal cancer (OPC) should occur only in the context of a clinical trial, according to a provisional clinical opinion released by the American Society of Clinical Oncology (ASCO).
“The hypothesis that deescalation of treatment intensity for patients with p16+ OPC can reduce long-term toxicity without compromising survival is compelling and necessitates careful study and the analysis of well-designed clinical trials before changing current treatment standards, wrote David J. Adelstein, MD, of Case Western Reserve University, Cleveland, along with his associates on the expert panel. Their report is in the Journal of Clinical Oncology.
The panel undertook a review of the literature for evidence pertaining to the treatment of patients with HPV-mediated p16+ OPC with radiation, transoral surgery, concomitant chemoradiotherapy, and chemotherapy, in addition to immunotherapy and targeted therapy. Both randomized and nonrandomized studies were included in the review, and expert consensus opinion was taken into consideration.
After the review, the panelists concluded that the presumption that deintensified treatment in patients with p16+ OPC can lower long-term adverse effects without impacting survival is still a hypothesis that warrants further testing. While early findings of deintensified treatment techniques show promise, current treatment recommendations have not changed, they said.
“The standard of care for the definitive nonoperative management of cisplatin-eligible patients with advanced disease is concurrent chemoradiation with high-dose cisplatin given every 3 weeks,” the panel wrote. “For patients undergoing initial surgical resection, adjuvant chemoradiation with concurrent high-dose cisplatin given every 3 weeks is recommended for patients with positive margins and/or extranodal tumor extension,” they added.
At present, they recommend that deintensified treatment for patients with p16+ OPC should occur only in the context of a clinical trial.
The panel acknowledged that establishing definitive recommendations for all possible clinical scenarios is challenging because of restrictive exclusion criteria in key clinical trials. As a result, the accuracy of outcome data may be limited to specific patient populations.
More information on the provisional clinical opinion is available on the ASCO website.
ASCO funded the study. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Genentech, PDS Biotechnology, and several others.
SOURCE: Adelstein DJ et al. J Clin Oncol. 2019 Apr 25. doi: 10.1200/JCO.19.00441.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The hypothesis that deintensified treatment in patients with p16+ oropharyngeal cancer (OPC) can lower long-term adverse effects without impacting survival warrants further evaluation.
Major finding: Deintensified treatment for patients with p16+ OPC should occur only in the context of a clinical trial.
Study details: A provisional clinical opinion released by ASCO.
Disclosures: ASCO funded the study. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Genentech, PDS Biotechnology, and several others.
Source: Adelstein DJ et al. J Clin Oncol. 2019 Apr 25. doi: 10.1200/JCO.19.00441.
Good news for ObGyns: Medical liability claims resulting in payment are decreasing!
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
CBO’s report on single-payer health care holds more questions than answers
Progressive Democrats have rallied around “Medicare-for-all,” a single-payer health plan popularized by Sen. Bernie Sanders (I-Vt.). Now, some of Washington’s official bean counters are trying to add a new framework around what it might look like. The picture they offer highlights just how complicated that shift might be.
A report released May 1 by the nonpartisan Congressional Budget Office outlined a laundry list of options and technicalities lawmakers would need to consider if they are serious about such a proposal.
“The conversation about single-payer is getting more in the weeds, more detailed, which is a good thing because it’s such a complicated issue,” said Jodi Liu, an associate policy researcher at the Rand Corp. who studies single-payer proposals.
The takeaway: There’s a lot left to be answered about the concepts of Medicare-for-all specifically and the category of single-payer more broadly before policymakers and voters can come close to understanding what it would mean in practice. The term “single-payer” generally refers to a system in which health care is paid for by a single public authority.
“Even single-payer systems around the globe vary from each other in many, many ways,” said John McDonough, a Harvard health policy professor who helped draft the Affordable Care Act. “There’s just so many aspects of it that differ from a Canada to a Sweden to a Taiwan – and those are all intensely consequential.”
The report comes as this once-lefty pipe dream becomes officially mainstream.
Medicare-for-all has been name-checked by Democrats running for president. On April 30, Democrats and Republicans alike put the proposal under the microscope at a House Rules Committee hearing. And that won’t be the last time that happens. House Ways and Means Committee Chairman Richard Neal (D-Mass.) said he, too, intends to hold a hearing on the issue this session. Meanwhile, Sanders’ latest Medicare-for-all bill, reintroduced in the Senate in April, and a similar House bill, have 14 and 108 cosponsors, respectively.
Let’s break down the most crucial issues raised by the CBO report – what single-payer might cover, why “what it would cost” isn’t easy to determine, and what it could mean for how Americans get their health care.
Medicare-for-all backers say the program would cover all medically necessary services. But what does that truly mean?
What may seem obvious – the notion of medical necessity – isn’t so easy to distill into policy rules. And different single-payer systems around the world handle the benefits question differently, the CBO noted.
For instance, Canada doesn’t cover prescription drugs, but the United Kingdom and Sweden do. Of those three, only Sweden fully covers long-term support services, according to the report.
There are two questions at the heart of it, said Robert Berenson, a health policy analyst at the Urban Institute, a left-leaning think tank.
What benefits would be covered? Would it include dental care or prescription drugs or vision, as Sanders’ bill would? And, how does one determine the discrete services included within those benefits categories?
Single-payer architects could look at existing standards, such as the so-called essential health benefits that govern Obamacare health plans, to determine what’s covered. They could be more generous by including long-term care, which isn’t currently covered by Medicare or most private insurance plans.
Even the two “Medicare-for-all” bills in Congress have slightly different takes. Though both provide for long-term support and services, they diverge on how to pay for it. Sanders’ bill covers only at-home long-term care and keeps Medicaid intact for services provided in institutions. The House bill by Rep. Pramila Jayapal (D-Wash.) covers both.
And there are questions about new medical treatments, and how to determine whether they provide added value. The CBO report suggested some kind of “cost-effectiveness criterion” could determine what the government is willing to cover. In practice, though, that standard could be difficult to develop and could fall victim to political lobbying or trigger contentious debate.
Separately from the CBO report, Mr. McDonough noted, controversial medical services could bring up different kinds of political baggage – whether this plan would cover abortion, for instance, likely would change the single-payer debate.
Next: Single-payer health care would probably require new taxes. Just what level of taxes, though, and who they would hit hardest remain open questions.
Notably, the CBO report avoids a question that critics frequently surface: How much would this cost? How would you pay for it?
That’s because there’s no uniform cost estimate for single-payer and no easy formula to apply.
For one thing, the price tag depends on what services are covered – something like long-term care would make the idea much more expensive.
There’s also the question of cost sharing. In some single-payer systems, people must pay a copay, meet a deductible, or pay a premium as part of the health plan. That would alleviate some need for new taxes.
“I don’t think you can put numbers on it until someone defines a benefit package and defines cost sharing,” Mr. Berenson said.
The current Medicare-for-all bills eschew cost sharing. Other health reform proposals would keep premiums intact to help foot some of the bill.
The CBO report suggests that new taxes would likely play a role in financing a new single-payer plan. But what kind of taxes – a payroll tax, an income tax, or a sales tax, for instance – has not yet been stipulated. And each would have different consequences.
The single-payer approach could bring down health expenses, or at least increase value. But how effectively it would do so – and its larger economic impact – would depend on other design choices.
Single-payer backers dismiss the “pay-for” questions because, the reasoning goes, this approach would save lots of money in other ways, ultimately making it a good deal.
Yet again, though, the CBO said, whether that actually happens depends on the system’s design.
By eliminating most private insurers, a single-payer system would likely slash hospitals’ administrative overhead. The government could then pay a rate that better reflects reduced hospital costs, according to the CBO report.
But, ultimately, the single-payer bottom line depends on what the system pays hospitals, doctors, and drug companies for different services and products. That answer also would inform other economic assessments – ascertaining, for instance, how single-payer affects a small town where the hospital is the main employer.
Even without clear answers, outlining those questions moves the ball, Liu said.
“This area is moving really fast,” she said. “To me, it seems like this is the beginning of a longer conversation.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Progressive Democrats have rallied around “Medicare-for-all,” a single-payer health plan popularized by Sen. Bernie Sanders (I-Vt.). Now, some of Washington’s official bean counters are trying to add a new framework around what it might look like. The picture they offer highlights just how complicated that shift might be.
A report released May 1 by the nonpartisan Congressional Budget Office outlined a laundry list of options and technicalities lawmakers would need to consider if they are serious about such a proposal.
“The conversation about single-payer is getting more in the weeds, more detailed, which is a good thing because it’s such a complicated issue,” said Jodi Liu, an associate policy researcher at the Rand Corp. who studies single-payer proposals.
The takeaway: There’s a lot left to be answered about the concepts of Medicare-for-all specifically and the category of single-payer more broadly before policymakers and voters can come close to understanding what it would mean in practice. The term “single-payer” generally refers to a system in which health care is paid for by a single public authority.
“Even single-payer systems around the globe vary from each other in many, many ways,” said John McDonough, a Harvard health policy professor who helped draft the Affordable Care Act. “There’s just so many aspects of it that differ from a Canada to a Sweden to a Taiwan – and those are all intensely consequential.”
The report comes as this once-lefty pipe dream becomes officially mainstream.
Medicare-for-all has been name-checked by Democrats running for president. On April 30, Democrats and Republicans alike put the proposal under the microscope at a House Rules Committee hearing. And that won’t be the last time that happens. House Ways and Means Committee Chairman Richard Neal (D-Mass.) said he, too, intends to hold a hearing on the issue this session. Meanwhile, Sanders’ latest Medicare-for-all bill, reintroduced in the Senate in April, and a similar House bill, have 14 and 108 cosponsors, respectively.
Let’s break down the most crucial issues raised by the CBO report – what single-payer might cover, why “what it would cost” isn’t easy to determine, and what it could mean for how Americans get their health care.
Medicare-for-all backers say the program would cover all medically necessary services. But what does that truly mean?
What may seem obvious – the notion of medical necessity – isn’t so easy to distill into policy rules. And different single-payer systems around the world handle the benefits question differently, the CBO noted.
For instance, Canada doesn’t cover prescription drugs, but the United Kingdom and Sweden do. Of those three, only Sweden fully covers long-term support services, according to the report.
There are two questions at the heart of it, said Robert Berenson, a health policy analyst at the Urban Institute, a left-leaning think tank.
What benefits would be covered? Would it include dental care or prescription drugs or vision, as Sanders’ bill would? And, how does one determine the discrete services included within those benefits categories?
Single-payer architects could look at existing standards, such as the so-called essential health benefits that govern Obamacare health plans, to determine what’s covered. They could be more generous by including long-term care, which isn’t currently covered by Medicare or most private insurance plans.
Even the two “Medicare-for-all” bills in Congress have slightly different takes. Though both provide for long-term support and services, they diverge on how to pay for it. Sanders’ bill covers only at-home long-term care and keeps Medicaid intact for services provided in institutions. The House bill by Rep. Pramila Jayapal (D-Wash.) covers both.
And there are questions about new medical treatments, and how to determine whether they provide added value. The CBO report suggested some kind of “cost-effectiveness criterion” could determine what the government is willing to cover. In practice, though, that standard could be difficult to develop and could fall victim to political lobbying or trigger contentious debate.
Separately from the CBO report, Mr. McDonough noted, controversial medical services could bring up different kinds of political baggage – whether this plan would cover abortion, for instance, likely would change the single-payer debate.
Next: Single-payer health care would probably require new taxes. Just what level of taxes, though, and who they would hit hardest remain open questions.
Notably, the CBO report avoids a question that critics frequently surface: How much would this cost? How would you pay for it?
That’s because there’s no uniform cost estimate for single-payer and no easy formula to apply.
For one thing, the price tag depends on what services are covered – something like long-term care would make the idea much more expensive.
There’s also the question of cost sharing. In some single-payer systems, people must pay a copay, meet a deductible, or pay a premium as part of the health plan. That would alleviate some need for new taxes.
“I don’t think you can put numbers on it until someone defines a benefit package and defines cost sharing,” Mr. Berenson said.
The current Medicare-for-all bills eschew cost sharing. Other health reform proposals would keep premiums intact to help foot some of the bill.
The CBO report suggests that new taxes would likely play a role in financing a new single-payer plan. But what kind of taxes – a payroll tax, an income tax, or a sales tax, for instance – has not yet been stipulated. And each would have different consequences.
The single-payer approach could bring down health expenses, or at least increase value. But how effectively it would do so – and its larger economic impact – would depend on other design choices.
Single-payer backers dismiss the “pay-for” questions because, the reasoning goes, this approach would save lots of money in other ways, ultimately making it a good deal.
Yet again, though, the CBO said, whether that actually happens depends on the system’s design.
By eliminating most private insurers, a single-payer system would likely slash hospitals’ administrative overhead. The government could then pay a rate that better reflects reduced hospital costs, according to the CBO report.
But, ultimately, the single-payer bottom line depends on what the system pays hospitals, doctors, and drug companies for different services and products. That answer also would inform other economic assessments – ascertaining, for instance, how single-payer affects a small town where the hospital is the main employer.
Even without clear answers, outlining those questions moves the ball, Liu said.
“This area is moving really fast,” she said. “To me, it seems like this is the beginning of a longer conversation.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Progressive Democrats have rallied around “Medicare-for-all,” a single-payer health plan popularized by Sen. Bernie Sanders (I-Vt.). Now, some of Washington’s official bean counters are trying to add a new framework around what it might look like. The picture they offer highlights just how complicated that shift might be.
A report released May 1 by the nonpartisan Congressional Budget Office outlined a laundry list of options and technicalities lawmakers would need to consider if they are serious about such a proposal.
“The conversation about single-payer is getting more in the weeds, more detailed, which is a good thing because it’s such a complicated issue,” said Jodi Liu, an associate policy researcher at the Rand Corp. who studies single-payer proposals.
The takeaway: There’s a lot left to be answered about the concepts of Medicare-for-all specifically and the category of single-payer more broadly before policymakers and voters can come close to understanding what it would mean in practice. The term “single-payer” generally refers to a system in which health care is paid for by a single public authority.
“Even single-payer systems around the globe vary from each other in many, many ways,” said John McDonough, a Harvard health policy professor who helped draft the Affordable Care Act. “There’s just so many aspects of it that differ from a Canada to a Sweden to a Taiwan – and those are all intensely consequential.”
The report comes as this once-lefty pipe dream becomes officially mainstream.
Medicare-for-all has been name-checked by Democrats running for president. On April 30, Democrats and Republicans alike put the proposal under the microscope at a House Rules Committee hearing. And that won’t be the last time that happens. House Ways and Means Committee Chairman Richard Neal (D-Mass.) said he, too, intends to hold a hearing on the issue this session. Meanwhile, Sanders’ latest Medicare-for-all bill, reintroduced in the Senate in April, and a similar House bill, have 14 and 108 cosponsors, respectively.
Let’s break down the most crucial issues raised by the CBO report – what single-payer might cover, why “what it would cost” isn’t easy to determine, and what it could mean for how Americans get their health care.
Medicare-for-all backers say the program would cover all medically necessary services. But what does that truly mean?
What may seem obvious – the notion of medical necessity – isn’t so easy to distill into policy rules. And different single-payer systems around the world handle the benefits question differently, the CBO noted.
For instance, Canada doesn’t cover prescription drugs, but the United Kingdom and Sweden do. Of those three, only Sweden fully covers long-term support services, according to the report.
There are two questions at the heart of it, said Robert Berenson, a health policy analyst at the Urban Institute, a left-leaning think tank.
What benefits would be covered? Would it include dental care or prescription drugs or vision, as Sanders’ bill would? And, how does one determine the discrete services included within those benefits categories?
Single-payer architects could look at existing standards, such as the so-called essential health benefits that govern Obamacare health plans, to determine what’s covered. They could be more generous by including long-term care, which isn’t currently covered by Medicare or most private insurance plans.
Even the two “Medicare-for-all” bills in Congress have slightly different takes. Though both provide for long-term support and services, they diverge on how to pay for it. Sanders’ bill covers only at-home long-term care and keeps Medicaid intact for services provided in institutions. The House bill by Rep. Pramila Jayapal (D-Wash.) covers both.
And there are questions about new medical treatments, and how to determine whether they provide added value. The CBO report suggested some kind of “cost-effectiveness criterion” could determine what the government is willing to cover. In practice, though, that standard could be difficult to develop and could fall victim to political lobbying or trigger contentious debate.
Separately from the CBO report, Mr. McDonough noted, controversial medical services could bring up different kinds of political baggage – whether this plan would cover abortion, for instance, likely would change the single-payer debate.
Next: Single-payer health care would probably require new taxes. Just what level of taxes, though, and who they would hit hardest remain open questions.
Notably, the CBO report avoids a question that critics frequently surface: How much would this cost? How would you pay for it?
That’s because there’s no uniform cost estimate for single-payer and no easy formula to apply.
For one thing, the price tag depends on what services are covered – something like long-term care would make the idea much more expensive.
There’s also the question of cost sharing. In some single-payer systems, people must pay a copay, meet a deductible, or pay a premium as part of the health plan. That would alleviate some need for new taxes.
“I don’t think you can put numbers on it until someone defines a benefit package and defines cost sharing,” Mr. Berenson said.
The current Medicare-for-all bills eschew cost sharing. Other health reform proposals would keep premiums intact to help foot some of the bill.
The CBO report suggests that new taxes would likely play a role in financing a new single-payer plan. But what kind of taxes – a payroll tax, an income tax, or a sales tax, for instance – has not yet been stipulated. And each would have different consequences.
The single-payer approach could bring down health expenses, or at least increase value. But how effectively it would do so – and its larger economic impact – would depend on other design choices.
Single-payer backers dismiss the “pay-for” questions because, the reasoning goes, this approach would save lots of money in other ways, ultimately making it a good deal.
Yet again, though, the CBO said, whether that actually happens depends on the system’s design.
By eliminating most private insurers, a single-payer system would likely slash hospitals’ administrative overhead. The government could then pay a rate that better reflects reduced hospital costs, according to the CBO report.
But, ultimately, the single-payer bottom line depends on what the system pays hospitals, doctors, and drug companies for different services and products. That answer also would inform other economic assessments – ascertaining, for instance, how single-payer affects a small town where the hospital is the main employer.
Even without clear answers, outlining those questions moves the ball, Liu said.
“This area is moving really fast,” she said. “To me, it seems like this is the beginning of a longer conversation.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Are sweeping efforts to reduce primary CD rates associated with an increase in maternal or neonatal AEs?
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Breaking the high-utilization cycle
Hospitalists know that a small percentage of patients account for a disproportionately large percentage of overall health care spending, much of which comes from inpatient admissions. Many programs have been developed around the country to work with this population, and most of these programs are – appropriately – outpatient based.
“However, a subset of frequently admitted patients either don’t make it to outpatient care or are unengaged with outpatient care and programs, for whom hospital stays can give us a unique opportunity to coordinate and streamline care, and to build trust that can then lead to increased patient engagement,” said Kirstin Knox, MD, PhD, of the Hospital of the University of Pennsylvania in Philadelphia, and lead author of an abstract describing a method to address this challenge. “Our program works with these patients, the ‘outliers among the outliers’ to re-engage them in care, streamline admissions, coordinate inpatient and outpatient care, and address the underlying barriers/drivers that lead to frequent hospitalization.”
Their program designed and implemented a multidisciplinary intervention targeting the highest utilizers on their inpatient general medicine service. Each was assigned an inpatient continuity team, and the patient case was then presented to a multidisciplinary high-utilizer care committee that included physicians, nurses, and social workers, as well as representatives from a community health worker program, home care, and risk management to develop a care plan.
Analysis comparing the 6 months before and after intervention showed admissions and total hospital days were reduced by 55% and 47% respectively, and 30-day readmissions were reduced by 65%. Total direct costs were reduced from $2,923,000 to $1,284,000.
The top takeaway, Dr. Knox said, is that, through efforts to coordinate care and address underlying drivers of high utilization, hospital-based programs for the most frequently admitted patients can streamline inpatient care and decrease utilization for many high-risk, high-cost patients.
“I hope that hospitalists will consider starting inpatient-based high-utilizer programs at their own institutions, if they haven’t already,” she said. “Even starting with one or two of your most frequently admitted patients can be incredibly eye opening, and streamlining/coordinating care (as well as working overtime to address the underlying drivers/barriers that lead to high utilization) for these patients is incredibly rewarding.”
Reference
Knox K et al. Breaking the cycle: a successful inpatient based intervention for hospital high utilizers. Abstract published at Hospital Medicine 2018; Apr 8-11; Orlando, Fla., Abstract 319. Accessed 2018 Oct 2.
Hospitalists know that a small percentage of patients account for a disproportionately large percentage of overall health care spending, much of which comes from inpatient admissions. Many programs have been developed around the country to work with this population, and most of these programs are – appropriately – outpatient based.
“However, a subset of frequently admitted patients either don’t make it to outpatient care or are unengaged with outpatient care and programs, for whom hospital stays can give us a unique opportunity to coordinate and streamline care, and to build trust that can then lead to increased patient engagement,” said Kirstin Knox, MD, PhD, of the Hospital of the University of Pennsylvania in Philadelphia, and lead author of an abstract describing a method to address this challenge. “Our program works with these patients, the ‘outliers among the outliers’ to re-engage them in care, streamline admissions, coordinate inpatient and outpatient care, and address the underlying barriers/drivers that lead to frequent hospitalization.”
Their program designed and implemented a multidisciplinary intervention targeting the highest utilizers on their inpatient general medicine service. Each was assigned an inpatient continuity team, and the patient case was then presented to a multidisciplinary high-utilizer care committee that included physicians, nurses, and social workers, as well as representatives from a community health worker program, home care, and risk management to develop a care plan.
Analysis comparing the 6 months before and after intervention showed admissions and total hospital days were reduced by 55% and 47% respectively, and 30-day readmissions were reduced by 65%. Total direct costs were reduced from $2,923,000 to $1,284,000.
The top takeaway, Dr. Knox said, is that, through efforts to coordinate care and address underlying drivers of high utilization, hospital-based programs for the most frequently admitted patients can streamline inpatient care and decrease utilization for many high-risk, high-cost patients.
“I hope that hospitalists will consider starting inpatient-based high-utilizer programs at their own institutions, if they haven’t already,” she said. “Even starting with one or two of your most frequently admitted patients can be incredibly eye opening, and streamlining/coordinating care (as well as working overtime to address the underlying drivers/barriers that lead to high utilization) for these patients is incredibly rewarding.”
Reference
Knox K et al. Breaking the cycle: a successful inpatient based intervention for hospital high utilizers. Abstract published at Hospital Medicine 2018; Apr 8-11; Orlando, Fla., Abstract 319. Accessed 2018 Oct 2.
Hospitalists know that a small percentage of patients account for a disproportionately large percentage of overall health care spending, much of which comes from inpatient admissions. Many programs have been developed around the country to work with this population, and most of these programs are – appropriately – outpatient based.
“However, a subset of frequently admitted patients either don’t make it to outpatient care or are unengaged with outpatient care and programs, for whom hospital stays can give us a unique opportunity to coordinate and streamline care, and to build trust that can then lead to increased patient engagement,” said Kirstin Knox, MD, PhD, of the Hospital of the University of Pennsylvania in Philadelphia, and lead author of an abstract describing a method to address this challenge. “Our program works with these patients, the ‘outliers among the outliers’ to re-engage them in care, streamline admissions, coordinate inpatient and outpatient care, and address the underlying barriers/drivers that lead to frequent hospitalization.”
Their program designed and implemented a multidisciplinary intervention targeting the highest utilizers on their inpatient general medicine service. Each was assigned an inpatient continuity team, and the patient case was then presented to a multidisciplinary high-utilizer care committee that included physicians, nurses, and social workers, as well as representatives from a community health worker program, home care, and risk management to develop a care plan.
Analysis comparing the 6 months before and after intervention showed admissions and total hospital days were reduced by 55% and 47% respectively, and 30-day readmissions were reduced by 65%. Total direct costs were reduced from $2,923,000 to $1,284,000.
The top takeaway, Dr. Knox said, is that, through efforts to coordinate care and address underlying drivers of high utilization, hospital-based programs for the most frequently admitted patients can streamline inpatient care and decrease utilization for many high-risk, high-cost patients.
“I hope that hospitalists will consider starting inpatient-based high-utilizer programs at their own institutions, if they haven’t already,” she said. “Even starting with one or two of your most frequently admitted patients can be incredibly eye opening, and streamlining/coordinating care (as well as working overtime to address the underlying drivers/barriers that lead to high utilization) for these patients is incredibly rewarding.”
Reference
Knox K et al. Breaking the cycle: a successful inpatient based intervention for hospital high utilizers. Abstract published at Hospital Medicine 2018; Apr 8-11; Orlando, Fla., Abstract 319. Accessed 2018 Oct 2.
Erythema on abdomen
The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Outpatient program successfully tackles substance use and chronic pain
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
REPORTING FROM APS 2019
Key clinical point: An intensive, 6-week joint substance use disorder and chronic pain intensive outpatient program significantly reduced both substance use and pain.
Major finding: Patients had less pain and reduced substance use after completing the program, compared with baseline (P = .013 and .041, respectively).
Study details: A quality improvement project including 66 patients at a military health facility.
Disclosures: The study was sponsored by the Defense Health Agency. Dr. Stockin reported no conflicts of interest.
Blast crisis, no crisis? Caring for the apathetic patient
The diagnosis was straightforward. My patient’s reaction was not.
One Saturday evening, I receive a call from the emergency room about a man with a very high white blood cell count. For the past 7 years, he had chronic myeloid leukemia – a cancer, but one of the few that can be well controlled for years. The discovery of the medications that can do it revolutionized care for the disease.
For the last 7 years, Mr. C didn’t take that medication regularly. He was young, with no other medical problems, and this was the only medication he was supposed to take. But his use was sporadic at best.
What was it, I wondered? Cost? Side effects? Not understanding the seriousness of having leukemia? No, the medication was fully covered by his insurance. No, he tolerated it well. Instead, his on-and-off medication schedule came across as a strange sense of apathy. He didn’t seem to recognize his agency in his own life.
Now, not only is his white count extremely high, but the majority are the cancerous cells. I look at his blood under the microscope – blasts everywhere. He has progressed from a chronic, indolent disease that can be kept at bay into the dreaded blast crisis, which is essentially an acute leukemia but even more challenging to treat.
It is very serious. I tell him this. “I am worried your leukemia has progressed into what we call a blast crisis,” I say. “Has anyone ever talked to you about this before?”
“Hmm, I think Dr. M may have said something,” he says. His medical chart over the last 7 years was populated with notes from his hematologist documenting their discussions of this possibility.
“This is serious,” I continue. “You will need to come into the hospital and we need to start medication to lower your white count. Otherwise you could have a stroke.”
“Okay.”
“As the white count comes down, your cells will break open and the chemicals in them can make you very sick. So we will have to check your blood often to watch for this.”
“Got it.”
“And we will change your chemotherapy pill.” I pause, letting it sink in, then repeat for emphasis: “This is very serious.”
“Sure thing, Doc.”
“I know I’ve said a lot. What are your thoughts?”
He looks at his wife, then back at me. He seems unfazed. Just as unfazed as when his hematologist warned this could happen. Just as unfazed as the day he learned his diagnosis.
He smiles and shrugs. “What will be, will be.”
As I listened to him, I honestly couldn’t tell if this was the best coping mechanism I had ever seen or the worst.
On one hand, his apathy had hurt him, clearly and indisputably. Refusing to acknowledge his agency in his medical outcomes allowed him to be cavalier about taking the cure. The cure was in a bottle on his kitchen shelf, an arm’s reach away, and he chose to reach elsewhere.
On the other hand, it was unusual to see someone so at peace with being so critically ill. His acceptance of his new reality was refreshing. There were no heartbreaking questions about whether this was his fault. There was no agonizing over what could have been. His apathy gave him closure and his loved ones comfort.
I’ve written before about how a cancer diagnosis involves holding two seemingly competing ideas in one’s mind at once. Last month, I wrote about how it is possible to be realistic about a grim prognosis while retaining hope that a treatment may work. I discussed that realism and hopefulness are compatible beliefs, and it’s okay – preferred, even – to hold them at once.
Mr. C’s strange sense of apathy made me think about another mental limbo, this one involving control. As doctors and patients, we like when we have agency over outcomes. Take these medications, and you will be okay. Undergo this procedure, and you will reduce your risk of recurrence. At the same time, poor outcomes still occur when everything is done “right.” When that happens, it can be psychologically beneficial to relinquish control. Doing so discards the unhelpful emotions of guilt and blame in favor of acceptance.
Mr. C’s apathy seemed to be present from day 1. But now, in a dire blast crisis, what was once a harmful attitude actually became a helpful one.
His “what will be, will be” attitude wasn’t inherently maladaptive; it was ill timed. Under the right circumstances, well-placed apathy can be leveraged as a positive coping mechanism.
But alas, if only there were a switch to turn on the right emotion at the right time. There’s no right or wrong or sensible reaction to cancer. There’s only a swirl of messy, overwhelming feelings. It’s trying to bring effective emotions to light at the right time while playing whack-a-mole with the others. It’s cognitive dissonance. It’s exhausting. Cancer doesn’t create personalities; it surfaces them.
It’s the last day of Mr. C’s hospitalization. His blast crisis is amazingly under good control.
“So,” I say. “Will you take your medications now?”
“Sure,” he says instinctively. I look at him. “I mean, honestly, Doc? I’m not sure.”
As we shake hands, I wonder if I’ll ever truly understand Mr. C’s motivations. But I can’t wonder too long. I can only control my part: I hand him his medications and wish him luck.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
The diagnosis was straightforward. My patient’s reaction was not.
One Saturday evening, I receive a call from the emergency room about a man with a very high white blood cell count. For the past 7 years, he had chronic myeloid leukemia – a cancer, but one of the few that can be well controlled for years. The discovery of the medications that can do it revolutionized care for the disease.
For the last 7 years, Mr. C didn’t take that medication regularly. He was young, with no other medical problems, and this was the only medication he was supposed to take. But his use was sporadic at best.
What was it, I wondered? Cost? Side effects? Not understanding the seriousness of having leukemia? No, the medication was fully covered by his insurance. No, he tolerated it well. Instead, his on-and-off medication schedule came across as a strange sense of apathy. He didn’t seem to recognize his agency in his own life.
Now, not only is his white count extremely high, but the majority are the cancerous cells. I look at his blood under the microscope – blasts everywhere. He has progressed from a chronic, indolent disease that can be kept at bay into the dreaded blast crisis, which is essentially an acute leukemia but even more challenging to treat.
It is very serious. I tell him this. “I am worried your leukemia has progressed into what we call a blast crisis,” I say. “Has anyone ever talked to you about this before?”
“Hmm, I think Dr. M may have said something,” he says. His medical chart over the last 7 years was populated with notes from his hematologist documenting their discussions of this possibility.
“This is serious,” I continue. “You will need to come into the hospital and we need to start medication to lower your white count. Otherwise you could have a stroke.”
“Okay.”
“As the white count comes down, your cells will break open and the chemicals in them can make you very sick. So we will have to check your blood often to watch for this.”
“Got it.”
“And we will change your chemotherapy pill.” I pause, letting it sink in, then repeat for emphasis: “This is very serious.”
“Sure thing, Doc.”
“I know I’ve said a lot. What are your thoughts?”
He looks at his wife, then back at me. He seems unfazed. Just as unfazed as when his hematologist warned this could happen. Just as unfazed as the day he learned his diagnosis.
He smiles and shrugs. “What will be, will be.”
As I listened to him, I honestly couldn’t tell if this was the best coping mechanism I had ever seen or the worst.
On one hand, his apathy had hurt him, clearly and indisputably. Refusing to acknowledge his agency in his medical outcomes allowed him to be cavalier about taking the cure. The cure was in a bottle on his kitchen shelf, an arm’s reach away, and he chose to reach elsewhere.
On the other hand, it was unusual to see someone so at peace with being so critically ill. His acceptance of his new reality was refreshing. There were no heartbreaking questions about whether this was his fault. There was no agonizing over what could have been. His apathy gave him closure and his loved ones comfort.
I’ve written before about how a cancer diagnosis involves holding two seemingly competing ideas in one’s mind at once. Last month, I wrote about how it is possible to be realistic about a grim prognosis while retaining hope that a treatment may work. I discussed that realism and hopefulness are compatible beliefs, and it’s okay – preferred, even – to hold them at once.
Mr. C’s strange sense of apathy made me think about another mental limbo, this one involving control. As doctors and patients, we like when we have agency over outcomes. Take these medications, and you will be okay. Undergo this procedure, and you will reduce your risk of recurrence. At the same time, poor outcomes still occur when everything is done “right.” When that happens, it can be psychologically beneficial to relinquish control. Doing so discards the unhelpful emotions of guilt and blame in favor of acceptance.
Mr. C’s apathy seemed to be present from day 1. But now, in a dire blast crisis, what was once a harmful attitude actually became a helpful one.
His “what will be, will be” attitude wasn’t inherently maladaptive; it was ill timed. Under the right circumstances, well-placed apathy can be leveraged as a positive coping mechanism.
But alas, if only there were a switch to turn on the right emotion at the right time. There’s no right or wrong or sensible reaction to cancer. There’s only a swirl of messy, overwhelming feelings. It’s trying to bring effective emotions to light at the right time while playing whack-a-mole with the others. It’s cognitive dissonance. It’s exhausting. Cancer doesn’t create personalities; it surfaces them.
It’s the last day of Mr. C’s hospitalization. His blast crisis is amazingly under good control.
“So,” I say. “Will you take your medications now?”
“Sure,” he says instinctively. I look at him. “I mean, honestly, Doc? I’m not sure.”
As we shake hands, I wonder if I’ll ever truly understand Mr. C’s motivations. But I can’t wonder too long. I can only control my part: I hand him his medications and wish him luck.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
The diagnosis was straightforward. My patient’s reaction was not.
One Saturday evening, I receive a call from the emergency room about a man with a very high white blood cell count. For the past 7 years, he had chronic myeloid leukemia – a cancer, but one of the few that can be well controlled for years. The discovery of the medications that can do it revolutionized care for the disease.
For the last 7 years, Mr. C didn’t take that medication regularly. He was young, with no other medical problems, and this was the only medication he was supposed to take. But his use was sporadic at best.
What was it, I wondered? Cost? Side effects? Not understanding the seriousness of having leukemia? No, the medication was fully covered by his insurance. No, he tolerated it well. Instead, his on-and-off medication schedule came across as a strange sense of apathy. He didn’t seem to recognize his agency in his own life.
Now, not only is his white count extremely high, but the majority are the cancerous cells. I look at his blood under the microscope – blasts everywhere. He has progressed from a chronic, indolent disease that can be kept at bay into the dreaded blast crisis, which is essentially an acute leukemia but even more challenging to treat.
It is very serious. I tell him this. “I am worried your leukemia has progressed into what we call a blast crisis,” I say. “Has anyone ever talked to you about this before?”
“Hmm, I think Dr. M may have said something,” he says. His medical chart over the last 7 years was populated with notes from his hematologist documenting their discussions of this possibility.
“This is serious,” I continue. “You will need to come into the hospital and we need to start medication to lower your white count. Otherwise you could have a stroke.”
“Okay.”
“As the white count comes down, your cells will break open and the chemicals in them can make you very sick. So we will have to check your blood often to watch for this.”
“Got it.”
“And we will change your chemotherapy pill.” I pause, letting it sink in, then repeat for emphasis: “This is very serious.”
“Sure thing, Doc.”
“I know I’ve said a lot. What are your thoughts?”
He looks at his wife, then back at me. He seems unfazed. Just as unfazed as when his hematologist warned this could happen. Just as unfazed as the day he learned his diagnosis.
He smiles and shrugs. “What will be, will be.”
As I listened to him, I honestly couldn’t tell if this was the best coping mechanism I had ever seen or the worst.
On one hand, his apathy had hurt him, clearly and indisputably. Refusing to acknowledge his agency in his medical outcomes allowed him to be cavalier about taking the cure. The cure was in a bottle on his kitchen shelf, an arm’s reach away, and he chose to reach elsewhere.
On the other hand, it was unusual to see someone so at peace with being so critically ill. His acceptance of his new reality was refreshing. There were no heartbreaking questions about whether this was his fault. There was no agonizing over what could have been. His apathy gave him closure and his loved ones comfort.
I’ve written before about how a cancer diagnosis involves holding two seemingly competing ideas in one’s mind at once. Last month, I wrote about how it is possible to be realistic about a grim prognosis while retaining hope that a treatment may work. I discussed that realism and hopefulness are compatible beliefs, and it’s okay – preferred, even – to hold them at once.
Mr. C’s strange sense of apathy made me think about another mental limbo, this one involving control. As doctors and patients, we like when we have agency over outcomes. Take these medications, and you will be okay. Undergo this procedure, and you will reduce your risk of recurrence. At the same time, poor outcomes still occur when everything is done “right.” When that happens, it can be psychologically beneficial to relinquish control. Doing so discards the unhelpful emotions of guilt and blame in favor of acceptance.
Mr. C’s apathy seemed to be present from day 1. But now, in a dire blast crisis, what was once a harmful attitude actually became a helpful one.
His “what will be, will be” attitude wasn’t inherently maladaptive; it was ill timed. Under the right circumstances, well-placed apathy can be leveraged as a positive coping mechanism.
But alas, if only there were a switch to turn on the right emotion at the right time. There’s no right or wrong or sensible reaction to cancer. There’s only a swirl of messy, overwhelming feelings. It’s trying to bring effective emotions to light at the right time while playing whack-a-mole with the others. It’s cognitive dissonance. It’s exhausting. Cancer doesn’t create personalities; it surfaces them.
It’s the last day of Mr. C’s hospitalization. His blast crisis is amazingly under good control.
“So,” I say. “Will you take your medications now?”
“Sure,” he says instinctively. I look at him. “I mean, honestly, Doc? I’m not sure.”
As we shake hands, I wonder if I’ll ever truly understand Mr. C’s motivations. But I can’t wonder too long. I can only control my part: I hand him his medications and wish him luck.
Minor details of this story were changed to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.