User login
Use of “complication” triggers Medicare denial
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
Establishing a Rapid Response Team
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

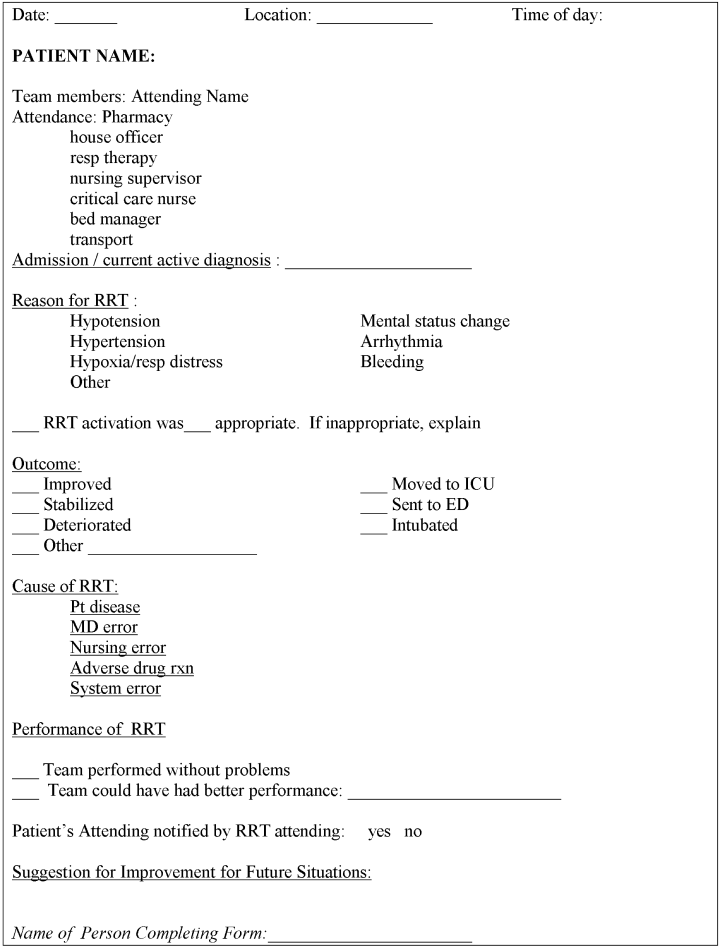
Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.
The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
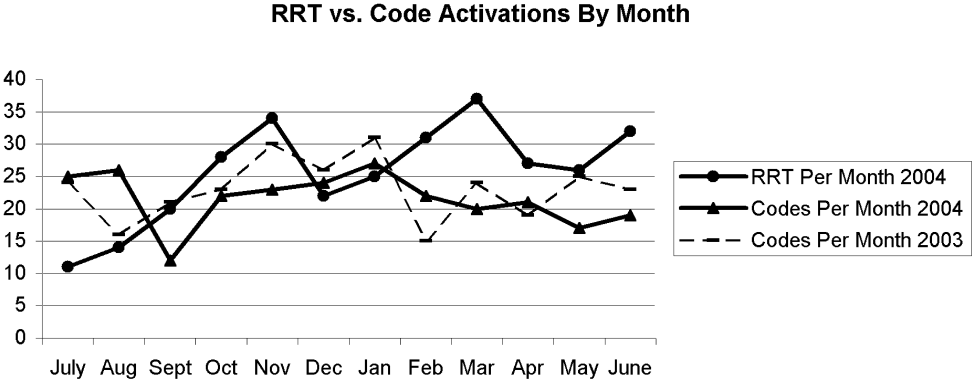
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).
Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Evidence‐Based Sepsis Therapy
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.
Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.
Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
Clinical Conundrum
A 35‐year‐old man presented to the emergency department of a community hospital with 3 days of nausea, vomiting, abdominal pain, and diarrhea. He had developed a sore throat, nasal congestion, and green sputum on a business trip to Las Vegas 10 days prior. He then traveled to Shanghai, China, where he developed frequent diarrhea with mucous and urgency. The stool was mustard colored without blood or melena. He became nauseated and unable to keep down food or fluids. He noted a 4‐kg weight loss since the beginning of his symptoms. He had not traveled outside Shanghai or eaten exotic foods. His travel companions remained unaffected. The patient had had type I diabetes mellitus for 23 years with known retinopathy and microalbuminuria. He had hypertension and hyperlipidemia but was otherwise healthy. He was a married traveling salesman with 3 healthy children. He did not smoke or drink and reported no drug use or extramarital relations. He had no known allergies. His medications included insulin, lisinopril, atorvastatin, and valsartan. He was afebrile, his vital signs were stable and the physical examination was unremarkable.
The patient presents with gastrointestinal symptoms following a trip to China. He may have an infection that began in the respiratory system and now is causing some gastrointestinal symptoms, like Legionnella. If he had received antibiotics, the diarrheal illness could be a complication. With his recent travel to China, typical enteric pathogens would have to be considered: enterotoxigenic E. coli, Shigella, Salmonella, Campylobacter, or perhaps Giardia.
He was admitted and treated with intravenous fluids and ciprofloxacin for presumed gastroenteritis. He was discharged the next day but returned 2 days later because of continued nausea and vomiting and limited oral intake. He was febrile, to 38.9C, with chills. The results of an abdominal exam were normal. Stool studies for Salmonella, Shigella, Campylobacter, Yersinia, enterotoxigenic E. coli, Giardia, and Cryptosporidium were negative. No blood parasites were seen. The white‐cell count was 3900/mm3 with a normal differential count. Hemoglobin level, platelet count, serum electrolytes and creatinine were normal. He was readmitted for intravenous fluids.
The patient was treated for traveler's diarrhea, although this is not a typical case and is now becoming a protracted illness. Amebiasis would be a consideration, as well as typhoid or perhaps an abdominal abscess. The normal platelet count reduces the likelihood of hemolytic uremic syndrome, as does the absence of bloody diarrhea. I would evaluate for a systemic illness: check for adenopathy, do a thorough abdominal exam, and get a chest radiogram, blood cultures, and liver function tests. I am also concerned about metabolic abnormalities that could occur as a consequence of the diarrhea.
Over 3 days, oliguria developed along with urinary hesitancy, a 9‐kg weight gain, and the development of marked edema. Blood pressure and heart rate remained normal, and a chest radiograph was clear. Liver function tests were normal. A urinary catheter was inserted. Urinalysis revealed a specific gravity of 1.031, protein of 100 mg/dL, and trace glucose but was otherwise negative; no casts or cells were seen in the sediment. Chemistries included sodium of 133 mmol/L, potassium of 3.9 mmol/L, and serum bicarbonate of 18.4 mmol/L. Blood and urine cultures were sterile. The creatinine increased from 1.0 mg/dL (88.4 mol/L) to 1.3 mg/dL (115 mol/L). He was transferred to a tertiary care hospital for renal consultation because of concerns of impending renal failure and for consideration of a kidney biopsy.
In a typical case of a malabsorptive diarrhea, the patient could be volume depleted, but in this case he has gained 9‐kg and is grossly edematous. The chest radiograph and liver tests point to renal rather than cardiac or hepatologic causes for the edema. A glomerulonephritis may be driving the salt and water retention.
The proteinuria could be related to hemodynamics, or it could be from a glomerular lesion secondary to immune complexes. The specific gravity of 1.031 indicates the kidney is able to concentrate, and we are not seeing acute tubular necrosis. There is only minimal elevation in creatinine at this point. Quantitation or estimation of the degree of proteinuria by a protein‐to‐creatinine ratio would be helpful.
A further workup should include additional blood cultures and a CT scan of the lungs and abdomen to look for occult infection. Is he unfortunate enough to have developed a malignancy? Is this a connective tissue disease? Reexamination of the urine sediment is important to evaluate for glomerulonephritis.
The patient reported ongoing nausea and vomiting, but his diarrhea resolved. He was tachypneic, with a respiratory rate of 26 breaths/minute and an oxygen saturation of 98% breathing ambient air. His temperature was 37.1C, heart rate 84 beats/minute, and blood pressure 120/65 mm Hg. His mucous membranes were moist, and his jugular venous pressure was 6 cm. No lymphadenopathy was present. The heart and lungs were normal. The abdomen was soft, nontender, and without organomegaly, masses, or shifting dullness; bowel sounds were hypoactive. Severe edema of his legs, sacrum, hands, arms, and orbits was noted. His right hand and wrist were painful with limited mobility and small joint effusions of the wrist and metacarpophalangeal joints, but without erythema or warmth. Small petechiae were noted on his eyelids; skin examination was otherwise unremarkable. He had been given ciprofloxacin, phenergan, calcium carbonate, and pantoprazole prior to transfer. Stool was negative for occult blood.
His lungs are clear, but it is possible to have early pulmonary congestion with normal breath sounds. As he is normotensive and has a normal JVP, I would not give further intravenous fluid. Unless he has evidence of symptomatic pulmonary edema, I would not give diuretics but would simply observe his course. At this point I would ultrasound his kidneys to make sure there is no obstruction. The proteinuria could be a result of underlying diabetic glomerulopathy that may predispose to fluid retention.
He has significant oliguria but only a mild rise in creatinine. As the referring physicians requested a biopsy, we should consider it. A decision to biopsy the kidney would rest on the degree of proteinuria and the activity of the sediment. For example, if red blood cell casts or dysmorphic red cells were present, postinfectious glomerulonephritis or IgA nephropathy would be more likely. However, if the proteinuria is in the non‐nephrotic range and the sediment is nonreactive, the yield of a biopsy would be low.
His right hand and wrist are painful with limited mobility. This could be a sequela of endocarditis, although the absence of a murmur and negative blood cultures make it unlikely. He could have an infectious arthritis, although this usually presents more dramatically. He could have gout or pseudogout, which could be determined by joint aspiration. Finally, could this be iatrogenic? A drug reaction could explain some of the features, including rash, fever, joint symptoms, and renal abnormalities.
Repeat urinalysis revealed protein of more than 300 mg/dL, hematuria (1+), mucous, renal tubular epithelial cells, renal tubular epithelial cell casts, and granular casts. Eosinophiluria was absent. Laboratory evaluation revealed a hemoglobin level of 10.7 g/dL (decreased from 13 g/dL on initial presentation), white‐cell count of 7300/mm3, platelet count of 337,000/mm3, serum creatinine of 1.2 mg/dL (106 mol/L), blood urea nitrogen of 23 mg/dL (8.2 mol.L), total serum protein of 6.3 g/dL (normal range, 6.3‐8.7), and albumin of 2.7 g/dL (normal range, 3.2‐5.2). Other liver tests and serum electrolytes were normal.
This degree of proteinuria is significant, but it is unclear whether this is related to the underlying disease process or his advancing diabetes. He has some hematuria, but that could be from the urinary catheter. It would be helpful to know if the red blood cells are dysmorphic, which would point to a glomerulonephritis. He has renal cells, renal cell casts, and granular casts, which are nonspecific. He has a mild anemia, which is unexplained, but could relate to phlebotomy or overhydration. The hypoalbuminemia may be a result of renal losses or a catabolic state.
A renal ultrasonogram was normal, apart from evidence of bilateral pleural effusions. Antinuclear antibody and rheumatoid factor test results were negative, as were those for hepatitis A, hepatitis B surface antigen, and hepatitis C antibodies. Antistreptolysin O and antideoxyribonuclease B titers were normal. Total complement Ch50 was low at 29 U/mL (normal range, 30‐75) as was complement factor C3 at 67 mg/dL (normal range, 90‐180). Complement factor C4 was normal. Serum and urine electrophoresis revealed no monoclonal protein spike. Vitamin B12 and serum folate were normal, serum ferritin was 584 ng/mL (normal range, 30‐400), iron serum was 29 g/dL (normal range, 45‐160), transferrin saturation was 16%, and total iron‐binding capacity was 164 g/dL (normal range, 250‐450). The reticulocyte count was 2.9% with an absolute reticulocyte count of 102/cm3 and a reticulocyte production index of 0.96 (normal range, 1.0‐2.0).
It is reassuring that his urinary tract ultrasound is normal. In addition to edema, he has bilateral effusions, which are probably transudative, related to fluid overload. The urinalysis does not suggest a rapidly progressive glomerulonephritis, but autoimmune disease is still in the differential.
He has a mild complement C3 deficiency. In nephrology we think of lupus, infective endocarditis, cryoglobulinemia, and specific glomerular lesions such as membranoproliferative glomerulonephritis and postinfectious glomerulonephritis as being associated with the development of circulating immune complexes that may lead to low complement levels. There is no evidence of a paraprotein, but testing for cryoglobulins should be considered. Cryoglobulins are associated with hepatitis C but may be induced by a variety of infections. Acting like immune complexes, they can lead to low complement levels and could cause some of this patient's symptoms. However, this whole illness seems most likely to be secondary to infection. The normal antistreptolysin O and antideoxyribonuclease B titers make streptococcal disease unlikely, but another bacterial infection could cause postinfectious glomerulonephritis.
Over the course of his 5‐day hospital stay, the patient received furosemide with increased urine output and normalization of his serum creatinine to its baseline level of 1.0 mg.dL (88.4 mol/L). Proteinuria resolved to 44 mg/dL. A kidney biopsy was not performed. The parvovirus IgG index, checked because of anemia and oligoarthralgias, was 3.67 (normal 0‐1.10), and the IgM index was 8.13 (normal 0‐1.10), suggesting recent infection. The patient was discharged after 5 days. His edema had resolved on discharge; he continued to be nauseated but was able to eat and drink normally. Six months after his hospitalization, his symptoms had completely resolved.
Parvovirus! It could cause the pulmonary infection and the gastroenteric symptoms. Parvovirus usually causes more anemia than nausea and vomiting. We see it occasionally in our transplant patients. The underlying diabetic nephropathy may have made him more symptomatic with a superimposed glomerulonephritis. The most important pedagogic point is that he did well with a very conservative approach, and the possible iatrogenic consequences of a kidney biopsy, had it been performed, were avoided.
COMMENTARY
Parvovirus B19 is endemic, with as many as 80% of adults showing serologic evidence of past infection. Although most adults with detectable B19‐specific IgG do not recall having had specific symptoms, a number of syndromes have been associated with acute infection.1, 2 Parvovirus B19 should be included in the differential for postinfectious glomerulonephritis, especially if a patient presents with marked edema with preserved renal function.
Human parvovirus B19, a member of the erythrovirus genus, is a nonenveloped single‐stranded DNA virus that propagates in erythroid progenitor cells, arresting erythropoiesis.3 The cellular receptor for the virus is globoside (erythrocyte P antigen), a neutral glycosphingolipid densely present on erythroid cells and also found on hepatocytes, nephrons, and bowel mucosa.3, 4
The most common clinical presentation of parvovirus B19 in children is erythema infectiosum, or fifth disease.3 In adults, the infection is known to cause symmetric polyarthropathy, rash, malaise, coryza, headache, and gastrointestinal symptoms (nausea, abdominal pain) and may mimic systemic lupus erythematosus.1, 3 In patients with sickle cell anemia or other chronic hemolytic disorders, parvovirus B19 can cause a transient aplastic crisis.3 Immunosuppressed patients (eg, organ transplant recipients, patients with certain cancers or advanced AIDS) may develop chronic infection and anemia because of an inability to mount an immune response to clear viremia. Mild anemia or pancytopenia is frequently observed in normal infected hosts.
The syndrome of renal involvement in parvovirus B19 includes the typical features of fever, a maculopapular or reticular erythematous rash on the face or extremities, and polyarthritis, accompanied by oliguria that leads to systemic edema. Mild pancytopenia, proteinuria, hematuria, and hypocomplementemia are often present. Creatinine is usually normal or near normal. These symptoms typically appear 1‐2 weeks after the initial viral syndrome.5, 6 With supportive care, most recover spontaneously, although chronic kidney disease has been reported.7, 8
Published kidney biopsy findings of parvovirus B19 show endocapillary or mesangial proliferative glomerulonephritis with subendothelial electron‐dense deposits and granular deposition of C3, IgG, or IgM along the capillary walls and mesangium. These lesions suggest immune complex deposition and are consistent with postinfectious glomerulonephritis.5, 9, 10 Indeed, increased levels of circulating immune complexes have been seen during acute parvovirus B19 infection.6, 9 It is likely that the protracted symptoms our patient experienced resulted from the formation, circulation, and deposition of immune complexes. The presence of globoside in the kidneys and bowel also raises the possibility of direct infection of these organs.
Postinfectious glomerulonephritis is often thought to be synonymous with poststreptococcal glomerulonephritis. However, viruses, including hepatitis B and C viruses, human immunodeficiency virus, cytomegalovirus, hantavirus, and parvovirus B19 may cause postinfectious glomerulonephritis. As with poststreptococcal glomerulonephritis, glomerular disease associated with viral infection appears to be mediated by the immune complexes. The pathogenic series of events leading to glomerular injury includes formation of circulating immune complexes with subsequent deposition in the glomerulus, or formation of in situ antigen‐antibody reactions.11 Immune complexes in the glomerulus lead to activation of the complement cascade, which in turn leads to hypocomplementemia, as the complement cascade is activated faster than the synthesis of new complement proteins.12 Histologically, a number of different renal lesions may be seen in postviral glomerulonephritis, including membranous, membranoproliferative, and mesangial glomerulonephritis, as well as focal segmental glomerulosclerosis.
Our patient presented with symptoms compatible with but not specific for parvovirus B19. Using a pattern recognition approach to diagnosis, our discussant correctly identified the disease pattern as a postinfectious glomerulonephritis but was unable to identify the correct pathogen, as bacterial infections were the main focus of concern, and viruses, parvovirus B19 in particular, were not considered. The clinical pattern of arthralgia, gastrointestinal symptoms, fever combined with anemia or pancytopenia, and hypocomplementemia is typical of the clues for parvovirus B19. Although renal involvement is unusual, the presence of oliguria, hematuria, and edema with minimal creatinine elevation is typical of parvovirus renal disease.
An essential part of clinical judgment is carefully determining which of a patient's often myriad complaints must be considered part of the disease process. Common and nonspecific signs and symptoms often fall off the clinician's radar screen. In this instance, several of the hallmark features of parvovirus B19 disease were dismissed by our discussant as due to the patient's previous medical conditions or hospital‐related interventions. Anemia (due to interruption of erythropoiesis by parvovirus B19 replication) was attributed to hydration or phlebotomy, fluid retention was attributed to advancing diabetes, and hematuria was attributed to a urinary catheter. It is important to evaluate the entire clinical picture prior to excluding potential clues to the diagnosis. Another reasonable approach would have been to choose a less general sign or symptom to narrow the possible diagnoses. For example, had the wrist arthralgia been more central in the discussant's thoughts, parvovirus B19 might have appeared on the differential.
Finally, the discussant wrestled with the decision to perform a renal biopsy for a definitive diagnosis versus the potential complications of the procedure. In this case, it was possible to achieve a clinical diagnosis, support it with serologic evidence, and thus avoid the need for biopsy. The current medical climate emphasizes the importance of reaching a definitive diagnosis as rapidly as possible. There are pressures to act quickly and utilize technology that may add both cost and risk. This case emphasizes the value of clinical reasoning and patience, which led to a correct diagnosis and a favorable outcome without the need for invasive procedures. Clinical acumen must occasionally include avoiding the temptation to perform the next test and merely standing at the patient's bedside instead.
- ,,, et al.Clinical manifestations of human parvovirus B19 in adults.Arch Intern Med.1989;149:1153‐1156.
- ,.The prevalence of antibody to human parvovirus B19 in England and Wales.J Med Microbiol.1999;25:25‐28.
- ,.Parvovirus B19.N Engl J Med.2004;350:586‐597.
- ,,.Multiple glycosphingolipids determine the tissue tropism of parvovirus B19.J Infect Dis.1995;172:1198‐1205.
- ,,,.Renal involvement induced by human parvovirus B19 infection.Nephron.2001;89:280‐285.
- ,,, et al.Association of parvovirus B19 infection with acute glomerulonephritis in the healthy adults: case report and review of the literature.Clin Nephrol.2002;57:69‐73.
- .Renal involvement in human parvovirus B19 infection.Pediatr Nephrol.2003;18:966‐967.
- ,,, et al.Acute glomerulonephritis after human parvovirus B19 infection.Am J Kidney Dis.2000;35:1‐8.
- ,,,,.Human parvovirus B19 and renal disease?Neth J Med.2000;56:163‐165.
- ,,, et al.Nephrotic syndrome associated with human parvovirus B19 infection.Pediatr Nephrol.2003;18:280‐282.
- ,.Glomerulonephritis.Lancet.2005;365:1797‐1806.
- .Complement and the kidney.J Immunol.2003;171:3319‐3324.
A 35‐year‐old man presented to the emergency department of a community hospital with 3 days of nausea, vomiting, abdominal pain, and diarrhea. He had developed a sore throat, nasal congestion, and green sputum on a business trip to Las Vegas 10 days prior. He then traveled to Shanghai, China, where he developed frequent diarrhea with mucous and urgency. The stool was mustard colored without blood or melena. He became nauseated and unable to keep down food or fluids. He noted a 4‐kg weight loss since the beginning of his symptoms. He had not traveled outside Shanghai or eaten exotic foods. His travel companions remained unaffected. The patient had had type I diabetes mellitus for 23 years with known retinopathy and microalbuminuria. He had hypertension and hyperlipidemia but was otherwise healthy. He was a married traveling salesman with 3 healthy children. He did not smoke or drink and reported no drug use or extramarital relations. He had no known allergies. His medications included insulin, lisinopril, atorvastatin, and valsartan. He was afebrile, his vital signs were stable and the physical examination was unremarkable.
The patient presents with gastrointestinal symptoms following a trip to China. He may have an infection that began in the respiratory system and now is causing some gastrointestinal symptoms, like Legionnella. If he had received antibiotics, the diarrheal illness could be a complication. With his recent travel to China, typical enteric pathogens would have to be considered: enterotoxigenic E. coli, Shigella, Salmonella, Campylobacter, or perhaps Giardia.
He was admitted and treated with intravenous fluids and ciprofloxacin for presumed gastroenteritis. He was discharged the next day but returned 2 days later because of continued nausea and vomiting and limited oral intake. He was febrile, to 38.9C, with chills. The results of an abdominal exam were normal. Stool studies for Salmonella, Shigella, Campylobacter, Yersinia, enterotoxigenic E. coli, Giardia, and Cryptosporidium were negative. No blood parasites were seen. The white‐cell count was 3900/mm3 with a normal differential count. Hemoglobin level, platelet count, serum electrolytes and creatinine were normal. He was readmitted for intravenous fluids.
The patient was treated for traveler's diarrhea, although this is not a typical case and is now becoming a protracted illness. Amebiasis would be a consideration, as well as typhoid or perhaps an abdominal abscess. The normal platelet count reduces the likelihood of hemolytic uremic syndrome, as does the absence of bloody diarrhea. I would evaluate for a systemic illness: check for adenopathy, do a thorough abdominal exam, and get a chest radiogram, blood cultures, and liver function tests. I am also concerned about metabolic abnormalities that could occur as a consequence of the diarrhea.
Over 3 days, oliguria developed along with urinary hesitancy, a 9‐kg weight gain, and the development of marked edema. Blood pressure and heart rate remained normal, and a chest radiograph was clear. Liver function tests were normal. A urinary catheter was inserted. Urinalysis revealed a specific gravity of 1.031, protein of 100 mg/dL, and trace glucose but was otherwise negative; no casts or cells were seen in the sediment. Chemistries included sodium of 133 mmol/L, potassium of 3.9 mmol/L, and serum bicarbonate of 18.4 mmol/L. Blood and urine cultures were sterile. The creatinine increased from 1.0 mg/dL (88.4 mol/L) to 1.3 mg/dL (115 mol/L). He was transferred to a tertiary care hospital for renal consultation because of concerns of impending renal failure and for consideration of a kidney biopsy.
In a typical case of a malabsorptive diarrhea, the patient could be volume depleted, but in this case he has gained 9‐kg and is grossly edematous. The chest radiograph and liver tests point to renal rather than cardiac or hepatologic causes for the edema. A glomerulonephritis may be driving the salt and water retention.
The proteinuria could be related to hemodynamics, or it could be from a glomerular lesion secondary to immune complexes. The specific gravity of 1.031 indicates the kidney is able to concentrate, and we are not seeing acute tubular necrosis. There is only minimal elevation in creatinine at this point. Quantitation or estimation of the degree of proteinuria by a protein‐to‐creatinine ratio would be helpful.
A further workup should include additional blood cultures and a CT scan of the lungs and abdomen to look for occult infection. Is he unfortunate enough to have developed a malignancy? Is this a connective tissue disease? Reexamination of the urine sediment is important to evaluate for glomerulonephritis.
The patient reported ongoing nausea and vomiting, but his diarrhea resolved. He was tachypneic, with a respiratory rate of 26 breaths/minute and an oxygen saturation of 98% breathing ambient air. His temperature was 37.1C, heart rate 84 beats/minute, and blood pressure 120/65 mm Hg. His mucous membranes were moist, and his jugular venous pressure was 6 cm. No lymphadenopathy was present. The heart and lungs were normal. The abdomen was soft, nontender, and without organomegaly, masses, or shifting dullness; bowel sounds were hypoactive. Severe edema of his legs, sacrum, hands, arms, and orbits was noted. His right hand and wrist were painful with limited mobility and small joint effusions of the wrist and metacarpophalangeal joints, but without erythema or warmth. Small petechiae were noted on his eyelids; skin examination was otherwise unremarkable. He had been given ciprofloxacin, phenergan, calcium carbonate, and pantoprazole prior to transfer. Stool was negative for occult blood.
His lungs are clear, but it is possible to have early pulmonary congestion with normal breath sounds. As he is normotensive and has a normal JVP, I would not give further intravenous fluid. Unless he has evidence of symptomatic pulmonary edema, I would not give diuretics but would simply observe his course. At this point I would ultrasound his kidneys to make sure there is no obstruction. The proteinuria could be a result of underlying diabetic glomerulopathy that may predispose to fluid retention.
He has significant oliguria but only a mild rise in creatinine. As the referring physicians requested a biopsy, we should consider it. A decision to biopsy the kidney would rest on the degree of proteinuria and the activity of the sediment. For example, if red blood cell casts or dysmorphic red cells were present, postinfectious glomerulonephritis or IgA nephropathy would be more likely. However, if the proteinuria is in the non‐nephrotic range and the sediment is nonreactive, the yield of a biopsy would be low.
His right hand and wrist are painful with limited mobility. This could be a sequela of endocarditis, although the absence of a murmur and negative blood cultures make it unlikely. He could have an infectious arthritis, although this usually presents more dramatically. He could have gout or pseudogout, which could be determined by joint aspiration. Finally, could this be iatrogenic? A drug reaction could explain some of the features, including rash, fever, joint symptoms, and renal abnormalities.
Repeat urinalysis revealed protein of more than 300 mg/dL, hematuria (1+), mucous, renal tubular epithelial cells, renal tubular epithelial cell casts, and granular casts. Eosinophiluria was absent. Laboratory evaluation revealed a hemoglobin level of 10.7 g/dL (decreased from 13 g/dL on initial presentation), white‐cell count of 7300/mm3, platelet count of 337,000/mm3, serum creatinine of 1.2 mg/dL (106 mol/L), blood urea nitrogen of 23 mg/dL (8.2 mol.L), total serum protein of 6.3 g/dL (normal range, 6.3‐8.7), and albumin of 2.7 g/dL (normal range, 3.2‐5.2). Other liver tests and serum electrolytes were normal.
This degree of proteinuria is significant, but it is unclear whether this is related to the underlying disease process or his advancing diabetes. He has some hematuria, but that could be from the urinary catheter. It would be helpful to know if the red blood cells are dysmorphic, which would point to a glomerulonephritis. He has renal cells, renal cell casts, and granular casts, which are nonspecific. He has a mild anemia, which is unexplained, but could relate to phlebotomy or overhydration. The hypoalbuminemia may be a result of renal losses or a catabolic state.
A renal ultrasonogram was normal, apart from evidence of bilateral pleural effusions. Antinuclear antibody and rheumatoid factor test results were negative, as were those for hepatitis A, hepatitis B surface antigen, and hepatitis C antibodies. Antistreptolysin O and antideoxyribonuclease B titers were normal. Total complement Ch50 was low at 29 U/mL (normal range, 30‐75) as was complement factor C3 at 67 mg/dL (normal range, 90‐180). Complement factor C4 was normal. Serum and urine electrophoresis revealed no monoclonal protein spike. Vitamin B12 and serum folate were normal, serum ferritin was 584 ng/mL (normal range, 30‐400), iron serum was 29 g/dL (normal range, 45‐160), transferrin saturation was 16%, and total iron‐binding capacity was 164 g/dL (normal range, 250‐450). The reticulocyte count was 2.9% with an absolute reticulocyte count of 102/cm3 and a reticulocyte production index of 0.96 (normal range, 1.0‐2.0).
It is reassuring that his urinary tract ultrasound is normal. In addition to edema, he has bilateral effusions, which are probably transudative, related to fluid overload. The urinalysis does not suggest a rapidly progressive glomerulonephritis, but autoimmune disease is still in the differential.
He has a mild complement C3 deficiency. In nephrology we think of lupus, infective endocarditis, cryoglobulinemia, and specific glomerular lesions such as membranoproliferative glomerulonephritis and postinfectious glomerulonephritis as being associated with the development of circulating immune complexes that may lead to low complement levels. There is no evidence of a paraprotein, but testing for cryoglobulins should be considered. Cryoglobulins are associated with hepatitis C but may be induced by a variety of infections. Acting like immune complexes, they can lead to low complement levels and could cause some of this patient's symptoms. However, this whole illness seems most likely to be secondary to infection. The normal antistreptolysin O and antideoxyribonuclease B titers make streptococcal disease unlikely, but another bacterial infection could cause postinfectious glomerulonephritis.
Over the course of his 5‐day hospital stay, the patient received furosemide with increased urine output and normalization of his serum creatinine to its baseline level of 1.0 mg.dL (88.4 mol/L). Proteinuria resolved to 44 mg/dL. A kidney biopsy was not performed. The parvovirus IgG index, checked because of anemia and oligoarthralgias, was 3.67 (normal 0‐1.10), and the IgM index was 8.13 (normal 0‐1.10), suggesting recent infection. The patient was discharged after 5 days. His edema had resolved on discharge; he continued to be nauseated but was able to eat and drink normally. Six months after his hospitalization, his symptoms had completely resolved.
Parvovirus! It could cause the pulmonary infection and the gastroenteric symptoms. Parvovirus usually causes more anemia than nausea and vomiting. We see it occasionally in our transplant patients. The underlying diabetic nephropathy may have made him more symptomatic with a superimposed glomerulonephritis. The most important pedagogic point is that he did well with a very conservative approach, and the possible iatrogenic consequences of a kidney biopsy, had it been performed, were avoided.
COMMENTARY
Parvovirus B19 is endemic, with as many as 80% of adults showing serologic evidence of past infection. Although most adults with detectable B19‐specific IgG do not recall having had specific symptoms, a number of syndromes have been associated with acute infection.1, 2 Parvovirus B19 should be included in the differential for postinfectious glomerulonephritis, especially if a patient presents with marked edema with preserved renal function.
Human parvovirus B19, a member of the erythrovirus genus, is a nonenveloped single‐stranded DNA virus that propagates in erythroid progenitor cells, arresting erythropoiesis.3 The cellular receptor for the virus is globoside (erythrocyte P antigen), a neutral glycosphingolipid densely present on erythroid cells and also found on hepatocytes, nephrons, and bowel mucosa.3, 4
The most common clinical presentation of parvovirus B19 in children is erythema infectiosum, or fifth disease.3 In adults, the infection is known to cause symmetric polyarthropathy, rash, malaise, coryza, headache, and gastrointestinal symptoms (nausea, abdominal pain) and may mimic systemic lupus erythematosus.1, 3 In patients with sickle cell anemia or other chronic hemolytic disorders, parvovirus B19 can cause a transient aplastic crisis.3 Immunosuppressed patients (eg, organ transplant recipients, patients with certain cancers or advanced AIDS) may develop chronic infection and anemia because of an inability to mount an immune response to clear viremia. Mild anemia or pancytopenia is frequently observed in normal infected hosts.
The syndrome of renal involvement in parvovirus B19 includes the typical features of fever, a maculopapular or reticular erythematous rash on the face or extremities, and polyarthritis, accompanied by oliguria that leads to systemic edema. Mild pancytopenia, proteinuria, hematuria, and hypocomplementemia are often present. Creatinine is usually normal or near normal. These symptoms typically appear 1‐2 weeks after the initial viral syndrome.5, 6 With supportive care, most recover spontaneously, although chronic kidney disease has been reported.7, 8
Published kidney biopsy findings of parvovirus B19 show endocapillary or mesangial proliferative glomerulonephritis with subendothelial electron‐dense deposits and granular deposition of C3, IgG, or IgM along the capillary walls and mesangium. These lesions suggest immune complex deposition and are consistent with postinfectious glomerulonephritis.5, 9, 10 Indeed, increased levels of circulating immune complexes have been seen during acute parvovirus B19 infection.6, 9 It is likely that the protracted symptoms our patient experienced resulted from the formation, circulation, and deposition of immune complexes. The presence of globoside in the kidneys and bowel also raises the possibility of direct infection of these organs.
Postinfectious glomerulonephritis is often thought to be synonymous with poststreptococcal glomerulonephritis. However, viruses, including hepatitis B and C viruses, human immunodeficiency virus, cytomegalovirus, hantavirus, and parvovirus B19 may cause postinfectious glomerulonephritis. As with poststreptococcal glomerulonephritis, glomerular disease associated with viral infection appears to be mediated by the immune complexes. The pathogenic series of events leading to glomerular injury includes formation of circulating immune complexes with subsequent deposition in the glomerulus, or formation of in situ antigen‐antibody reactions.11 Immune complexes in the glomerulus lead to activation of the complement cascade, which in turn leads to hypocomplementemia, as the complement cascade is activated faster than the synthesis of new complement proteins.12 Histologically, a number of different renal lesions may be seen in postviral glomerulonephritis, including membranous, membranoproliferative, and mesangial glomerulonephritis, as well as focal segmental glomerulosclerosis.
Our patient presented with symptoms compatible with but not specific for parvovirus B19. Using a pattern recognition approach to diagnosis, our discussant correctly identified the disease pattern as a postinfectious glomerulonephritis but was unable to identify the correct pathogen, as bacterial infections were the main focus of concern, and viruses, parvovirus B19 in particular, were not considered. The clinical pattern of arthralgia, gastrointestinal symptoms, fever combined with anemia or pancytopenia, and hypocomplementemia is typical of the clues for parvovirus B19. Although renal involvement is unusual, the presence of oliguria, hematuria, and edema with minimal creatinine elevation is typical of parvovirus renal disease.
An essential part of clinical judgment is carefully determining which of a patient's often myriad complaints must be considered part of the disease process. Common and nonspecific signs and symptoms often fall off the clinician's radar screen. In this instance, several of the hallmark features of parvovirus B19 disease were dismissed by our discussant as due to the patient's previous medical conditions or hospital‐related interventions. Anemia (due to interruption of erythropoiesis by parvovirus B19 replication) was attributed to hydration or phlebotomy, fluid retention was attributed to advancing diabetes, and hematuria was attributed to a urinary catheter. It is important to evaluate the entire clinical picture prior to excluding potential clues to the diagnosis. Another reasonable approach would have been to choose a less general sign or symptom to narrow the possible diagnoses. For example, had the wrist arthralgia been more central in the discussant's thoughts, parvovirus B19 might have appeared on the differential.
Finally, the discussant wrestled with the decision to perform a renal biopsy for a definitive diagnosis versus the potential complications of the procedure. In this case, it was possible to achieve a clinical diagnosis, support it with serologic evidence, and thus avoid the need for biopsy. The current medical climate emphasizes the importance of reaching a definitive diagnosis as rapidly as possible. There are pressures to act quickly and utilize technology that may add both cost and risk. This case emphasizes the value of clinical reasoning and patience, which led to a correct diagnosis and a favorable outcome without the need for invasive procedures. Clinical acumen must occasionally include avoiding the temptation to perform the next test and merely standing at the patient's bedside instead.
A 35‐year‐old man presented to the emergency department of a community hospital with 3 days of nausea, vomiting, abdominal pain, and diarrhea. He had developed a sore throat, nasal congestion, and green sputum on a business trip to Las Vegas 10 days prior. He then traveled to Shanghai, China, where he developed frequent diarrhea with mucous and urgency. The stool was mustard colored without blood or melena. He became nauseated and unable to keep down food or fluids. He noted a 4‐kg weight loss since the beginning of his symptoms. He had not traveled outside Shanghai or eaten exotic foods. His travel companions remained unaffected. The patient had had type I diabetes mellitus for 23 years with known retinopathy and microalbuminuria. He had hypertension and hyperlipidemia but was otherwise healthy. He was a married traveling salesman with 3 healthy children. He did not smoke or drink and reported no drug use or extramarital relations. He had no known allergies. His medications included insulin, lisinopril, atorvastatin, and valsartan. He was afebrile, his vital signs were stable and the physical examination was unremarkable.
The patient presents with gastrointestinal symptoms following a trip to China. He may have an infection that began in the respiratory system and now is causing some gastrointestinal symptoms, like Legionnella. If he had received antibiotics, the diarrheal illness could be a complication. With his recent travel to China, typical enteric pathogens would have to be considered: enterotoxigenic E. coli, Shigella, Salmonella, Campylobacter, or perhaps Giardia.
He was admitted and treated with intravenous fluids and ciprofloxacin for presumed gastroenteritis. He was discharged the next day but returned 2 days later because of continued nausea and vomiting and limited oral intake. He was febrile, to 38.9C, with chills. The results of an abdominal exam were normal. Stool studies for Salmonella, Shigella, Campylobacter, Yersinia, enterotoxigenic E. coli, Giardia, and Cryptosporidium were negative. No blood parasites were seen. The white‐cell count was 3900/mm3 with a normal differential count. Hemoglobin level, platelet count, serum electrolytes and creatinine were normal. He was readmitted for intravenous fluids.
The patient was treated for traveler's diarrhea, although this is not a typical case and is now becoming a protracted illness. Amebiasis would be a consideration, as well as typhoid or perhaps an abdominal abscess. The normal platelet count reduces the likelihood of hemolytic uremic syndrome, as does the absence of bloody diarrhea. I would evaluate for a systemic illness: check for adenopathy, do a thorough abdominal exam, and get a chest radiogram, blood cultures, and liver function tests. I am also concerned about metabolic abnormalities that could occur as a consequence of the diarrhea.
Over 3 days, oliguria developed along with urinary hesitancy, a 9‐kg weight gain, and the development of marked edema. Blood pressure and heart rate remained normal, and a chest radiograph was clear. Liver function tests were normal. A urinary catheter was inserted. Urinalysis revealed a specific gravity of 1.031, protein of 100 mg/dL, and trace glucose but was otherwise negative; no casts or cells were seen in the sediment. Chemistries included sodium of 133 mmol/L, potassium of 3.9 mmol/L, and serum bicarbonate of 18.4 mmol/L. Blood and urine cultures were sterile. The creatinine increased from 1.0 mg/dL (88.4 mol/L) to 1.3 mg/dL (115 mol/L). He was transferred to a tertiary care hospital for renal consultation because of concerns of impending renal failure and for consideration of a kidney biopsy.
In a typical case of a malabsorptive diarrhea, the patient could be volume depleted, but in this case he has gained 9‐kg and is grossly edematous. The chest radiograph and liver tests point to renal rather than cardiac or hepatologic causes for the edema. A glomerulonephritis may be driving the salt and water retention.
The proteinuria could be related to hemodynamics, or it could be from a glomerular lesion secondary to immune complexes. The specific gravity of 1.031 indicates the kidney is able to concentrate, and we are not seeing acute tubular necrosis. There is only minimal elevation in creatinine at this point. Quantitation or estimation of the degree of proteinuria by a protein‐to‐creatinine ratio would be helpful.
A further workup should include additional blood cultures and a CT scan of the lungs and abdomen to look for occult infection. Is he unfortunate enough to have developed a malignancy? Is this a connective tissue disease? Reexamination of the urine sediment is important to evaluate for glomerulonephritis.
The patient reported ongoing nausea and vomiting, but his diarrhea resolved. He was tachypneic, with a respiratory rate of 26 breaths/minute and an oxygen saturation of 98% breathing ambient air. His temperature was 37.1C, heart rate 84 beats/minute, and blood pressure 120/65 mm Hg. His mucous membranes were moist, and his jugular venous pressure was 6 cm. No lymphadenopathy was present. The heart and lungs were normal. The abdomen was soft, nontender, and without organomegaly, masses, or shifting dullness; bowel sounds were hypoactive. Severe edema of his legs, sacrum, hands, arms, and orbits was noted. His right hand and wrist were painful with limited mobility and small joint effusions of the wrist and metacarpophalangeal joints, but without erythema or warmth. Small petechiae were noted on his eyelids; skin examination was otherwise unremarkable. He had been given ciprofloxacin, phenergan, calcium carbonate, and pantoprazole prior to transfer. Stool was negative for occult blood.
His lungs are clear, but it is possible to have early pulmonary congestion with normal breath sounds. As he is normotensive and has a normal JVP, I would not give further intravenous fluid. Unless he has evidence of symptomatic pulmonary edema, I would not give diuretics but would simply observe his course. At this point I would ultrasound his kidneys to make sure there is no obstruction. The proteinuria could be a result of underlying diabetic glomerulopathy that may predispose to fluid retention.
He has significant oliguria but only a mild rise in creatinine. As the referring physicians requested a biopsy, we should consider it. A decision to biopsy the kidney would rest on the degree of proteinuria and the activity of the sediment. For example, if red blood cell casts or dysmorphic red cells were present, postinfectious glomerulonephritis or IgA nephropathy would be more likely. However, if the proteinuria is in the non‐nephrotic range and the sediment is nonreactive, the yield of a biopsy would be low.
His right hand and wrist are painful with limited mobility. This could be a sequela of endocarditis, although the absence of a murmur and negative blood cultures make it unlikely. He could have an infectious arthritis, although this usually presents more dramatically. He could have gout or pseudogout, which could be determined by joint aspiration. Finally, could this be iatrogenic? A drug reaction could explain some of the features, including rash, fever, joint symptoms, and renal abnormalities.
Repeat urinalysis revealed protein of more than 300 mg/dL, hematuria (1+), mucous, renal tubular epithelial cells, renal tubular epithelial cell casts, and granular casts. Eosinophiluria was absent. Laboratory evaluation revealed a hemoglobin level of 10.7 g/dL (decreased from 13 g/dL on initial presentation), white‐cell count of 7300/mm3, platelet count of 337,000/mm3, serum creatinine of 1.2 mg/dL (106 mol/L), blood urea nitrogen of 23 mg/dL (8.2 mol.L), total serum protein of 6.3 g/dL (normal range, 6.3‐8.7), and albumin of 2.7 g/dL (normal range, 3.2‐5.2). Other liver tests and serum electrolytes were normal.
This degree of proteinuria is significant, but it is unclear whether this is related to the underlying disease process or his advancing diabetes. He has some hematuria, but that could be from the urinary catheter. It would be helpful to know if the red blood cells are dysmorphic, which would point to a glomerulonephritis. He has renal cells, renal cell casts, and granular casts, which are nonspecific. He has a mild anemia, which is unexplained, but could relate to phlebotomy or overhydration. The hypoalbuminemia may be a result of renal losses or a catabolic state.
A renal ultrasonogram was normal, apart from evidence of bilateral pleural effusions. Antinuclear antibody and rheumatoid factor test results were negative, as were those for hepatitis A, hepatitis B surface antigen, and hepatitis C antibodies. Antistreptolysin O and antideoxyribonuclease B titers were normal. Total complement Ch50 was low at 29 U/mL (normal range, 30‐75) as was complement factor C3 at 67 mg/dL (normal range, 90‐180). Complement factor C4 was normal. Serum and urine electrophoresis revealed no monoclonal protein spike. Vitamin B12 and serum folate were normal, serum ferritin was 584 ng/mL (normal range, 30‐400), iron serum was 29 g/dL (normal range, 45‐160), transferrin saturation was 16%, and total iron‐binding capacity was 164 g/dL (normal range, 250‐450). The reticulocyte count was 2.9% with an absolute reticulocyte count of 102/cm3 and a reticulocyte production index of 0.96 (normal range, 1.0‐2.0).
It is reassuring that his urinary tract ultrasound is normal. In addition to edema, he has bilateral effusions, which are probably transudative, related to fluid overload. The urinalysis does not suggest a rapidly progressive glomerulonephritis, but autoimmune disease is still in the differential.
He has a mild complement C3 deficiency. In nephrology we think of lupus, infective endocarditis, cryoglobulinemia, and specific glomerular lesions such as membranoproliferative glomerulonephritis and postinfectious glomerulonephritis as being associated with the development of circulating immune complexes that may lead to low complement levels. There is no evidence of a paraprotein, but testing for cryoglobulins should be considered. Cryoglobulins are associated with hepatitis C but may be induced by a variety of infections. Acting like immune complexes, they can lead to low complement levels and could cause some of this patient's symptoms. However, this whole illness seems most likely to be secondary to infection. The normal antistreptolysin O and antideoxyribonuclease B titers make streptococcal disease unlikely, but another bacterial infection could cause postinfectious glomerulonephritis.
Over the course of his 5‐day hospital stay, the patient received furosemide with increased urine output and normalization of his serum creatinine to its baseline level of 1.0 mg.dL (88.4 mol/L). Proteinuria resolved to 44 mg/dL. A kidney biopsy was not performed. The parvovirus IgG index, checked because of anemia and oligoarthralgias, was 3.67 (normal 0‐1.10), and the IgM index was 8.13 (normal 0‐1.10), suggesting recent infection. The patient was discharged after 5 days. His edema had resolved on discharge; he continued to be nauseated but was able to eat and drink normally. Six months after his hospitalization, his symptoms had completely resolved.
Parvovirus! It could cause the pulmonary infection and the gastroenteric symptoms. Parvovirus usually causes more anemia than nausea and vomiting. We see it occasionally in our transplant patients. The underlying diabetic nephropathy may have made him more symptomatic with a superimposed glomerulonephritis. The most important pedagogic point is that he did well with a very conservative approach, and the possible iatrogenic consequences of a kidney biopsy, had it been performed, were avoided.
COMMENTARY
Parvovirus B19 is endemic, with as many as 80% of adults showing serologic evidence of past infection. Although most adults with detectable B19‐specific IgG do not recall having had specific symptoms, a number of syndromes have been associated with acute infection.1, 2 Parvovirus B19 should be included in the differential for postinfectious glomerulonephritis, especially if a patient presents with marked edema with preserved renal function.
Human parvovirus B19, a member of the erythrovirus genus, is a nonenveloped single‐stranded DNA virus that propagates in erythroid progenitor cells, arresting erythropoiesis.3 The cellular receptor for the virus is globoside (erythrocyte P antigen), a neutral glycosphingolipid densely present on erythroid cells and also found on hepatocytes, nephrons, and bowel mucosa.3, 4
The most common clinical presentation of parvovirus B19 in children is erythema infectiosum, or fifth disease.3 In adults, the infection is known to cause symmetric polyarthropathy, rash, malaise, coryza, headache, and gastrointestinal symptoms (nausea, abdominal pain) and may mimic systemic lupus erythematosus.1, 3 In patients with sickle cell anemia or other chronic hemolytic disorders, parvovirus B19 can cause a transient aplastic crisis.3 Immunosuppressed patients (eg, organ transplant recipients, patients with certain cancers or advanced AIDS) may develop chronic infection and anemia because of an inability to mount an immune response to clear viremia. Mild anemia or pancytopenia is frequently observed in normal infected hosts.
The syndrome of renal involvement in parvovirus B19 includes the typical features of fever, a maculopapular or reticular erythematous rash on the face or extremities, and polyarthritis, accompanied by oliguria that leads to systemic edema. Mild pancytopenia, proteinuria, hematuria, and hypocomplementemia are often present. Creatinine is usually normal or near normal. These symptoms typically appear 1‐2 weeks after the initial viral syndrome.5, 6 With supportive care, most recover spontaneously, although chronic kidney disease has been reported.7, 8
Published kidney biopsy findings of parvovirus B19 show endocapillary or mesangial proliferative glomerulonephritis with subendothelial electron‐dense deposits and granular deposition of C3, IgG, or IgM along the capillary walls and mesangium. These lesions suggest immune complex deposition and are consistent with postinfectious glomerulonephritis.5, 9, 10 Indeed, increased levels of circulating immune complexes have been seen during acute parvovirus B19 infection.6, 9 It is likely that the protracted symptoms our patient experienced resulted from the formation, circulation, and deposition of immune complexes. The presence of globoside in the kidneys and bowel also raises the possibility of direct infection of these organs.
Postinfectious glomerulonephritis is often thought to be synonymous with poststreptococcal glomerulonephritis. However, viruses, including hepatitis B and C viruses, human immunodeficiency virus, cytomegalovirus, hantavirus, and parvovirus B19 may cause postinfectious glomerulonephritis. As with poststreptococcal glomerulonephritis, glomerular disease associated with viral infection appears to be mediated by the immune complexes. The pathogenic series of events leading to glomerular injury includes formation of circulating immune complexes with subsequent deposition in the glomerulus, or formation of in situ antigen‐antibody reactions.11 Immune complexes in the glomerulus lead to activation of the complement cascade, which in turn leads to hypocomplementemia, as the complement cascade is activated faster than the synthesis of new complement proteins.12 Histologically, a number of different renal lesions may be seen in postviral glomerulonephritis, including membranous, membranoproliferative, and mesangial glomerulonephritis, as well as focal segmental glomerulosclerosis.
Our patient presented with symptoms compatible with but not specific for parvovirus B19. Using a pattern recognition approach to diagnosis, our discussant correctly identified the disease pattern as a postinfectious glomerulonephritis but was unable to identify the correct pathogen, as bacterial infections were the main focus of concern, and viruses, parvovirus B19 in particular, were not considered. The clinical pattern of arthralgia, gastrointestinal symptoms, fever combined with anemia or pancytopenia, and hypocomplementemia is typical of the clues for parvovirus B19. Although renal involvement is unusual, the presence of oliguria, hematuria, and edema with minimal creatinine elevation is typical of parvovirus renal disease.
An essential part of clinical judgment is carefully determining which of a patient's often myriad complaints must be considered part of the disease process. Common and nonspecific signs and symptoms often fall off the clinician's radar screen. In this instance, several of the hallmark features of parvovirus B19 disease were dismissed by our discussant as due to the patient's previous medical conditions or hospital‐related interventions. Anemia (due to interruption of erythropoiesis by parvovirus B19 replication) was attributed to hydration or phlebotomy, fluid retention was attributed to advancing diabetes, and hematuria was attributed to a urinary catheter. It is important to evaluate the entire clinical picture prior to excluding potential clues to the diagnosis. Another reasonable approach would have been to choose a less general sign or symptom to narrow the possible diagnoses. For example, had the wrist arthralgia been more central in the discussant's thoughts, parvovirus B19 might have appeared on the differential.
Finally, the discussant wrestled with the decision to perform a renal biopsy for a definitive diagnosis versus the potential complications of the procedure. In this case, it was possible to achieve a clinical diagnosis, support it with serologic evidence, and thus avoid the need for biopsy. The current medical climate emphasizes the importance of reaching a definitive diagnosis as rapidly as possible. There are pressures to act quickly and utilize technology that may add both cost and risk. This case emphasizes the value of clinical reasoning and patience, which led to a correct diagnosis and a favorable outcome without the need for invasive procedures. Clinical acumen must occasionally include avoiding the temptation to perform the next test and merely standing at the patient's bedside instead.
- ,,, et al.Clinical manifestations of human parvovirus B19 in adults.Arch Intern Med.1989;149:1153‐1156.
- ,.The prevalence of antibody to human parvovirus B19 in England and Wales.J Med Microbiol.1999;25:25‐28.
- ,.Parvovirus B19.N Engl J Med.2004;350:586‐597.
- ,,.Multiple glycosphingolipids determine the tissue tropism of parvovirus B19.J Infect Dis.1995;172:1198‐1205.
- ,,,.Renal involvement induced by human parvovirus B19 infection.Nephron.2001;89:280‐285.
- ,,, et al.Association of parvovirus B19 infection with acute glomerulonephritis in the healthy adults: case report and review of the literature.Clin Nephrol.2002;57:69‐73.
- .Renal involvement in human parvovirus B19 infection.Pediatr Nephrol.2003;18:966‐967.
- ,,, et al.Acute glomerulonephritis after human parvovirus B19 infection.Am J Kidney Dis.2000;35:1‐8.
- ,,,,.Human parvovirus B19 and renal disease?Neth J Med.2000;56:163‐165.
- ,,, et al.Nephrotic syndrome associated with human parvovirus B19 infection.Pediatr Nephrol.2003;18:280‐282.
- ,.Glomerulonephritis.Lancet.2005;365:1797‐1806.
- .Complement and the kidney.J Immunol.2003;171:3319‐3324.
- ,,, et al.Clinical manifestations of human parvovirus B19 in adults.Arch Intern Med.1989;149:1153‐1156.
- ,.The prevalence of antibody to human parvovirus B19 in England and Wales.J Med Microbiol.1999;25:25‐28.
- ,.Parvovirus B19.N Engl J Med.2004;350:586‐597.
- ,,.Multiple glycosphingolipids determine the tissue tropism of parvovirus B19.J Infect Dis.1995;172:1198‐1205.
- ,,,.Renal involvement induced by human parvovirus B19 infection.Nephron.2001;89:280‐285.
- ,,, et al.Association of parvovirus B19 infection with acute glomerulonephritis in the healthy adults: case report and review of the literature.Clin Nephrol.2002;57:69‐73.
- .Renal involvement in human parvovirus B19 infection.Pediatr Nephrol.2003;18:966‐967.
- ,,, et al.Acute glomerulonephritis after human parvovirus B19 infection.Am J Kidney Dis.2000;35:1‐8.
- ,,,,.Human parvovirus B19 and renal disease?Neth J Med.2000;56:163‐165.
- ,,, et al.Nephrotic syndrome associated with human parvovirus B19 infection.Pediatr Nephrol.2003;18:280‐282.
- ,.Glomerulonephritis.Lancet.2005;365:1797‐1806.
- .Complement and the kidney.J Immunol.2003;171:3319‐3324.
Discharge Summary Survey
Twelve percent of patients have been reported to have preventable or ameliorable adverse events in the period immediately following hospital discharge.1, 2 A potential contributor to the number of adverse events is inadequate transfer of clinical information at hospital discharge. The discharge summary is a vital component of the transfer of information from the inpatient to the outpatient setting. Unfortunately, discharge summaries are often unavailable when follow‐up care occurs and often lack important content.36
Many hospitals are implementing an electronic medical record systems. This creates the opportunity at hospital discharge to immediately assemble the major components of a discharge summary. With enhanced communication systems, this information can be delivered in a variety of ways with minimal delay. We report the results and evaluation of a survey of medicine faculty at an urban academic medical center about the timeliness and quality of discharge summaries, the perceived incidence of adverse events related to suboptimal information transfer at discharge, and the need for the electronically generated discharge summary we plan to design.
METHODS
Study Site
The study was conducted at a 753‐bed academic hospital in Chicago, Illinois. Discharge summaries have traditionally been dictated by inpatient physicians and delivered to outpatient physicians by both mail and facsimile via the medical records department. The hospital has used an electronic medical record and computerized physician order entry system (PowerChart Millennium from Cerner Corporation) since August 2004. Although all history and physicals and progress notes were documented in the electronic medical record, the system did not provide a method for delivering the discharge summaries contained in the electronic medical record to outpatient physician offices. Because of this, inpatient physicians continued to dictate discharge summaries during this study.
Participants
An advisory board consisting of 16 physicians from the Department of Medicine was convened. The advisory board gave input on needs assessment and helped to create a survey to be administered to all 425 medicine faculty who have an outpatient practice. All respondents who had at least 1 patient admitted to the hospital within the 6 months prior to the survey were eligible.
Survey Content
Our survey consisted of 2 parts. In the first part, we asked respondents to estimate how many of their patients had been discharged from the hospital in the past 6 months and to reflect on these patients as they completed the survey. Satisfaction with the timeliness and quality of discharge summaries was assessed using a 5‐point Likert scale, from 5, very satisfied, to 1, very unsatisfied. The frequency of hospital follow‐up of a patient occurring prior to arrival of the discharge summary was assessed as the percentage of times this occurred in 20% increments (0%‐19%, 20%‐39%, 40%‐59%, 60%‐79%, and 80%‐100%). The number of discharge summaries missing critical information and the number of summaries containing unnecessary information were similarly assessed using 20% increments. We then asked respondents to estimate the number of patients who had sustained a preventable adverse event related to suboptimal transfer of information at discharge. We defined a preventable adverse event as a preventable medical problem or worsening of an existing problem.
In the second part of the survey, we elicited preferences for discharge summary content and method of delivery. We assessed preferences for discharge summary content by asking respondents to rank items on a scale from 1 to 10, from 10, most important, to 1, least important. Preferences for delivery of discharge summaries were assessed by asking respondents to indicate one or more delivery methods, including facsimile, mail, the electronic medical record, and E‐mail.
Survey Process
The survey was sent out in March 2005. A postcard reminder was sent out approximately 2 weeks after the initial survey was mailed. A second survey was sent to nonresponders 6 weeks after the initial survey. Simultaneously, the survey was also sent in Web‐based format to nonresponders via email.
Data Analysis
Physician characteristics, including practice type, faculty appointment type, and year of medical school graduation, were provided by the hospital's medical staff office. Physician respondents and nonrespondents were compared using the chi‐square test and logistic regression to determine potential response biases. We calculated means and standard deviations and percentages for categorical variables. Logistic regression was used to examine the likelihood of participants reporting any preventable adverse event related to suboptimal transfer of information. The regression model tested the likelihood of one or more preventable adverse events reported with the frequency of seeing patients for follow‐up prior to the arrival of discharge summaries, controlling for participant characteristics and the number of hospitalized patients each physician had in the previous 6 months.
RESULTS
Physician Characteristics
The survey was sent to 425 physicians, 9 of whom were excluded because they had had no patients admitted within the past 6 months. Of the 416 eligible respondents, 2 returned a survey that was incomplete and not usable, and 226 returned a completed survey (response rate of 54%). The characteristics of responders and nonresponders are shown in Table 1. General medicine physicians completed the survey more often than specialist physicians (56% vs. 44%, P < .001). Affiliated faculty were also more likely to complete the survey than full‐time faculty; multivariate logistic regression revealed this was purely a function of the larger number of specialists among the full‐time faculty.
| Responders (N = 226) | Nonresponders (N = 188) | P value | |
|---|---|---|---|
| |||
| Practice type | |||
| Generalist, N (%) | 127 (56.2) | 65 (34.6) | < .001 |
| Specialist, N (%) | 99 (43.8) | 123 (65.4) | |
| Faculty appointment | |||
| Full‐time, N (%) | 104 (46.0 | 106 (56.4) | .04 |
| Affiliated, N (%) | 122 (54.0) | 82 (43.6) | |
| Year of medical school graduation | |||
| Before 1990, N (%) | 131 (58.0) | 127 (67.6) | .04 |
| 1990 or later, N (%) | 95 (42.0) | 61 (32.4) | |
| Number of patients hospitalized in last 6 monthsa | |||
| 1‐4, N (%) | 15 (7.9) | ||
| 5‐10, N (%) | 62 (32.5) | ||
| 11‐19, N (%) | 35 (18.3) | ||
| 20 or more, N (%) | 79 (41.4) | ||
Timeliness and Content
Only 19% of the participants were satisfied or very satisfied with the timeliness of discharge summaries. Among all participants, 33% indicated that 60% or more of their patients were seen for their follow‐up outpatient visit prior to the arrival of the discharge summary, and 22% indicated that for 60% or more of their patients they never received a discharge summary at all.
Only 32% of the participants were satisfied or very satisfied with the quality of discharge summaries. Among all participants, 17% believed that 60% or more of discharge summaries missed critical information. Unnecessary information in the discharge summary was less problematic; only 9% of participants indicated that 60% or more of discharge summaries contained unnecessary information.
Preventable Adverse Events
Overall, 41% of participants believed that in the previous 6 months at least one of their patients had sustained a preventable adverse event related to poor transfer of information at hospital discharge. Reporting one or more preventable adverse events was positively associated with physicians' reports of how often they saw patients for a first postdischarge follow‐up without having a discharge summary available. After adjusting for participant characteristics and the number of patients hospitalized by each physician, logistic regression results indicated that each 20% increase in the frequency of discharge summaries not arriving prior to patient follow‐up appointments was associated with a 28% increase in the odds of a reported preventable adverse event (adjusted OR = 1.28, P = .04).
Preferences for Content and Delivery
The mean rating for importance of discharge summary elements is shown in Table 2. No discharge summary element had a mean rating of less than 5. Participants preferred discharge summaries be delivered via the following methods: facsimile, 48%; mail, 30%; electronic medical record, 41%; and E‐mail, 30%.
| Mean rating (scale of 1‐10) | |
|---|---|
| Medications at discharge | 9.69 |
| Follow‐up issues | 9.09 |
| Discharge diagnosis | 9.02 |
| List of procedures performed | 8.79 |
| Pathology reports | 8.78 |
| Pending test results | 8.68 |
| Procedure reports | 8.16 |
| Stress test reports | 8.07 |
| Dates of admission and discharge | 8.01 |
| Problem list | 7.99 |
| List of radiology tests performed | 7.84 |
| Echocardiogram reports | 7.79 |
| Follow‐up appointments | 7.79 |
| Radiology reports | 7.76 |
| Names of consulting attendings | 7.64 |
| Name of inpatient attending | 7.28 |
| Labs from last hospital day | 7.08 |
| Medications at admission | 6.91 |
| Allergies | 6.56 |
| All lab results | 6.22 |
| Code status | 6.09 |
| Names of inpatient house officers | 5.64 |
DISCUSSION
Our study found that outpatient physicians were not satisfied with the timeliness or the quality of current discharge summaries. Our findings are in agreement with previous studies demonstrating that discharge summaries were often not available to outpatient physicians3,4 and were often of poor quality.5, 6
Preventable or ameliorable adverse events have been reported to occur in 12% of patients in the period immediately following hospital discharge.1, 2 No studies have evaluated the relationship between discharge summaries and preventable adverse events following discharge. Our study found that 41% of outpatient physicians believed that at least one of their patients in the 6 months prior to the survey had sustained a preventable adverse event related to the suboptimal transfer of information at hospital discharge. In addition, the likelihood of physicians reporting one or more preventable adverse events increased with the frequency of seeing patients for follow‐up prior to discharge summary arrival.
In preparation for revising the discharge summary, we asked outpatient physicians to rate the importance of discharge summary content and their preference for method of delivery of discharge summaries. As in previous studies, the outpatient physicians rated discharge medications, discharge diagnosis, test results, and follow‐up plans as highly important.7, 8 Much of this clinical data is now available in the electronic medical record. Therefore, it is possible to electronically assemble much, if not all, of discharge summary content. One recent study demonstrated that database‐generated discharge summaries significantly increased the likelihood that a discharge summary was generated within 4 weeks of hospital discharge.9 The database used in that study required manual data input from a handwritten form. To our knowledge, no study has reported the experience of discharge summaries generated from an electronic medical record.
Our study had several limitations. First, our study used physician survey to assess the timeliness of receiving discharge summaries. Measuring the time to actual receipt of discharge summaries by physicians was beyond the scope of our study. Second, our study did not measure adverse events directly. Instead, we asked outpatient physicians to estimate how many of their patients discharged in the last 6 months had sustained a preventable adverse event related to suboptimal information transfer at discharge. We had limited space in the questionnaire to define the meaning of a preventable adverse event; therefore, the description in the survey does not exactly match previous definitions.1, 2 Our study had a response rate of 54%. It is possible that nonresponders may have been more satisfied with the quality and timeliness of discharge summaries and may have believed fewer patients experienced preventable adverse events related to suboptimal information transfer at discharge.
The results of our study suggest that the use of systems to improve the quality and delivery of discharge summaries has the potential to improve outpatient physician satisfaction and to reduce the number of preventable adverse events that occur during the vulnerable period following hospital discharge. With the use of electronic medical records, we now have the potential to automate the process of assembling and delivering clinical information with minimal delay. We are now using the information from this study to design a partially automated, high‐quality discharge summary that can be delivered to outpatient physicians immediately on discharge.
- ,,, et al.Adverse events among medical patient after hospital discharge.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21:104–108.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur.1998;11:90–95.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
Twelve percent of patients have been reported to have preventable or ameliorable adverse events in the period immediately following hospital discharge.1, 2 A potential contributor to the number of adverse events is inadequate transfer of clinical information at hospital discharge. The discharge summary is a vital component of the transfer of information from the inpatient to the outpatient setting. Unfortunately, discharge summaries are often unavailable when follow‐up care occurs and often lack important content.36
Many hospitals are implementing an electronic medical record systems. This creates the opportunity at hospital discharge to immediately assemble the major components of a discharge summary. With enhanced communication systems, this information can be delivered in a variety of ways with minimal delay. We report the results and evaluation of a survey of medicine faculty at an urban academic medical center about the timeliness and quality of discharge summaries, the perceived incidence of adverse events related to suboptimal information transfer at discharge, and the need for the electronically generated discharge summary we plan to design.
METHODS
Study Site
The study was conducted at a 753‐bed academic hospital in Chicago, Illinois. Discharge summaries have traditionally been dictated by inpatient physicians and delivered to outpatient physicians by both mail and facsimile via the medical records department. The hospital has used an electronic medical record and computerized physician order entry system (PowerChart Millennium from Cerner Corporation) since August 2004. Although all history and physicals and progress notes were documented in the electronic medical record, the system did not provide a method for delivering the discharge summaries contained in the electronic medical record to outpatient physician offices. Because of this, inpatient physicians continued to dictate discharge summaries during this study.
Participants
An advisory board consisting of 16 physicians from the Department of Medicine was convened. The advisory board gave input on needs assessment and helped to create a survey to be administered to all 425 medicine faculty who have an outpatient practice. All respondents who had at least 1 patient admitted to the hospital within the 6 months prior to the survey were eligible.
Survey Content
Our survey consisted of 2 parts. In the first part, we asked respondents to estimate how many of their patients had been discharged from the hospital in the past 6 months and to reflect on these patients as they completed the survey. Satisfaction with the timeliness and quality of discharge summaries was assessed using a 5‐point Likert scale, from 5, very satisfied, to 1, very unsatisfied. The frequency of hospital follow‐up of a patient occurring prior to arrival of the discharge summary was assessed as the percentage of times this occurred in 20% increments (0%‐19%, 20%‐39%, 40%‐59%, 60%‐79%, and 80%‐100%). The number of discharge summaries missing critical information and the number of summaries containing unnecessary information were similarly assessed using 20% increments. We then asked respondents to estimate the number of patients who had sustained a preventable adverse event related to suboptimal transfer of information at discharge. We defined a preventable adverse event as a preventable medical problem or worsening of an existing problem.
In the second part of the survey, we elicited preferences for discharge summary content and method of delivery. We assessed preferences for discharge summary content by asking respondents to rank items on a scale from 1 to 10, from 10, most important, to 1, least important. Preferences for delivery of discharge summaries were assessed by asking respondents to indicate one or more delivery methods, including facsimile, mail, the electronic medical record, and E‐mail.
Survey Process
The survey was sent out in March 2005. A postcard reminder was sent out approximately 2 weeks after the initial survey was mailed. A second survey was sent to nonresponders 6 weeks after the initial survey. Simultaneously, the survey was also sent in Web‐based format to nonresponders via email.
Data Analysis
Physician characteristics, including practice type, faculty appointment type, and year of medical school graduation, were provided by the hospital's medical staff office. Physician respondents and nonrespondents were compared using the chi‐square test and logistic regression to determine potential response biases. We calculated means and standard deviations and percentages for categorical variables. Logistic regression was used to examine the likelihood of participants reporting any preventable adverse event related to suboptimal transfer of information. The regression model tested the likelihood of one or more preventable adverse events reported with the frequency of seeing patients for follow‐up prior to the arrival of discharge summaries, controlling for participant characteristics and the number of hospitalized patients each physician had in the previous 6 months.
RESULTS
Physician Characteristics
The survey was sent to 425 physicians, 9 of whom were excluded because they had had no patients admitted within the past 6 months. Of the 416 eligible respondents, 2 returned a survey that was incomplete and not usable, and 226 returned a completed survey (response rate of 54%). The characteristics of responders and nonresponders are shown in Table 1. General medicine physicians completed the survey more often than specialist physicians (56% vs. 44%, P < .001). Affiliated faculty were also more likely to complete the survey than full‐time faculty; multivariate logistic regression revealed this was purely a function of the larger number of specialists among the full‐time faculty.
| Responders (N = 226) | Nonresponders (N = 188) | P value | |
|---|---|---|---|
| |||
| Practice type | |||
| Generalist, N (%) | 127 (56.2) | 65 (34.6) | < .001 |
| Specialist, N (%) | 99 (43.8) | 123 (65.4) | |
| Faculty appointment | |||
| Full‐time, N (%) | 104 (46.0 | 106 (56.4) | .04 |
| Affiliated, N (%) | 122 (54.0) | 82 (43.6) | |
| Year of medical school graduation | |||
| Before 1990, N (%) | 131 (58.0) | 127 (67.6) | .04 |
| 1990 or later, N (%) | 95 (42.0) | 61 (32.4) | |
| Number of patients hospitalized in last 6 monthsa | |||
| 1‐4, N (%) | 15 (7.9) | ||
| 5‐10, N (%) | 62 (32.5) | ||
| 11‐19, N (%) | 35 (18.3) | ||
| 20 or more, N (%) | 79 (41.4) | ||
Timeliness and Content
Only 19% of the participants were satisfied or very satisfied with the timeliness of discharge summaries. Among all participants, 33% indicated that 60% or more of their patients were seen for their follow‐up outpatient visit prior to the arrival of the discharge summary, and 22% indicated that for 60% or more of their patients they never received a discharge summary at all.
Only 32% of the participants were satisfied or very satisfied with the quality of discharge summaries. Among all participants, 17% believed that 60% or more of discharge summaries missed critical information. Unnecessary information in the discharge summary was less problematic; only 9% of participants indicated that 60% or more of discharge summaries contained unnecessary information.
Preventable Adverse Events
Overall, 41% of participants believed that in the previous 6 months at least one of their patients had sustained a preventable adverse event related to poor transfer of information at hospital discharge. Reporting one or more preventable adverse events was positively associated with physicians' reports of how often they saw patients for a first postdischarge follow‐up without having a discharge summary available. After adjusting for participant characteristics and the number of patients hospitalized by each physician, logistic regression results indicated that each 20% increase in the frequency of discharge summaries not arriving prior to patient follow‐up appointments was associated with a 28% increase in the odds of a reported preventable adverse event (adjusted OR = 1.28, P = .04).
Preferences for Content and Delivery
The mean rating for importance of discharge summary elements is shown in Table 2. No discharge summary element had a mean rating of less than 5. Participants preferred discharge summaries be delivered via the following methods: facsimile, 48%; mail, 30%; electronic medical record, 41%; and E‐mail, 30%.
| Mean rating (scale of 1‐10) | |
|---|---|
| Medications at discharge | 9.69 |
| Follow‐up issues | 9.09 |
| Discharge diagnosis | 9.02 |
| List of procedures performed | 8.79 |
| Pathology reports | 8.78 |
| Pending test results | 8.68 |
| Procedure reports | 8.16 |
| Stress test reports | 8.07 |
| Dates of admission and discharge | 8.01 |
| Problem list | 7.99 |
| List of radiology tests performed | 7.84 |
| Echocardiogram reports | 7.79 |
| Follow‐up appointments | 7.79 |
| Radiology reports | 7.76 |
| Names of consulting attendings | 7.64 |
| Name of inpatient attending | 7.28 |
| Labs from last hospital day | 7.08 |
| Medications at admission | 6.91 |
| Allergies | 6.56 |
| All lab results | 6.22 |
| Code status | 6.09 |
| Names of inpatient house officers | 5.64 |
DISCUSSION
Our study found that outpatient physicians were not satisfied with the timeliness or the quality of current discharge summaries. Our findings are in agreement with previous studies demonstrating that discharge summaries were often not available to outpatient physicians3,4 and were often of poor quality.5, 6
Preventable or ameliorable adverse events have been reported to occur in 12% of patients in the period immediately following hospital discharge.1, 2 No studies have evaluated the relationship between discharge summaries and preventable adverse events following discharge. Our study found that 41% of outpatient physicians believed that at least one of their patients in the 6 months prior to the survey had sustained a preventable adverse event related to the suboptimal transfer of information at hospital discharge. In addition, the likelihood of physicians reporting one or more preventable adverse events increased with the frequency of seeing patients for follow‐up prior to discharge summary arrival.
In preparation for revising the discharge summary, we asked outpatient physicians to rate the importance of discharge summary content and their preference for method of delivery of discharge summaries. As in previous studies, the outpatient physicians rated discharge medications, discharge diagnosis, test results, and follow‐up plans as highly important.7, 8 Much of this clinical data is now available in the electronic medical record. Therefore, it is possible to electronically assemble much, if not all, of discharge summary content. One recent study demonstrated that database‐generated discharge summaries significantly increased the likelihood that a discharge summary was generated within 4 weeks of hospital discharge.9 The database used in that study required manual data input from a handwritten form. To our knowledge, no study has reported the experience of discharge summaries generated from an electronic medical record.
Our study had several limitations. First, our study used physician survey to assess the timeliness of receiving discharge summaries. Measuring the time to actual receipt of discharge summaries by physicians was beyond the scope of our study. Second, our study did not measure adverse events directly. Instead, we asked outpatient physicians to estimate how many of their patients discharged in the last 6 months had sustained a preventable adverse event related to suboptimal information transfer at discharge. We had limited space in the questionnaire to define the meaning of a preventable adverse event; therefore, the description in the survey does not exactly match previous definitions.1, 2 Our study had a response rate of 54%. It is possible that nonresponders may have been more satisfied with the quality and timeliness of discharge summaries and may have believed fewer patients experienced preventable adverse events related to suboptimal information transfer at discharge.
The results of our study suggest that the use of systems to improve the quality and delivery of discharge summaries has the potential to improve outpatient physician satisfaction and to reduce the number of preventable adverse events that occur during the vulnerable period following hospital discharge. With the use of electronic medical records, we now have the potential to automate the process of assembling and delivering clinical information with minimal delay. We are now using the information from this study to design a partially automated, high‐quality discharge summary that can be delivered to outpatient physicians immediately on discharge.
Twelve percent of patients have been reported to have preventable or ameliorable adverse events in the period immediately following hospital discharge.1, 2 A potential contributor to the number of adverse events is inadequate transfer of clinical information at hospital discharge. The discharge summary is a vital component of the transfer of information from the inpatient to the outpatient setting. Unfortunately, discharge summaries are often unavailable when follow‐up care occurs and often lack important content.36
Many hospitals are implementing an electronic medical record systems. This creates the opportunity at hospital discharge to immediately assemble the major components of a discharge summary. With enhanced communication systems, this information can be delivered in a variety of ways with minimal delay. We report the results and evaluation of a survey of medicine faculty at an urban academic medical center about the timeliness and quality of discharge summaries, the perceived incidence of adverse events related to suboptimal information transfer at discharge, and the need for the electronically generated discharge summary we plan to design.
METHODS
Study Site
The study was conducted at a 753‐bed academic hospital in Chicago, Illinois. Discharge summaries have traditionally been dictated by inpatient physicians and delivered to outpatient physicians by both mail and facsimile via the medical records department. The hospital has used an electronic medical record and computerized physician order entry system (PowerChart Millennium from Cerner Corporation) since August 2004. Although all history and physicals and progress notes were documented in the electronic medical record, the system did not provide a method for delivering the discharge summaries contained in the electronic medical record to outpatient physician offices. Because of this, inpatient physicians continued to dictate discharge summaries during this study.
Participants
An advisory board consisting of 16 physicians from the Department of Medicine was convened. The advisory board gave input on needs assessment and helped to create a survey to be administered to all 425 medicine faculty who have an outpatient practice. All respondents who had at least 1 patient admitted to the hospital within the 6 months prior to the survey were eligible.
Survey Content
Our survey consisted of 2 parts. In the first part, we asked respondents to estimate how many of their patients had been discharged from the hospital in the past 6 months and to reflect on these patients as they completed the survey. Satisfaction with the timeliness and quality of discharge summaries was assessed using a 5‐point Likert scale, from 5, very satisfied, to 1, very unsatisfied. The frequency of hospital follow‐up of a patient occurring prior to arrival of the discharge summary was assessed as the percentage of times this occurred in 20% increments (0%‐19%, 20%‐39%, 40%‐59%, 60%‐79%, and 80%‐100%). The number of discharge summaries missing critical information and the number of summaries containing unnecessary information were similarly assessed using 20% increments. We then asked respondents to estimate the number of patients who had sustained a preventable adverse event related to suboptimal transfer of information at discharge. We defined a preventable adverse event as a preventable medical problem or worsening of an existing problem.
In the second part of the survey, we elicited preferences for discharge summary content and method of delivery. We assessed preferences for discharge summary content by asking respondents to rank items on a scale from 1 to 10, from 10, most important, to 1, least important. Preferences for delivery of discharge summaries were assessed by asking respondents to indicate one or more delivery methods, including facsimile, mail, the electronic medical record, and E‐mail.
Survey Process
The survey was sent out in March 2005. A postcard reminder was sent out approximately 2 weeks after the initial survey was mailed. A second survey was sent to nonresponders 6 weeks after the initial survey. Simultaneously, the survey was also sent in Web‐based format to nonresponders via email.
Data Analysis
Physician characteristics, including practice type, faculty appointment type, and year of medical school graduation, were provided by the hospital's medical staff office. Physician respondents and nonrespondents were compared using the chi‐square test and logistic regression to determine potential response biases. We calculated means and standard deviations and percentages for categorical variables. Logistic regression was used to examine the likelihood of participants reporting any preventable adverse event related to suboptimal transfer of information. The regression model tested the likelihood of one or more preventable adverse events reported with the frequency of seeing patients for follow‐up prior to the arrival of discharge summaries, controlling for participant characteristics and the number of hospitalized patients each physician had in the previous 6 months.
RESULTS
Physician Characteristics
The survey was sent to 425 physicians, 9 of whom were excluded because they had had no patients admitted within the past 6 months. Of the 416 eligible respondents, 2 returned a survey that was incomplete and not usable, and 226 returned a completed survey (response rate of 54%). The characteristics of responders and nonresponders are shown in Table 1. General medicine physicians completed the survey more often than specialist physicians (56% vs. 44%, P < .001). Affiliated faculty were also more likely to complete the survey than full‐time faculty; multivariate logistic regression revealed this was purely a function of the larger number of specialists among the full‐time faculty.
| Responders (N = 226) | Nonresponders (N = 188) | P value | |
|---|---|---|---|
| |||
| Practice type | |||
| Generalist, N (%) | 127 (56.2) | 65 (34.6) | < .001 |
| Specialist, N (%) | 99 (43.8) | 123 (65.4) | |
| Faculty appointment | |||
| Full‐time, N (%) | 104 (46.0 | 106 (56.4) | .04 |
| Affiliated, N (%) | 122 (54.0) | 82 (43.6) | |
| Year of medical school graduation | |||
| Before 1990, N (%) | 131 (58.0) | 127 (67.6) | .04 |
| 1990 or later, N (%) | 95 (42.0) | 61 (32.4) | |
| Number of patients hospitalized in last 6 monthsa | |||
| 1‐4, N (%) | 15 (7.9) | ||
| 5‐10, N (%) | 62 (32.5) | ||
| 11‐19, N (%) | 35 (18.3) | ||
| 20 or more, N (%) | 79 (41.4) | ||
Timeliness and Content
Only 19% of the participants were satisfied or very satisfied with the timeliness of discharge summaries. Among all participants, 33% indicated that 60% or more of their patients were seen for their follow‐up outpatient visit prior to the arrival of the discharge summary, and 22% indicated that for 60% or more of their patients they never received a discharge summary at all.
Only 32% of the participants were satisfied or very satisfied with the quality of discharge summaries. Among all participants, 17% believed that 60% or more of discharge summaries missed critical information. Unnecessary information in the discharge summary was less problematic; only 9% of participants indicated that 60% or more of discharge summaries contained unnecessary information.
Preventable Adverse Events
Overall, 41% of participants believed that in the previous 6 months at least one of their patients had sustained a preventable adverse event related to poor transfer of information at hospital discharge. Reporting one or more preventable adverse events was positively associated with physicians' reports of how often they saw patients for a first postdischarge follow‐up without having a discharge summary available. After adjusting for participant characteristics and the number of patients hospitalized by each physician, logistic regression results indicated that each 20% increase in the frequency of discharge summaries not arriving prior to patient follow‐up appointments was associated with a 28% increase in the odds of a reported preventable adverse event (adjusted OR = 1.28, P = .04).
Preferences for Content and Delivery
The mean rating for importance of discharge summary elements is shown in Table 2. No discharge summary element had a mean rating of less than 5. Participants preferred discharge summaries be delivered via the following methods: facsimile, 48%; mail, 30%; electronic medical record, 41%; and E‐mail, 30%.
| Mean rating (scale of 1‐10) | |
|---|---|
| Medications at discharge | 9.69 |
| Follow‐up issues | 9.09 |
| Discharge diagnosis | 9.02 |
| List of procedures performed | 8.79 |
| Pathology reports | 8.78 |
| Pending test results | 8.68 |
| Procedure reports | 8.16 |
| Stress test reports | 8.07 |
| Dates of admission and discharge | 8.01 |
| Problem list | 7.99 |
| List of radiology tests performed | 7.84 |
| Echocardiogram reports | 7.79 |
| Follow‐up appointments | 7.79 |
| Radiology reports | 7.76 |
| Names of consulting attendings | 7.64 |
| Name of inpatient attending | 7.28 |
| Labs from last hospital day | 7.08 |
| Medications at admission | 6.91 |
| Allergies | 6.56 |
| All lab results | 6.22 |
| Code status | 6.09 |
| Names of inpatient house officers | 5.64 |
DISCUSSION
Our study found that outpatient physicians were not satisfied with the timeliness or the quality of current discharge summaries. Our findings are in agreement with previous studies demonstrating that discharge summaries were often not available to outpatient physicians3,4 and were often of poor quality.5, 6
Preventable or ameliorable adverse events have been reported to occur in 12% of patients in the period immediately following hospital discharge.1, 2 No studies have evaluated the relationship between discharge summaries and preventable adverse events following discharge. Our study found that 41% of outpatient physicians believed that at least one of their patients in the 6 months prior to the survey had sustained a preventable adverse event related to the suboptimal transfer of information at hospital discharge. In addition, the likelihood of physicians reporting one or more preventable adverse events increased with the frequency of seeing patients for follow‐up prior to discharge summary arrival.
In preparation for revising the discharge summary, we asked outpatient physicians to rate the importance of discharge summary content and their preference for method of delivery of discharge summaries. As in previous studies, the outpatient physicians rated discharge medications, discharge diagnosis, test results, and follow‐up plans as highly important.7, 8 Much of this clinical data is now available in the electronic medical record. Therefore, it is possible to electronically assemble much, if not all, of discharge summary content. One recent study demonstrated that database‐generated discharge summaries significantly increased the likelihood that a discharge summary was generated within 4 weeks of hospital discharge.9 The database used in that study required manual data input from a handwritten form. To our knowledge, no study has reported the experience of discharge summaries generated from an electronic medical record.
Our study had several limitations. First, our study used physician survey to assess the timeliness of receiving discharge summaries. Measuring the time to actual receipt of discharge summaries by physicians was beyond the scope of our study. Second, our study did not measure adverse events directly. Instead, we asked outpatient physicians to estimate how many of their patients discharged in the last 6 months had sustained a preventable adverse event related to suboptimal information transfer at discharge. We had limited space in the questionnaire to define the meaning of a preventable adverse event; therefore, the description in the survey does not exactly match previous definitions.1, 2 Our study had a response rate of 54%. It is possible that nonresponders may have been more satisfied with the quality and timeliness of discharge summaries and may have believed fewer patients experienced preventable adverse events related to suboptimal information transfer at discharge.
The results of our study suggest that the use of systems to improve the quality and delivery of discharge summaries has the potential to improve outpatient physician satisfaction and to reduce the number of preventable adverse events that occur during the vulnerable period following hospital discharge. With the use of electronic medical records, we now have the potential to automate the process of assembling and delivering clinical information with minimal delay. We are now using the information from this study to design a partially automated, high‐quality discharge summary that can be delivered to outpatient physicians immediately on discharge.
- ,,, et al.Adverse events among medical patient after hospital discharge.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21:104–108.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur.1998;11:90–95.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,, et al.Adverse events among medical patient after hospital discharge.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21:104–108.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur.1998;11:90–95.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14:160–169.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
Handoffs
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.
Octreotide Scan for Carcinoids
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.
The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.
DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.

The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.

DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.

The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.

DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.
Infected pneumatocele
A 49‐year‐old woman with a history of asthma and a pneumatocele secondary to a past pneumonia (Fig. 1, prior baseline chest X‐ray) presented with fever, cough, and shortness of breath. This was her third admission for similar symptoms. In the past, these symptoms had resolved with antibiotics and percutaneous drainage of the infected pneumatocele. Her admission chest X‐ray revealed a fluid‐filled pneumatocele (Fig. 2). Given the recurrent nature of her disease, a right middle lobectomy was performed. Her recovery after surgery was uneventful, and she continues to do well 4 months later.
Pneumatoceles are air‐filled cysts that occur because of trauma or inflammation in the lung parenchyma. The most common cause is pneumonia and they occur most frequently in children. Complications of pneumatocele include pneumothorax and, as in this patient, secondary infection. In most instances the pneumatocele resolves after treating the underlying pneumonia, but surgical intervention is sometimes required to have a definitive cure.
A 49‐year‐old woman with a history of asthma and a pneumatocele secondary to a past pneumonia (Fig. 1, prior baseline chest X‐ray) presented with fever, cough, and shortness of breath. This was her third admission for similar symptoms. In the past, these symptoms had resolved with antibiotics and percutaneous drainage of the infected pneumatocele. Her admission chest X‐ray revealed a fluid‐filled pneumatocele (Fig. 2). Given the recurrent nature of her disease, a right middle lobectomy was performed. Her recovery after surgery was uneventful, and she continues to do well 4 months later.


Pneumatoceles are air‐filled cysts that occur because of trauma or inflammation in the lung parenchyma. The most common cause is pneumonia and they occur most frequently in children. Complications of pneumatocele include pneumothorax and, as in this patient, secondary infection. In most instances the pneumatocele resolves after treating the underlying pneumonia, but surgical intervention is sometimes required to have a definitive cure.
A 49‐year‐old woman with a history of asthma and a pneumatocele secondary to a past pneumonia (Fig. 1, prior baseline chest X‐ray) presented with fever, cough, and shortness of breath. This was her third admission for similar symptoms. In the past, these symptoms had resolved with antibiotics and percutaneous drainage of the infected pneumatocele. Her admission chest X‐ray revealed a fluid‐filled pneumatocele (Fig. 2). Given the recurrent nature of her disease, a right middle lobectomy was performed. Her recovery after surgery was uneventful, and she continues to do well 4 months later.


Pneumatoceles are air‐filled cysts that occur because of trauma or inflammation in the lung parenchyma. The most common cause is pneumonia and they occur most frequently in children. Complications of pneumatocele include pneumothorax and, as in this patient, secondary infection. In most instances the pneumatocele resolves after treating the underlying pneumonia, but surgical intervention is sometimes required to have a definitive cure.
Predictors of Regaining Ambulatory Ability
Functional decline, defined as loss of the ability to care for oneself, commonly occurs during hospitalization, being experienced by up to 65% of older adults.14 Frequently, recovery of functional ability does not occur by the time of discharge from the hospital, despite resolution of the medical condition responsible for admission to the hospital.1, 5 Causes of the declining ability to perform activities of daily living (ADLs) are multiple and include both acute illness and adverse events associated with hospitalization.4, 6, 7 The functional decline experienced by older persons during hospitalization is a strong predictor of length of stay, nursing home placement, and mortality.8 Loss of ambulatory ability specifically has been noted to occur in 17%‐65% of hospitalized older patients, usually within days of admission, with few recovering this ability prior to discharge.2, 4, 5, 9 Importantly, ambulatory ability is significantly associated with a decline in other ADLs.9
Although several studies have explored risk factors associated with general functional decline during hospitalization,4, 7, 10, 11 only one study specifically examined risk factors associated with loss of ambulatory ability. In a cohort of participants who were largely independently performing ADLs on admission to the hospital, Mahoney et al. found age 85 years, white race, use of a walker, and functional impairment prior to being hospitalized were significant predictors of newly having walking dependence.12
Ambulatory ability could also be affected by a variety of other factors not examined in the Mahoney et al. study; these include severity of illness, bed rest, and hospital‐related treatments such as restraints or urinary catheters. In addition, little is known about predictors of recovery of ambulatory ability in patients expected to have activity limitations on admission who are dependent in most or all ADLs. The deconditioning associated with bed rest and reduced mobility has been described as one of the most predictable causes of functional decline, including loss of ambulatory ability, observed in older hospitalized patients.13 In one study, patients whose activity was limited to a bed or chair during hospitalization were 5.6 times more likely to develop functional decline than those who walked at all, even after controlling for other covariates including severity of illness and comorbidity.14 Those patients with both activity limitations and dependence in most or all ADLs represent an important subset of all hospitalized older patients who might be expected to be at higher risk of developing new ambulatory dependence. The ability to identify, at admission, those patients who will recover ambulatory ability may have important implications for discharge planning as well as for the development of preventive strategies.
The objective of the present study was to define patient demographic, illness severity, comorbid illness, and hospital‐related variables that are independent and significant predictors of regaining ambulatory ability prior to hospital discharge in a cohort of patients who had significant activity limitations and functional impairment at the time of admission to the hospital.
METHODS
Study Design
This study was part of a larger prospective cohort study conducted at a tertiary‐care teaching hospital that examined risk factors for pressure ulcers among patients with activity limitations.15 All patients admitted to the medical wards from December 1988 to June 1991 were screened, and research nurses confirmed eligibility within 3 days. Candidates were at least 55 years of age and were expected to be limited to a bed or chair for at least the first 5 days of hospitalization according to the assessments of their primary nurses. Participants were also eligible if admitted with a hip fracture. In all, 286 patients were included in the present analysis. These patients were included because they had been ambulatory in the 4 weeks before admission, and so they would be expected to have the potential to either maintain or regain ambulatory ability prior to discharge. Thirty‐one patients who otherwise would have been eligible for the present analysis were excluded because they had unusually long hospital stays, defined as longer than 31 days. These patients were excluded in order to remove outliers of this variable and because only the effects of relatively acute hospitalization were being studied. Study procedures were approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB).
Baseline Data Collection
For each patient, baseline data were collected from interviews with physicians and nurses at admission and were abstracted from the medical record. Chart review provided information on length of stay; the demographic variables age, sex, race, and marital status; and the presence of specified medical conditions or diseases that might affect a patient's ability to ambulate. These medical conditions and diseases were hip fracture, hypotension, deep vein thrombosis, major surgery and neurological disease defined as a history of hemiparesis regardless of cause, cerebrovascular accident without residual weakness, transient ischemic attack, Parkinson's disease, or seizures. Quartiles of the Comorbidity Damage Index of the Charlson16 and the Acute Physiology Score (APS) of the APACHE II17 were used as global measures of comorbidity and illness severity, respectively. Each patient's primary physician was asked to estimate the patient's life expectancy on a 4‐point scale (<6 months, from 6 months to <1 year, 1‐5 years, >5 years). It was ascertained from each patient's primary nurse whether a urinary catheter or physical restraints were in use. Confusion was assessed according to how nurses gauged patient mental status on a 4‐point scale, from 1 = stuporous/comatose to 4 = alert, defined as being fully responsive and oriented. Any score other than 4 was coded as having altered mental status. Nurses classified patients as either independent or dependent for each of the 7 ADLs (feeding, bathing, dressing, grooming, toileting, transferring, and walking).18 Admission bed mobility was assessed by nurse rating on a 4‐point scale, from 1 = immobile to 4 = fully mobile.
In‐Hospital Outcome Assessment
Throughout the hospitalization, the primary nurse of each patient was interviewed weekly about whether the patient was expected to remain limited to a bed or chair for at least the next week. Whether patients had regained mobility was determined on the basis of the nurses' reports. Patients were defined as ambulatory if their activity was no longer confined to a bed or chair. Patients who died were included in the analysis, as the purpose of this study was to determine characteristics at admission that would predict who would likely regain ambulatory ability.
Statistical Analysis
Appropriate descriptive statistics, including means, standard deviations, and proportions, were used to describe the characteristics of those in the study group. For each variable of interest, logistic regression with dummy coding was used to examine unadjusted relationships with recovery of ambulatory ability. The independent contribution of each of the predictor variables to recovery of ambulatory ability was then tested in a series of multivariate logistic regression models that sequentially adjusted for factors considered important covariables. This was done by adding groups of similar covariates into the model in separate stages. These covariate groups were length of stay, demographics, global health measures and specific medical problems, hospital‐related factors, and admission bed mobility. All statistical analyses were performed using the Statistical Analyses System (SAS Institute, Cary, NC), and P < .05 was considered statistically significant.
RESULTS
For this study, 286 participants met all eligibility criteria, with 119 (42%) regaining ambulatory ability during hospitalization. Mean age of study participants was 73 9 years, with 12% of participants more than 85 years old. On admission, 214 patients (75%) were dependent in all 7 ADLs. Mean length of stay ( SD) was 12.3 6.5 days, with a range of 1‐31 days. Table 1 presents the cohort characteristics and the unadjusted effects of each variable for predicting those who did and did not recover ambulatory ability by characteristic. The P value of a variable in Table 1 indicates how significant that variable was, as determined with a simple logistic regression analysis. The unadjusted odds ratio of each variable is presented in Table 2 in order to facilitate comparisons with the adjusted odds ratios from the multivariate models. Analysis of the effects of the unadjusted single variables showed that age was a significant predictor, with older patients less likely to regain ambulatory ability. Several global health measures and hospital‐related factors were also significantly different between those who recovered and those who did not recover ambulatory ability, as summarized in Table 1. Importantly, length of stay was not significantly associated with recovery.
| Characteristic | No. of Subjects | Regained ambulatory ability | P valuea | ||
|---|---|---|---|---|---|
| Yes N (%) | No N(%) | ||||
| |||||
| Demographics | |||||
| Age | 55‐64 years | 46 | 19 (41) | 27 (59) | .009 |
| 65‐74 years | 118 | 63 (53) | 55 (47) | ||
| 75‐84 years | 88 | 29 (33) | 59 (67) | ||
| 85 years | 34 | 8 (24) | 26 (76) | ||
| Sex | Female | 163 | 72 (44) | 91 (56) | .31 |
| Male | 123 | 47 (38) | 76 (62) | ||
| Race | White | 168 | 72 (43) | 96 (57) | .61 |
| Black/other | 118 | 47 (40) | 71 (60) | ||
| Married | 164 | 64 (39) | 55 (45) | .30 | |
| Not married | 122 | 55 (61) | 67 (55) | ||
| Global health measures/specific medical problems | |||||
| Life expectancy | <6 months | 28 | 3 (11) | 25 (89) | < .0001 |
| 6 months‐1 year | 43 | 11 (26) | 32 (74) | ||
| 1‐5 years | 127 | 47 (37) | 80 (63) | ||
| >5 years | 88 | 58 (66) | 30 (34) | ||
| Acute Physiology Score | 0‐6 | 71 | 35 (49) | 36 (51) | < .0001 |
| 7‐10 | 83 | 46 (55) | 37 (45) | ||
| 11‐13 | 62 | 19 (31) | 43 (69) | ||
| 14+ | 61 | 13 (21) | 48 (79) | ||
| Comorbidity Index | 0‐1.0 | 89 | 48 (54) | 41 (46) | .01 |
| 1.1‐2.5 | 65 | 24 (37) | 41 (63) | ||
| 2.6‐4.0 | 55 | 23 (42) | 32 (58) | ||
| 4+ | 63 | 20 (32) | 43 (68) | ||
| Hip fracture present | Yes | 30 | 14 (47) | 16 (53) | .55 |
| No | 256 | 105 (41) | 151 (59) | ||
| Neurological disease present | Yes | 152 | 49 (32) | 103 (68) | .0007 |
| No | 134 | 70 (52) | 64 (48) | ||
| Hypotension present | Yes | 35 | 18 (51) | 17 (49) | .21 |
| No | 251 | 101 (40) | 150 (60) | ||
| Deep vein thrombosis present | Yes | 9 | 1 (11) | 8 (89) | .10 |
| No | 277 | 118 (43) | 159 (57) | ||
| Had major surgery | Yes | 73 | 47 (64) | 26 (36) | < .0001 |
| No | 213 | 72 (34) | 141 (66) | ||
| Level of consciousness | Altered mental status | 123 | 30 (24) | 93 (76) | < .0001 |
| No altered mental status | 163 | 89 (55) | 74 (45) | ||
| Hospital‐related factors | |||||
| Urinary catheter | Yes | 172 | 66 (38) | 106 (62) | .17 |
| No | 114 | 53 (46) | 61 (54) | ||
| Restraints in use | Yes | 93 | 21 (23) | 72 (77) | < .0001 |
| No | 193 | 98 (51) | 95 (49) | ||
| Initial bed mobility | Immobile | 25 | 5 (20) | 20 (80) | < .0001 |
| Very limited | 100 | 26 (26) | 74 (74) | ||
| Slightly limited | 131 | 73 (56) | 58 (44) | ||
| Fully mobile | 30 | 15 (50) | 15 (50) | ||
| Variables | Model 1 Demographics ORa (95% CI) | Model 2 Global health/specific diseases OR (95% CI) | Model 3 Hospital factors OR (95% CI) | Model 4 Mobility OR (95% CI) | Unadjusted Results OR (95% CI) |
|---|---|---|---|---|---|
| |||||
| Length of stay | 0.9 (0.9‐0.99)b | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) |
| Age | 0.6 (0.4‐0.8)b | 0.8 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐0.9)b |
| Sexfemale | 1.1 (0.7‐1.9) | 0.9 (0.5‐1.7) | 0.8 (0.4‐1.6) | 0.9 (0.4‐1.6) | 1.3 (0.8‐2.1) |
| Racewhite | 1.3 (0.8‐2.2) | 1.3 (0.7‐2.5) | 1.3 (0.7‐2.4) | 1.2 (0.6‐2.2) | 1.1 (0.7‐1.8) |
| Not married | 1.7 (1.0‐2.9) | 2.5 (1.3‐5.0)b | 2.7 (1.3‐5.5)b | 3.0 (1.4‐6.2)b | 1.3 (0.8‐2.1) |
| APS quartilesc | 0.7 (0.5‐1.0)b | 0.8 (0.5‐1.0) | 0.8 (0.6‐1.1) | 0.6 (0.5‐0.8)b | |
| MD‐rated life expectancyc | 1.9 (1.3‐2.8)b | 1.9 (1.3‐2.9)b | 1.9 (1.3‐2.8)b | 2.5 (1.8‐3.5)b | |
| Comorbidity quartilesc | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 0.8 (0.6‐0.9)b | |
| No deep vein thrombosis | 14.8 (1.6‐138.1)b | 13.1 (1.4‐121.1)b | 11.4 (1.2‐105.1)b | 5.9 (0.7‐48.1) | |
| No hip fracture | 2.1 (0.8‐5.6) | 2.1 (0.8‐6.0) | 2.2 (0.8‐6.3) | 0.8 (0.4‐1.7) | |
| No neurological diseasesc | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.4) | 2.3 (1.4‐3.7)b | |
| No hypotension | 0.8 (0.3‐2.1) | 0.8 (0.3‐2.2) | 0.8 (0.3‐2.3) | 0.6 (0.3‐1.3) | |
| Having major surgery | 1.7 (0.8‐3.6) | 1.8 (0.8‐3.9) | 1.9 (0.9‐4.0) | 3.5 (2.0‐6.2)b | |
| Having normal mental statusc | 2.1 (1.1‐4.0)b | 2.0 (0.8‐4.0) | 1.6 (0.8‐3.3) | 3.7 (2.2‐6.2)b | |
| No urinary catheter | 2.1 (1.0‐4.2)b | 2.2 (1.2‐5.5)b | 1.4 (0.9‐2.3) | ||
| Not in restraints | 2.2 (1.0‐4.6)b | 2.5 (1.2‐5.5)b | 3.5 (2.0‐6.2)b | ||
| Bed mobility on admissionc | 1.7 (1.1‐2.6)b | 2.0 (1.5‐2.8)b | |||
Table 2 shows the effects of adjusting the model for important covariables in a sequential fashion. Model 1 shows that longer length of stay and older age are associated with reduced odds of regaining ambulatory ability after adjusting for other demographic variables. However, age and length of stay were no longer significant after adjusting for global health measures and specific medical problems (Model 2). As demonstrated by the full model (Model 4), the participants who regained ambulatory ability were more likely to be unmarried, have a longer physician‐rated life expectancy, not have a diagnosis of deep vein thrombosis, not have physical restraints or a urinary catheter in use, and have greater bed mobility at admission.
Predictors that remained significant in the final multivariate model (Model 4) were summed in order to determine the proportion of patients who had one predictor versus those who had more than one predictor. Figure 1 shows the percentage of those who recovered ambulatory ability according to number of predictors, ranging from one to all 6 predictors. The results demonstrate a graded relationship, with number of predictors positively correlated with percentage of those who recovered ambulatory ability (P < .0001).
Patients who died prior to discharge were more likely to be male and have altered mental status, a urinary catheter, restraints, a shorter physician‐rated life expectancy, lower admission bed mobility, and increased severity of illness compared to those who survived. When the 34 patients who died were excluded from the multivariate analysis, the factors predicting ambulatory recovery were unchanged from those reported in Model 4 of Table 2.
DISCUSSION
In this study of older hospitalized patients, approximately 40% were able to regain their ambulatory ability despite being limited to the bed or chair on admission, having significant severity of illness, high level of use of restraints, and functional dependence on admission. Predictors of regaining ambulatory ability were identifiable at hospital admission. These predictors also were correlated with recovery of ambulatory ability in a graded fashion, lending support for the direct relationship between these predictors and recovery.
Physician‐rated life expectancy, a simple assessment that combines a physician's medical knowledge and clinical acumen, was demonstrated in our study to be a better predictor of recovery of ambulatory ability than more elaborate measures like the APACHE II17 and Charlson Comorbidity Index.16 This assessment can easily be done at the bedside and may help to guide discharge planning for the hospitalist physician. Nurse‐rated bed mobility at time of admission, which may reflect aspects of illness severity and cognitive status, was also able to predict recovery.
Of the 6 factors found to be independent predictors of recovery of ambulatory ability, 4 were related to mobility: lack of a DVT diagnosis, absence of a urinary catheter, absence of restraints, and nurse‐rated bed mobility at admission. In the group of patients initially expected to be confined to a bed or chair, those with additional mobility‐reducing factors, such as catheters and restraints, were less likely to recover, even after controlling for illness severity and comorbidity.
Marital status was not found to predict recovery of ambulatory ability in the simple unadjusted bivariate analysis, but after adjusting for other demographic, global health, and disease‐related variables, those who were unmarried were more likely to recover ambulatory ability. These interesting covariate‐adjusted effects for marital status have not been previously reported in the literature. One large study of the impact of marital status on hospital outcomes demonstrated those who were unmarried were more likely to require discharge to a nursing home and had slightly higher hospital costs and longer length of stay.19 Our findings may be related to such patients lacking support other than from themselves, with the possibility of being discharged to a nursing home an incentive to get up and walk.
Although age, race, and previous ADL status were found to be significant predictors in a previous study,12 we did not find this in our sample. This may be because, unlike in other studies, most of our patients had significant functional impairment on admission. The importance of age in our cohort disappeared when illness severity and comorbidities were added to the model.
The strengths of this study include having comprehensive patient‐related data on demographic, illness severity, comorbidity, and hospital‐related factors available, which enabled detailed analyses of predictors for regaining ambulatory ability. In particular, the ability to examine such factors as bed rest and hospital‐related treatments like restraint and catheter use, enabled this study to add significantly to the available knowledge of predictors of ambulatory recovery. The use of nurse interviews to obtain patient‐related data has been demonstrated in previous studies to be a preferred method of collecting data when compared to patient self‐report.20, 21 Examination of these factors in a cohort of patients who would be expected to be at very high risk for remaining bed‐ or chair bound, given their admission activity limitations and functional dependence, is also noteworthy.
Several important limitations deserve comment. Since the data were collected, average length of hospital stay generally has decreased. However, the patient population we studied continue to experience longer hospital stays than functionally intact patients. One recent study demonstrated that the length of hospital stay of patients who were dependent in one or more ADLs on admission was 35% longer than that of those not ADL dependent at admission.22 Seventy‐five percent of those in the present study cohort were dependent in all 7 ADLs and had a mean length of stay of 12.3 days. Despite the longer mean length of stay, 70% of those in the study cohort were discharged within 2 weeks of admission. In the university hospital where this research was conducted, mean length of stay ( SD) in 2004 was 6 8.6 days, but 10% of patients 55 years of age and older remained in the hospital for more than 2 weeks.23 This suggests there continue to be long‐stay patients in the current hospital environment, to which these findings may apply.
Standards of practice, such as for use of restraints, have also changed. In 1992, between 7.4% and 17% of all hospitalized medical patients were restrained, according to a literature review.24 A 1998 survey of 3 hospitals found the prevalence of restraints still ranged from 3.9% to 8.2% and noted that among the most common reasons reported for using restraints were to prevent patient disruption of therapy, to confine confused patients, and to reduce the number of falls.25 Thus, our study cohort would be more likely to be restrained, even in the current hospital environment, given that 43% of the cohort had altered mental status and that most were at risk for falls because of their poor functional status.26
Nevertheless, even though the use of restraints has declined since the data were collected for this study, this should affect neither the internal validity of the results nor the ability to address the question of what factors predict recovery of ambulatory ability. Indeed, the inclusion of patients on whom restraints are frequently used emphasizes the need for continued diligence in creating a restraint‐free environment in our hospitals. Data about the use of physical therapy services were not available in the study. Therefore, it is unknown to what extent the use of these services encouraged ambulation.
In this observational study, we found 6 factors associated with regaining ambulatory ability among hospitalized patients who had significant activity limitations and functional dependence on admission. These findings suggest predictors easily assessed by the hospitalist physician can help to identify those patients most likely to recover ambulatory ability prior to discharge. It also demonstrates the importance of mobility in maintaining function, given that many of the predictors are factors that either impede mobility such as restraints and urinary catheters or measure mobility such as admission bed mobility. Last, recognizing physician‐rated life expectancy as a strong independent predictor of recovery of ambulatory ability should encourage hospitalist physicians to continue to use their greatest tool, their clinical judgment, to determine who will recover ambulatory ability.
As most of these predictors can be identified on admission or shortly thereafter, these factors may be useful in helping physicians and other health care providers to predict the potential patients have to recover ambulatory ability. This information may help physicians identify patients who might benefit from early mobility programs, placement on hospital units where mobility will be enhanced, or the early initiation of discharge planning for those patients identified as unlikely to regain ambulation ability. In addition, addressing factors that are potentially modifiable, such as low bed mobility and the use of urinary catheters and restraints may not only improve the chance of recovering ambulatory ability but would also improve the quality of care provided to older patients.
- ,,, et al.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79‐84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847‐850.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451‐458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645‐652.
- ,,, et al.The natural history of functional morbidity in hospitalized older patients.J Am Geriatr Soc.1990;38:1296‐1303.
- ,,, et al.Hospital diagnosis, Medicare charges and nursing home admissions in the year when older persons become severely disabled.JAMA.1997;277:728‐734.
- ,,.A predictive index for functional decline in hospitalized elderly medical patients.J Gen Intern Med.1993;8:645‐652.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients: the importance of functional status.J Am Geriatr Soc.1988;36:775‐783.
- ,.Admission and discharge mobility of frail hospitalized older adults.Medsurg Nurs.2004;13:156‐163.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization.J Am Geriatr Soc.1996;44:251‐257.
- ,,.New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol Med Sci.1998;53A:M307–M312.
- ,,,.Geriatric hospital medicine.Med Clin North Am.2002;86:707‐729.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263‐1270.
- ,,, et al.Pressure ulcer risk factors among hospitalized patients with activity limitations.JAMA.1995;273:865‐870.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1986;40:373‐383.
- ,,,.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818‐829.
- ,,, et al.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial functioning.JAMA.1963;185:914‐919.
- ,.Impact of marital status on outcomes in hospitalized patients.Arch Intern Med.1995;155:2465‐2471.
- ,,.Current concepts in geriatrics: instruments for the functional assessment of older patients.N Engl J Med.1990;322:1207‐1214.
- ,,,,,.Comparison of subjective ratings of function with observed functional ability of frail older persons.Am J Public Health.1991;81:1127‐1130.
- ,,,,,.Diagnosis‐related group‐adjusted hospital costs are higher in older medical patients with lower functional status.J Am Geriatr Soc.2003;51:1729‐1734.
- UAB Hospital data,2005.
- .Physical restraints in the practice of medicine. Current concepts.Arch Intern Med.1992;152:2203‐2206.
- et al.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28:19‐24.
- Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664‐672.
Functional decline, defined as loss of the ability to care for oneself, commonly occurs during hospitalization, being experienced by up to 65% of older adults.14 Frequently, recovery of functional ability does not occur by the time of discharge from the hospital, despite resolution of the medical condition responsible for admission to the hospital.1, 5 Causes of the declining ability to perform activities of daily living (ADLs) are multiple and include both acute illness and adverse events associated with hospitalization.4, 6, 7 The functional decline experienced by older persons during hospitalization is a strong predictor of length of stay, nursing home placement, and mortality.8 Loss of ambulatory ability specifically has been noted to occur in 17%‐65% of hospitalized older patients, usually within days of admission, with few recovering this ability prior to discharge.2, 4, 5, 9 Importantly, ambulatory ability is significantly associated with a decline in other ADLs.9
Although several studies have explored risk factors associated with general functional decline during hospitalization,4, 7, 10, 11 only one study specifically examined risk factors associated with loss of ambulatory ability. In a cohort of participants who were largely independently performing ADLs on admission to the hospital, Mahoney et al. found age 85 years, white race, use of a walker, and functional impairment prior to being hospitalized were significant predictors of newly having walking dependence.12
Ambulatory ability could also be affected by a variety of other factors not examined in the Mahoney et al. study; these include severity of illness, bed rest, and hospital‐related treatments such as restraints or urinary catheters. In addition, little is known about predictors of recovery of ambulatory ability in patients expected to have activity limitations on admission who are dependent in most or all ADLs. The deconditioning associated with bed rest and reduced mobility has been described as one of the most predictable causes of functional decline, including loss of ambulatory ability, observed in older hospitalized patients.13 In one study, patients whose activity was limited to a bed or chair during hospitalization were 5.6 times more likely to develop functional decline than those who walked at all, even after controlling for other covariates including severity of illness and comorbidity.14 Those patients with both activity limitations and dependence in most or all ADLs represent an important subset of all hospitalized older patients who might be expected to be at higher risk of developing new ambulatory dependence. The ability to identify, at admission, those patients who will recover ambulatory ability may have important implications for discharge planning as well as for the development of preventive strategies.
The objective of the present study was to define patient demographic, illness severity, comorbid illness, and hospital‐related variables that are independent and significant predictors of regaining ambulatory ability prior to hospital discharge in a cohort of patients who had significant activity limitations and functional impairment at the time of admission to the hospital.
METHODS
Study Design
This study was part of a larger prospective cohort study conducted at a tertiary‐care teaching hospital that examined risk factors for pressure ulcers among patients with activity limitations.15 All patients admitted to the medical wards from December 1988 to June 1991 were screened, and research nurses confirmed eligibility within 3 days. Candidates were at least 55 years of age and were expected to be limited to a bed or chair for at least the first 5 days of hospitalization according to the assessments of their primary nurses. Participants were also eligible if admitted with a hip fracture. In all, 286 patients were included in the present analysis. These patients were included because they had been ambulatory in the 4 weeks before admission, and so they would be expected to have the potential to either maintain or regain ambulatory ability prior to discharge. Thirty‐one patients who otherwise would have been eligible for the present analysis were excluded because they had unusually long hospital stays, defined as longer than 31 days. These patients were excluded in order to remove outliers of this variable and because only the effects of relatively acute hospitalization were being studied. Study procedures were approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB).
Baseline Data Collection
For each patient, baseline data were collected from interviews with physicians and nurses at admission and were abstracted from the medical record. Chart review provided information on length of stay; the demographic variables age, sex, race, and marital status; and the presence of specified medical conditions or diseases that might affect a patient's ability to ambulate. These medical conditions and diseases were hip fracture, hypotension, deep vein thrombosis, major surgery and neurological disease defined as a history of hemiparesis regardless of cause, cerebrovascular accident without residual weakness, transient ischemic attack, Parkinson's disease, or seizures. Quartiles of the Comorbidity Damage Index of the Charlson16 and the Acute Physiology Score (APS) of the APACHE II17 were used as global measures of comorbidity and illness severity, respectively. Each patient's primary physician was asked to estimate the patient's life expectancy on a 4‐point scale (<6 months, from 6 months to <1 year, 1‐5 years, >5 years). It was ascertained from each patient's primary nurse whether a urinary catheter or physical restraints were in use. Confusion was assessed according to how nurses gauged patient mental status on a 4‐point scale, from 1 = stuporous/comatose to 4 = alert, defined as being fully responsive and oriented. Any score other than 4 was coded as having altered mental status. Nurses classified patients as either independent or dependent for each of the 7 ADLs (feeding, bathing, dressing, grooming, toileting, transferring, and walking).18 Admission bed mobility was assessed by nurse rating on a 4‐point scale, from 1 = immobile to 4 = fully mobile.
In‐Hospital Outcome Assessment
Throughout the hospitalization, the primary nurse of each patient was interviewed weekly about whether the patient was expected to remain limited to a bed or chair for at least the next week. Whether patients had regained mobility was determined on the basis of the nurses' reports. Patients were defined as ambulatory if their activity was no longer confined to a bed or chair. Patients who died were included in the analysis, as the purpose of this study was to determine characteristics at admission that would predict who would likely regain ambulatory ability.
Statistical Analysis
Appropriate descriptive statistics, including means, standard deviations, and proportions, were used to describe the characteristics of those in the study group. For each variable of interest, logistic regression with dummy coding was used to examine unadjusted relationships with recovery of ambulatory ability. The independent contribution of each of the predictor variables to recovery of ambulatory ability was then tested in a series of multivariate logistic regression models that sequentially adjusted for factors considered important covariables. This was done by adding groups of similar covariates into the model in separate stages. These covariate groups were length of stay, demographics, global health measures and specific medical problems, hospital‐related factors, and admission bed mobility. All statistical analyses were performed using the Statistical Analyses System (SAS Institute, Cary, NC), and P < .05 was considered statistically significant.
RESULTS
For this study, 286 participants met all eligibility criteria, with 119 (42%) regaining ambulatory ability during hospitalization. Mean age of study participants was 73 9 years, with 12% of participants more than 85 years old. On admission, 214 patients (75%) were dependent in all 7 ADLs. Mean length of stay ( SD) was 12.3 6.5 days, with a range of 1‐31 days. Table 1 presents the cohort characteristics and the unadjusted effects of each variable for predicting those who did and did not recover ambulatory ability by characteristic. The P value of a variable in Table 1 indicates how significant that variable was, as determined with a simple logistic regression analysis. The unadjusted odds ratio of each variable is presented in Table 2 in order to facilitate comparisons with the adjusted odds ratios from the multivariate models. Analysis of the effects of the unadjusted single variables showed that age was a significant predictor, with older patients less likely to regain ambulatory ability. Several global health measures and hospital‐related factors were also significantly different between those who recovered and those who did not recover ambulatory ability, as summarized in Table 1. Importantly, length of stay was not significantly associated with recovery.
| Characteristic | No. of Subjects | Regained ambulatory ability | P valuea | ||
|---|---|---|---|---|---|
| Yes N (%) | No N(%) | ||||
| |||||
| Demographics | |||||
| Age | 55‐64 years | 46 | 19 (41) | 27 (59) | .009 |
| 65‐74 years | 118 | 63 (53) | 55 (47) | ||
| 75‐84 years | 88 | 29 (33) | 59 (67) | ||
| 85 years | 34 | 8 (24) | 26 (76) | ||
| Sex | Female | 163 | 72 (44) | 91 (56) | .31 |
| Male | 123 | 47 (38) | 76 (62) | ||
| Race | White | 168 | 72 (43) | 96 (57) | .61 |
| Black/other | 118 | 47 (40) | 71 (60) | ||
| Married | 164 | 64 (39) | 55 (45) | .30 | |
| Not married | 122 | 55 (61) | 67 (55) | ||
| Global health measures/specific medical problems | |||||
| Life expectancy | <6 months | 28 | 3 (11) | 25 (89) | < .0001 |
| 6 months‐1 year | 43 | 11 (26) | 32 (74) | ||
| 1‐5 years | 127 | 47 (37) | 80 (63) | ||
| >5 years | 88 | 58 (66) | 30 (34) | ||
| Acute Physiology Score | 0‐6 | 71 | 35 (49) | 36 (51) | < .0001 |
| 7‐10 | 83 | 46 (55) | 37 (45) | ||
| 11‐13 | 62 | 19 (31) | 43 (69) | ||
| 14+ | 61 | 13 (21) | 48 (79) | ||
| Comorbidity Index | 0‐1.0 | 89 | 48 (54) | 41 (46) | .01 |
| 1.1‐2.5 | 65 | 24 (37) | 41 (63) | ||
| 2.6‐4.0 | 55 | 23 (42) | 32 (58) | ||
| 4+ | 63 | 20 (32) | 43 (68) | ||
| Hip fracture present | Yes | 30 | 14 (47) | 16 (53) | .55 |
| No | 256 | 105 (41) | 151 (59) | ||
| Neurological disease present | Yes | 152 | 49 (32) | 103 (68) | .0007 |
| No | 134 | 70 (52) | 64 (48) | ||
| Hypotension present | Yes | 35 | 18 (51) | 17 (49) | .21 |
| No | 251 | 101 (40) | 150 (60) | ||
| Deep vein thrombosis present | Yes | 9 | 1 (11) | 8 (89) | .10 |
| No | 277 | 118 (43) | 159 (57) | ||
| Had major surgery | Yes | 73 | 47 (64) | 26 (36) | < .0001 |
| No | 213 | 72 (34) | 141 (66) | ||
| Level of consciousness | Altered mental status | 123 | 30 (24) | 93 (76) | < .0001 |
| No altered mental status | 163 | 89 (55) | 74 (45) | ||
| Hospital‐related factors | |||||
| Urinary catheter | Yes | 172 | 66 (38) | 106 (62) | .17 |
| No | 114 | 53 (46) | 61 (54) | ||
| Restraints in use | Yes | 93 | 21 (23) | 72 (77) | < .0001 |
| No | 193 | 98 (51) | 95 (49) | ||
| Initial bed mobility | Immobile | 25 | 5 (20) | 20 (80) | < .0001 |
| Very limited | 100 | 26 (26) | 74 (74) | ||
| Slightly limited | 131 | 73 (56) | 58 (44) | ||
| Fully mobile | 30 | 15 (50) | 15 (50) | ||
| Variables | Model 1 Demographics ORa (95% CI) | Model 2 Global health/specific diseases OR (95% CI) | Model 3 Hospital factors OR (95% CI) | Model 4 Mobility OR (95% CI) | Unadjusted Results OR (95% CI) |
|---|---|---|---|---|---|
| |||||
| Length of stay | 0.9 (0.9‐0.99)b | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) |
| Age | 0.6 (0.4‐0.8)b | 0.8 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐0.9)b |
| Sexfemale | 1.1 (0.7‐1.9) | 0.9 (0.5‐1.7) | 0.8 (0.4‐1.6) | 0.9 (0.4‐1.6) | 1.3 (0.8‐2.1) |
| Racewhite | 1.3 (0.8‐2.2) | 1.3 (0.7‐2.5) | 1.3 (0.7‐2.4) | 1.2 (0.6‐2.2) | 1.1 (0.7‐1.8) |
| Not married | 1.7 (1.0‐2.9) | 2.5 (1.3‐5.0)b | 2.7 (1.3‐5.5)b | 3.0 (1.4‐6.2)b | 1.3 (0.8‐2.1) |
| APS quartilesc | 0.7 (0.5‐1.0)b | 0.8 (0.5‐1.0) | 0.8 (0.6‐1.1) | 0.6 (0.5‐0.8)b | |
| MD‐rated life expectancyc | 1.9 (1.3‐2.8)b | 1.9 (1.3‐2.9)b | 1.9 (1.3‐2.8)b | 2.5 (1.8‐3.5)b | |
| Comorbidity quartilesc | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 0.8 (0.6‐0.9)b | |
| No deep vein thrombosis | 14.8 (1.6‐138.1)b | 13.1 (1.4‐121.1)b | 11.4 (1.2‐105.1)b | 5.9 (0.7‐48.1) | |
| No hip fracture | 2.1 (0.8‐5.6) | 2.1 (0.8‐6.0) | 2.2 (0.8‐6.3) | 0.8 (0.4‐1.7) | |
| No neurological diseasesc | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.4) | 2.3 (1.4‐3.7)b | |
| No hypotension | 0.8 (0.3‐2.1) | 0.8 (0.3‐2.2) | 0.8 (0.3‐2.3) | 0.6 (0.3‐1.3) | |
| Having major surgery | 1.7 (0.8‐3.6) | 1.8 (0.8‐3.9) | 1.9 (0.9‐4.0) | 3.5 (2.0‐6.2)b | |
| Having normal mental statusc | 2.1 (1.1‐4.0)b | 2.0 (0.8‐4.0) | 1.6 (0.8‐3.3) | 3.7 (2.2‐6.2)b | |
| No urinary catheter | 2.1 (1.0‐4.2)b | 2.2 (1.2‐5.5)b | 1.4 (0.9‐2.3) | ||
| Not in restraints | 2.2 (1.0‐4.6)b | 2.5 (1.2‐5.5)b | 3.5 (2.0‐6.2)b | ||
| Bed mobility on admissionc | 1.7 (1.1‐2.6)b | 2.0 (1.5‐2.8)b | |||
Table 2 shows the effects of adjusting the model for important covariables in a sequential fashion. Model 1 shows that longer length of stay and older age are associated with reduced odds of regaining ambulatory ability after adjusting for other demographic variables. However, age and length of stay were no longer significant after adjusting for global health measures and specific medical problems (Model 2). As demonstrated by the full model (Model 4), the participants who regained ambulatory ability were more likely to be unmarried, have a longer physician‐rated life expectancy, not have a diagnosis of deep vein thrombosis, not have physical restraints or a urinary catheter in use, and have greater bed mobility at admission.
Predictors that remained significant in the final multivariate model (Model 4) were summed in order to determine the proportion of patients who had one predictor versus those who had more than one predictor. Figure 1 shows the percentage of those who recovered ambulatory ability according to number of predictors, ranging from one to all 6 predictors. The results demonstrate a graded relationship, with number of predictors positively correlated with percentage of those who recovered ambulatory ability (P < .0001).

Patients who died prior to discharge were more likely to be male and have altered mental status, a urinary catheter, restraints, a shorter physician‐rated life expectancy, lower admission bed mobility, and increased severity of illness compared to those who survived. When the 34 patients who died were excluded from the multivariate analysis, the factors predicting ambulatory recovery were unchanged from those reported in Model 4 of Table 2.
DISCUSSION
In this study of older hospitalized patients, approximately 40% were able to regain their ambulatory ability despite being limited to the bed or chair on admission, having significant severity of illness, high level of use of restraints, and functional dependence on admission. Predictors of regaining ambulatory ability were identifiable at hospital admission. These predictors also were correlated with recovery of ambulatory ability in a graded fashion, lending support for the direct relationship between these predictors and recovery.
Physician‐rated life expectancy, a simple assessment that combines a physician's medical knowledge and clinical acumen, was demonstrated in our study to be a better predictor of recovery of ambulatory ability than more elaborate measures like the APACHE II17 and Charlson Comorbidity Index.16 This assessment can easily be done at the bedside and may help to guide discharge planning for the hospitalist physician. Nurse‐rated bed mobility at time of admission, which may reflect aspects of illness severity and cognitive status, was also able to predict recovery.
Of the 6 factors found to be independent predictors of recovery of ambulatory ability, 4 were related to mobility: lack of a DVT diagnosis, absence of a urinary catheter, absence of restraints, and nurse‐rated bed mobility at admission. In the group of patients initially expected to be confined to a bed or chair, those with additional mobility‐reducing factors, such as catheters and restraints, were less likely to recover, even after controlling for illness severity and comorbidity.
Marital status was not found to predict recovery of ambulatory ability in the simple unadjusted bivariate analysis, but after adjusting for other demographic, global health, and disease‐related variables, those who were unmarried were more likely to recover ambulatory ability. These interesting covariate‐adjusted effects for marital status have not been previously reported in the literature. One large study of the impact of marital status on hospital outcomes demonstrated those who were unmarried were more likely to require discharge to a nursing home and had slightly higher hospital costs and longer length of stay.19 Our findings may be related to such patients lacking support other than from themselves, with the possibility of being discharged to a nursing home an incentive to get up and walk.
Although age, race, and previous ADL status were found to be significant predictors in a previous study,12 we did not find this in our sample. This may be because, unlike in other studies, most of our patients had significant functional impairment on admission. The importance of age in our cohort disappeared when illness severity and comorbidities were added to the model.
The strengths of this study include having comprehensive patient‐related data on demographic, illness severity, comorbidity, and hospital‐related factors available, which enabled detailed analyses of predictors for regaining ambulatory ability. In particular, the ability to examine such factors as bed rest and hospital‐related treatments like restraint and catheter use, enabled this study to add significantly to the available knowledge of predictors of ambulatory recovery. The use of nurse interviews to obtain patient‐related data has been demonstrated in previous studies to be a preferred method of collecting data when compared to patient self‐report.20, 21 Examination of these factors in a cohort of patients who would be expected to be at very high risk for remaining bed‐ or chair bound, given their admission activity limitations and functional dependence, is also noteworthy.
Several important limitations deserve comment. Since the data were collected, average length of hospital stay generally has decreased. However, the patient population we studied continue to experience longer hospital stays than functionally intact patients. One recent study demonstrated that the length of hospital stay of patients who were dependent in one or more ADLs on admission was 35% longer than that of those not ADL dependent at admission.22 Seventy‐five percent of those in the present study cohort were dependent in all 7 ADLs and had a mean length of stay of 12.3 days. Despite the longer mean length of stay, 70% of those in the study cohort were discharged within 2 weeks of admission. In the university hospital where this research was conducted, mean length of stay ( SD) in 2004 was 6 8.6 days, but 10% of patients 55 years of age and older remained in the hospital for more than 2 weeks.23 This suggests there continue to be long‐stay patients in the current hospital environment, to which these findings may apply.
Standards of practice, such as for use of restraints, have also changed. In 1992, between 7.4% and 17% of all hospitalized medical patients were restrained, according to a literature review.24 A 1998 survey of 3 hospitals found the prevalence of restraints still ranged from 3.9% to 8.2% and noted that among the most common reasons reported for using restraints were to prevent patient disruption of therapy, to confine confused patients, and to reduce the number of falls.25 Thus, our study cohort would be more likely to be restrained, even in the current hospital environment, given that 43% of the cohort had altered mental status and that most were at risk for falls because of their poor functional status.26
Nevertheless, even though the use of restraints has declined since the data were collected for this study, this should affect neither the internal validity of the results nor the ability to address the question of what factors predict recovery of ambulatory ability. Indeed, the inclusion of patients on whom restraints are frequently used emphasizes the need for continued diligence in creating a restraint‐free environment in our hospitals. Data about the use of physical therapy services were not available in the study. Therefore, it is unknown to what extent the use of these services encouraged ambulation.
In this observational study, we found 6 factors associated with regaining ambulatory ability among hospitalized patients who had significant activity limitations and functional dependence on admission. These findings suggest predictors easily assessed by the hospitalist physician can help to identify those patients most likely to recover ambulatory ability prior to discharge. It also demonstrates the importance of mobility in maintaining function, given that many of the predictors are factors that either impede mobility such as restraints and urinary catheters or measure mobility such as admission bed mobility. Last, recognizing physician‐rated life expectancy as a strong independent predictor of recovery of ambulatory ability should encourage hospitalist physicians to continue to use their greatest tool, their clinical judgment, to determine who will recover ambulatory ability.
As most of these predictors can be identified on admission or shortly thereafter, these factors may be useful in helping physicians and other health care providers to predict the potential patients have to recover ambulatory ability. This information may help physicians identify patients who might benefit from early mobility programs, placement on hospital units where mobility will be enhanced, or the early initiation of discharge planning for those patients identified as unlikely to regain ambulation ability. In addition, addressing factors that are potentially modifiable, such as low bed mobility and the use of urinary catheters and restraints may not only improve the chance of recovering ambulatory ability but would also improve the quality of care provided to older patients.
Functional decline, defined as loss of the ability to care for oneself, commonly occurs during hospitalization, being experienced by up to 65% of older adults.14 Frequently, recovery of functional ability does not occur by the time of discharge from the hospital, despite resolution of the medical condition responsible for admission to the hospital.1, 5 Causes of the declining ability to perform activities of daily living (ADLs) are multiple and include both acute illness and adverse events associated with hospitalization.4, 6, 7 The functional decline experienced by older persons during hospitalization is a strong predictor of length of stay, nursing home placement, and mortality.8 Loss of ambulatory ability specifically has been noted to occur in 17%‐65% of hospitalized older patients, usually within days of admission, with few recovering this ability prior to discharge.2, 4, 5, 9 Importantly, ambulatory ability is significantly associated with a decline in other ADLs.9
Although several studies have explored risk factors associated with general functional decline during hospitalization,4, 7, 10, 11 only one study specifically examined risk factors associated with loss of ambulatory ability. In a cohort of participants who were largely independently performing ADLs on admission to the hospital, Mahoney et al. found age 85 years, white race, use of a walker, and functional impairment prior to being hospitalized were significant predictors of newly having walking dependence.12
Ambulatory ability could also be affected by a variety of other factors not examined in the Mahoney et al. study; these include severity of illness, bed rest, and hospital‐related treatments such as restraints or urinary catheters. In addition, little is known about predictors of recovery of ambulatory ability in patients expected to have activity limitations on admission who are dependent in most or all ADLs. The deconditioning associated with bed rest and reduced mobility has been described as one of the most predictable causes of functional decline, including loss of ambulatory ability, observed in older hospitalized patients.13 In one study, patients whose activity was limited to a bed or chair during hospitalization were 5.6 times more likely to develop functional decline than those who walked at all, even after controlling for other covariates including severity of illness and comorbidity.14 Those patients with both activity limitations and dependence in most or all ADLs represent an important subset of all hospitalized older patients who might be expected to be at higher risk of developing new ambulatory dependence. The ability to identify, at admission, those patients who will recover ambulatory ability may have important implications for discharge planning as well as for the development of preventive strategies.
The objective of the present study was to define patient demographic, illness severity, comorbid illness, and hospital‐related variables that are independent and significant predictors of regaining ambulatory ability prior to hospital discharge in a cohort of patients who had significant activity limitations and functional impairment at the time of admission to the hospital.
METHODS
Study Design
This study was part of a larger prospective cohort study conducted at a tertiary‐care teaching hospital that examined risk factors for pressure ulcers among patients with activity limitations.15 All patients admitted to the medical wards from December 1988 to June 1991 were screened, and research nurses confirmed eligibility within 3 days. Candidates were at least 55 years of age and were expected to be limited to a bed or chair for at least the first 5 days of hospitalization according to the assessments of their primary nurses. Participants were also eligible if admitted with a hip fracture. In all, 286 patients were included in the present analysis. These patients were included because they had been ambulatory in the 4 weeks before admission, and so they would be expected to have the potential to either maintain or regain ambulatory ability prior to discharge. Thirty‐one patients who otherwise would have been eligible for the present analysis were excluded because they had unusually long hospital stays, defined as longer than 31 days. These patients were excluded in order to remove outliers of this variable and because only the effects of relatively acute hospitalization were being studied. Study procedures were approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB).
Baseline Data Collection
For each patient, baseline data were collected from interviews with physicians and nurses at admission and were abstracted from the medical record. Chart review provided information on length of stay; the demographic variables age, sex, race, and marital status; and the presence of specified medical conditions or diseases that might affect a patient's ability to ambulate. These medical conditions and diseases were hip fracture, hypotension, deep vein thrombosis, major surgery and neurological disease defined as a history of hemiparesis regardless of cause, cerebrovascular accident without residual weakness, transient ischemic attack, Parkinson's disease, or seizures. Quartiles of the Comorbidity Damage Index of the Charlson16 and the Acute Physiology Score (APS) of the APACHE II17 were used as global measures of comorbidity and illness severity, respectively. Each patient's primary physician was asked to estimate the patient's life expectancy on a 4‐point scale (<6 months, from 6 months to <1 year, 1‐5 years, >5 years). It was ascertained from each patient's primary nurse whether a urinary catheter or physical restraints were in use. Confusion was assessed according to how nurses gauged patient mental status on a 4‐point scale, from 1 = stuporous/comatose to 4 = alert, defined as being fully responsive and oriented. Any score other than 4 was coded as having altered mental status. Nurses classified patients as either independent or dependent for each of the 7 ADLs (feeding, bathing, dressing, grooming, toileting, transferring, and walking).18 Admission bed mobility was assessed by nurse rating on a 4‐point scale, from 1 = immobile to 4 = fully mobile.
In‐Hospital Outcome Assessment
Throughout the hospitalization, the primary nurse of each patient was interviewed weekly about whether the patient was expected to remain limited to a bed or chair for at least the next week. Whether patients had regained mobility was determined on the basis of the nurses' reports. Patients were defined as ambulatory if their activity was no longer confined to a bed or chair. Patients who died were included in the analysis, as the purpose of this study was to determine characteristics at admission that would predict who would likely regain ambulatory ability.
Statistical Analysis
Appropriate descriptive statistics, including means, standard deviations, and proportions, were used to describe the characteristics of those in the study group. For each variable of interest, logistic regression with dummy coding was used to examine unadjusted relationships with recovery of ambulatory ability. The independent contribution of each of the predictor variables to recovery of ambulatory ability was then tested in a series of multivariate logistic regression models that sequentially adjusted for factors considered important covariables. This was done by adding groups of similar covariates into the model in separate stages. These covariate groups were length of stay, demographics, global health measures and specific medical problems, hospital‐related factors, and admission bed mobility. All statistical analyses were performed using the Statistical Analyses System (SAS Institute, Cary, NC), and P < .05 was considered statistically significant.
RESULTS
For this study, 286 participants met all eligibility criteria, with 119 (42%) regaining ambulatory ability during hospitalization. Mean age of study participants was 73 9 years, with 12% of participants more than 85 years old. On admission, 214 patients (75%) were dependent in all 7 ADLs. Mean length of stay ( SD) was 12.3 6.5 days, with a range of 1‐31 days. Table 1 presents the cohort characteristics and the unadjusted effects of each variable for predicting those who did and did not recover ambulatory ability by characteristic. The P value of a variable in Table 1 indicates how significant that variable was, as determined with a simple logistic regression analysis. The unadjusted odds ratio of each variable is presented in Table 2 in order to facilitate comparisons with the adjusted odds ratios from the multivariate models. Analysis of the effects of the unadjusted single variables showed that age was a significant predictor, with older patients less likely to regain ambulatory ability. Several global health measures and hospital‐related factors were also significantly different between those who recovered and those who did not recover ambulatory ability, as summarized in Table 1. Importantly, length of stay was not significantly associated with recovery.
| Characteristic | No. of Subjects | Regained ambulatory ability | P valuea | ||
|---|---|---|---|---|---|
| Yes N (%) | No N(%) | ||||
| |||||
| Demographics | |||||
| Age | 55‐64 years | 46 | 19 (41) | 27 (59) | .009 |
| 65‐74 years | 118 | 63 (53) | 55 (47) | ||
| 75‐84 years | 88 | 29 (33) | 59 (67) | ||
| 85 years | 34 | 8 (24) | 26 (76) | ||
| Sex | Female | 163 | 72 (44) | 91 (56) | .31 |
| Male | 123 | 47 (38) | 76 (62) | ||
| Race | White | 168 | 72 (43) | 96 (57) | .61 |
| Black/other | 118 | 47 (40) | 71 (60) | ||
| Married | 164 | 64 (39) | 55 (45) | .30 | |
| Not married | 122 | 55 (61) | 67 (55) | ||
| Global health measures/specific medical problems | |||||
| Life expectancy | <6 months | 28 | 3 (11) | 25 (89) | < .0001 |
| 6 months‐1 year | 43 | 11 (26) | 32 (74) | ||
| 1‐5 years | 127 | 47 (37) | 80 (63) | ||
| >5 years | 88 | 58 (66) | 30 (34) | ||
| Acute Physiology Score | 0‐6 | 71 | 35 (49) | 36 (51) | < .0001 |
| 7‐10 | 83 | 46 (55) | 37 (45) | ||
| 11‐13 | 62 | 19 (31) | 43 (69) | ||
| 14+ | 61 | 13 (21) | 48 (79) | ||
| Comorbidity Index | 0‐1.0 | 89 | 48 (54) | 41 (46) | .01 |
| 1.1‐2.5 | 65 | 24 (37) | 41 (63) | ||
| 2.6‐4.0 | 55 | 23 (42) | 32 (58) | ||
| 4+ | 63 | 20 (32) | 43 (68) | ||
| Hip fracture present | Yes | 30 | 14 (47) | 16 (53) | .55 |
| No | 256 | 105 (41) | 151 (59) | ||
| Neurological disease present | Yes | 152 | 49 (32) | 103 (68) | .0007 |
| No | 134 | 70 (52) | 64 (48) | ||
| Hypotension present | Yes | 35 | 18 (51) | 17 (49) | .21 |
| No | 251 | 101 (40) | 150 (60) | ||
| Deep vein thrombosis present | Yes | 9 | 1 (11) | 8 (89) | .10 |
| No | 277 | 118 (43) | 159 (57) | ||
| Had major surgery | Yes | 73 | 47 (64) | 26 (36) | < .0001 |
| No | 213 | 72 (34) | 141 (66) | ||
| Level of consciousness | Altered mental status | 123 | 30 (24) | 93 (76) | < .0001 |
| No altered mental status | 163 | 89 (55) | 74 (45) | ||
| Hospital‐related factors | |||||
| Urinary catheter | Yes | 172 | 66 (38) | 106 (62) | .17 |
| No | 114 | 53 (46) | 61 (54) | ||
| Restraints in use | Yes | 93 | 21 (23) | 72 (77) | < .0001 |
| No | 193 | 98 (51) | 95 (49) | ||
| Initial bed mobility | Immobile | 25 | 5 (20) | 20 (80) | < .0001 |
| Very limited | 100 | 26 (26) | 74 (74) | ||
| Slightly limited | 131 | 73 (56) | 58 (44) | ||
| Fully mobile | 30 | 15 (50) | 15 (50) | ||
| Variables | Model 1 Demographics ORa (95% CI) | Model 2 Global health/specific diseases OR (95% CI) | Model 3 Hospital factors OR (95% CI) | Model 4 Mobility OR (95% CI) | Unadjusted Results OR (95% CI) |
|---|---|---|---|---|---|
| |||||
| Length of stay | 0.9 (0.9‐0.99)b | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) | 1.0 (0.9‐1.0) |
| Age | 0.6 (0.4‐0.8)b | 0.8 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐1.1) | 0.7 (0.5‐0.9)b |
| Sexfemale | 1.1 (0.7‐1.9) | 0.9 (0.5‐1.7) | 0.8 (0.4‐1.6) | 0.9 (0.4‐1.6) | 1.3 (0.8‐2.1) |
| Racewhite | 1.3 (0.8‐2.2) | 1.3 (0.7‐2.5) | 1.3 (0.7‐2.4) | 1.2 (0.6‐2.2) | 1.1 (0.7‐1.8) |
| Not married | 1.7 (1.0‐2.9) | 2.5 (1.3‐5.0)b | 2.7 (1.3‐5.5)b | 3.0 (1.4‐6.2)b | 1.3 (0.8‐2.1) |
| APS quartilesc | 0.7 (0.5‐1.0)b | 0.8 (0.5‐1.0) | 0.8 (0.6‐1.1) | 0.6 (0.5‐0.8)b | |
| MD‐rated life expectancyc | 1.9 (1.3‐2.8)b | 1.9 (1.3‐2.9)b | 1.9 (1.3‐2.8)b | 2.5 (1.8‐3.5)b | |
| Comorbidity quartilesc | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 1.0 (0.7‐1.3) | 0.8 (0.6‐0.9)b | |
| No deep vein thrombosis | 14.8 (1.6‐138.1)b | 13.1 (1.4‐121.1)b | 11.4 (1.2‐105.1)b | 5.9 (0.7‐48.1) | |
| No hip fracture | 2.1 (0.8‐5.6) | 2.1 (0.8‐6.0) | 2.2 (0.8‐6.3) | 0.8 (0.4‐1.7) | |
| No neurological diseasesc | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.3) | 1.7 (0.9‐3.4) | 2.3 (1.4‐3.7)b | |
| No hypotension | 0.8 (0.3‐2.1) | 0.8 (0.3‐2.2) | 0.8 (0.3‐2.3) | 0.6 (0.3‐1.3) | |
| Having major surgery | 1.7 (0.8‐3.6) | 1.8 (0.8‐3.9) | 1.9 (0.9‐4.0) | 3.5 (2.0‐6.2)b | |
| Having normal mental statusc | 2.1 (1.1‐4.0)b | 2.0 (0.8‐4.0) | 1.6 (0.8‐3.3) | 3.7 (2.2‐6.2)b | |
| No urinary catheter | 2.1 (1.0‐4.2)b | 2.2 (1.2‐5.5)b | 1.4 (0.9‐2.3) | ||
| Not in restraints | 2.2 (1.0‐4.6)b | 2.5 (1.2‐5.5)b | 3.5 (2.0‐6.2)b | ||
| Bed mobility on admissionc | 1.7 (1.1‐2.6)b | 2.0 (1.5‐2.8)b | |||
Table 2 shows the effects of adjusting the model for important covariables in a sequential fashion. Model 1 shows that longer length of stay and older age are associated with reduced odds of regaining ambulatory ability after adjusting for other demographic variables. However, age and length of stay were no longer significant after adjusting for global health measures and specific medical problems (Model 2). As demonstrated by the full model (Model 4), the participants who regained ambulatory ability were more likely to be unmarried, have a longer physician‐rated life expectancy, not have a diagnosis of deep vein thrombosis, not have physical restraints or a urinary catheter in use, and have greater bed mobility at admission.
Predictors that remained significant in the final multivariate model (Model 4) were summed in order to determine the proportion of patients who had one predictor versus those who had more than one predictor. Figure 1 shows the percentage of those who recovered ambulatory ability according to number of predictors, ranging from one to all 6 predictors. The results demonstrate a graded relationship, with number of predictors positively correlated with percentage of those who recovered ambulatory ability (P < .0001).

Patients who died prior to discharge were more likely to be male and have altered mental status, a urinary catheter, restraints, a shorter physician‐rated life expectancy, lower admission bed mobility, and increased severity of illness compared to those who survived. When the 34 patients who died were excluded from the multivariate analysis, the factors predicting ambulatory recovery were unchanged from those reported in Model 4 of Table 2.
DISCUSSION
In this study of older hospitalized patients, approximately 40% were able to regain their ambulatory ability despite being limited to the bed or chair on admission, having significant severity of illness, high level of use of restraints, and functional dependence on admission. Predictors of regaining ambulatory ability were identifiable at hospital admission. These predictors also were correlated with recovery of ambulatory ability in a graded fashion, lending support for the direct relationship between these predictors and recovery.
Physician‐rated life expectancy, a simple assessment that combines a physician's medical knowledge and clinical acumen, was demonstrated in our study to be a better predictor of recovery of ambulatory ability than more elaborate measures like the APACHE II17 and Charlson Comorbidity Index.16 This assessment can easily be done at the bedside and may help to guide discharge planning for the hospitalist physician. Nurse‐rated bed mobility at time of admission, which may reflect aspects of illness severity and cognitive status, was also able to predict recovery.
Of the 6 factors found to be independent predictors of recovery of ambulatory ability, 4 were related to mobility: lack of a DVT diagnosis, absence of a urinary catheter, absence of restraints, and nurse‐rated bed mobility at admission. In the group of patients initially expected to be confined to a bed or chair, those with additional mobility‐reducing factors, such as catheters and restraints, were less likely to recover, even after controlling for illness severity and comorbidity.
Marital status was not found to predict recovery of ambulatory ability in the simple unadjusted bivariate analysis, but after adjusting for other demographic, global health, and disease‐related variables, those who were unmarried were more likely to recover ambulatory ability. These interesting covariate‐adjusted effects for marital status have not been previously reported in the literature. One large study of the impact of marital status on hospital outcomes demonstrated those who were unmarried were more likely to require discharge to a nursing home and had slightly higher hospital costs and longer length of stay.19 Our findings may be related to such patients lacking support other than from themselves, with the possibility of being discharged to a nursing home an incentive to get up and walk.
Although age, race, and previous ADL status were found to be significant predictors in a previous study,12 we did not find this in our sample. This may be because, unlike in other studies, most of our patients had significant functional impairment on admission. The importance of age in our cohort disappeared when illness severity and comorbidities were added to the model.
The strengths of this study include having comprehensive patient‐related data on demographic, illness severity, comorbidity, and hospital‐related factors available, which enabled detailed analyses of predictors for regaining ambulatory ability. In particular, the ability to examine such factors as bed rest and hospital‐related treatments like restraint and catheter use, enabled this study to add significantly to the available knowledge of predictors of ambulatory recovery. The use of nurse interviews to obtain patient‐related data has been demonstrated in previous studies to be a preferred method of collecting data when compared to patient self‐report.20, 21 Examination of these factors in a cohort of patients who would be expected to be at very high risk for remaining bed‐ or chair bound, given their admission activity limitations and functional dependence, is also noteworthy.
Several important limitations deserve comment. Since the data were collected, average length of hospital stay generally has decreased. However, the patient population we studied continue to experience longer hospital stays than functionally intact patients. One recent study demonstrated that the length of hospital stay of patients who were dependent in one or more ADLs on admission was 35% longer than that of those not ADL dependent at admission.22 Seventy‐five percent of those in the present study cohort were dependent in all 7 ADLs and had a mean length of stay of 12.3 days. Despite the longer mean length of stay, 70% of those in the study cohort were discharged within 2 weeks of admission. In the university hospital where this research was conducted, mean length of stay ( SD) in 2004 was 6 8.6 days, but 10% of patients 55 years of age and older remained in the hospital for more than 2 weeks.23 This suggests there continue to be long‐stay patients in the current hospital environment, to which these findings may apply.
Standards of practice, such as for use of restraints, have also changed. In 1992, between 7.4% and 17% of all hospitalized medical patients were restrained, according to a literature review.24 A 1998 survey of 3 hospitals found the prevalence of restraints still ranged from 3.9% to 8.2% and noted that among the most common reasons reported for using restraints were to prevent patient disruption of therapy, to confine confused patients, and to reduce the number of falls.25 Thus, our study cohort would be more likely to be restrained, even in the current hospital environment, given that 43% of the cohort had altered mental status and that most were at risk for falls because of their poor functional status.26
Nevertheless, even though the use of restraints has declined since the data were collected for this study, this should affect neither the internal validity of the results nor the ability to address the question of what factors predict recovery of ambulatory ability. Indeed, the inclusion of patients on whom restraints are frequently used emphasizes the need for continued diligence in creating a restraint‐free environment in our hospitals. Data about the use of physical therapy services were not available in the study. Therefore, it is unknown to what extent the use of these services encouraged ambulation.
In this observational study, we found 6 factors associated with regaining ambulatory ability among hospitalized patients who had significant activity limitations and functional dependence on admission. These findings suggest predictors easily assessed by the hospitalist physician can help to identify those patients most likely to recover ambulatory ability prior to discharge. It also demonstrates the importance of mobility in maintaining function, given that many of the predictors are factors that either impede mobility such as restraints and urinary catheters or measure mobility such as admission bed mobility. Last, recognizing physician‐rated life expectancy as a strong independent predictor of recovery of ambulatory ability should encourage hospitalist physicians to continue to use their greatest tool, their clinical judgment, to determine who will recover ambulatory ability.
As most of these predictors can be identified on admission or shortly thereafter, these factors may be useful in helping physicians and other health care providers to predict the potential patients have to recover ambulatory ability. This information may help physicians identify patients who might benefit from early mobility programs, placement on hospital units where mobility will be enhanced, or the early initiation of discharge planning for those patients identified as unlikely to regain ambulation ability. In addition, addressing factors that are potentially modifiable, such as low bed mobility and the use of urinary catheters and restraints may not only improve the chance of recovering ambulatory ability but would also improve the quality of care provided to older patients.
- ,,, et al.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79‐84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847‐850.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451‐458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645‐652.
- ,,, et al.The natural history of functional morbidity in hospitalized older patients.J Am Geriatr Soc.1990;38:1296‐1303.
- ,,, et al.Hospital diagnosis, Medicare charges and nursing home admissions in the year when older persons become severely disabled.JAMA.1997;277:728‐734.
- ,,.A predictive index for functional decline in hospitalized elderly medical patients.J Gen Intern Med.1993;8:645‐652.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients: the importance of functional status.J Am Geriatr Soc.1988;36:775‐783.
- ,.Admission and discharge mobility of frail hospitalized older adults.Medsurg Nurs.2004;13:156‐163.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization.J Am Geriatr Soc.1996;44:251‐257.
- ,,.New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol Med Sci.1998;53A:M307–M312.
- ,,,.Geriatric hospital medicine.Med Clin North Am.2002;86:707‐729.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263‐1270.
- ,,, et al.Pressure ulcer risk factors among hospitalized patients with activity limitations.JAMA.1995;273:865‐870.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1986;40:373‐383.
- ,,,.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818‐829.
- ,,, et al.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial functioning.JAMA.1963;185:914‐919.
- ,.Impact of marital status on outcomes in hospitalized patients.Arch Intern Med.1995;155:2465‐2471.
- ,,.Current concepts in geriatrics: instruments for the functional assessment of older patients.N Engl J Med.1990;322:1207‐1214.
- ,,,,,.Comparison of subjective ratings of function with observed functional ability of frail older persons.Am J Public Health.1991;81:1127‐1130.
- ,,,,,.Diagnosis‐related group‐adjusted hospital costs are higher in older medical patients with lower functional status.J Am Geriatr Soc.2003;51:1729‐1734.
- UAB Hospital data,2005.
- .Physical restraints in the practice of medicine. Current concepts.Arch Intern Med.1992;152:2203‐2206.
- et al.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28:19‐24.
- Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664‐672.
- ,,, et al.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79‐84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847‐850.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451‐458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645‐652.
- ,,, et al.The natural history of functional morbidity in hospitalized older patients.J Am Geriatr Soc.1990;38:1296‐1303.
- ,,, et al.Hospital diagnosis, Medicare charges and nursing home admissions in the year when older persons become severely disabled.JAMA.1997;277:728‐734.
- ,,.A predictive index for functional decline in hospitalized elderly medical patients.J Gen Intern Med.1993;8:645‐652.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients: the importance of functional status.J Am Geriatr Soc.1988;36:775‐783.
- ,.Admission and discharge mobility of frail hospitalized older adults.Medsurg Nurs.2004;13:156‐163.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization.J Am Geriatr Soc.1996;44:251‐257.
- ,,.New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol Med Sci.1998;53A:M307–M312.
- ,,,.Geriatric hospital medicine.Med Clin North Am.2002;86:707‐729.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263‐1270.
- ,,, et al.Pressure ulcer risk factors among hospitalized patients with activity limitations.JAMA.1995;273:865‐870.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1986;40:373‐383.
- ,,,.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818‐829.
- ,,, et al.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial functioning.JAMA.1963;185:914‐919.
- ,.Impact of marital status on outcomes in hospitalized patients.Arch Intern Med.1995;155:2465‐2471.
- ,,.Current concepts in geriatrics: instruments for the functional assessment of older patients.N Engl J Med.1990;322:1207‐1214.
- ,,,,,.Comparison of subjective ratings of function with observed functional ability of frail older persons.Am J Public Health.1991;81:1127‐1130.
- ,,,,,.Diagnosis‐related group‐adjusted hospital costs are higher in older medical patients with lower functional status.J Am Geriatr Soc.2003;51:1729‐1734.
- UAB Hospital data,2005.
- .Physical restraints in the practice of medicine. Current concepts.Arch Intern Med.1992;152:2203‐2206.
- et al.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28:19‐24.
- Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664‐672.
Copyright © 2006 Society of Hospital Medicine
NIPPV effective for pulmonary edema
-
CLINICAL QUESTION: Is noninvasive positive pressure ventilation effective in managing patients with acute cardiogenic pulmonary edema?
-
BOTTOM LINE: Patients with acute cardiogenic pulmonary edema treated with noninvasive positive pressure ventilation (NIPPV) are less likely than those receiving standard care to die in the hospital or to require mechanical ventilation. (LOE = 1a)
-
REFERENCE: Peter JV, Moran JL, Phillips‐Hughes J, Graham P, Bersten AD. Effect of non‐invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta‐analysis. Lancet 2006;367:1155‐1163.
-
STUDY DESIGN: Meta‐analysis (randomized controlled trials)
-
SETTING: Inpatient (any location)
-
SYNOPSIS: This team systematically reviewed multiple databases, using a sensible search strategy,q to find 23 small randomized controlled trials of NIPPV. NIPPV included continuous positive airway pressure or bilevel ventilation. The data were extracted independently by 2 investigators, with discrepancies resolved by consensus. The authors don't report if the decision to include or exclude studies was similarly done independently. Additionally, they don't say if they looked for unpublished studies. The eligible studies included more than 1300 patients with cardiogenic pulmonary edema. NIPPV was more effective than standard care in preventing in‐hospital mortality (11.7% vs 21.3%; number needed to treat [NNT] =11; 95% CI, 7 ‐ 21), with no difference between continuous positive airway pressure and bilevel ventilation. Similarly, patients receiving NIPPV required mechanical ventilation less frequently (11.9% vs 28.1%; NNT = 7; 5 ‐ 9), with no difference in outcomes between continuous positive airway pressure and bilevel ventilation. The data were fairly consistent across the studies. Since there is a possibility of publication bias in favor of positive results, the results of a mega‐trial (if one occurs) may not look this good.
-
CLINICAL QUESTION: Is noninvasive positive pressure ventilation effective in managing patients with acute cardiogenic pulmonary edema?
-
BOTTOM LINE: Patients with acute cardiogenic pulmonary edema treated with noninvasive positive pressure ventilation (NIPPV) are less likely than those receiving standard care to die in the hospital or to require mechanical ventilation. (LOE = 1a)
-
REFERENCE: Peter JV, Moran JL, Phillips‐Hughes J, Graham P, Bersten AD. Effect of non‐invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta‐analysis. Lancet 2006;367:1155‐1163.
-
STUDY DESIGN: Meta‐analysis (randomized controlled trials)
-
SETTING: Inpatient (any location)
-
SYNOPSIS: This team systematically reviewed multiple databases, using a sensible search strategy,q to find 23 small randomized controlled trials of NIPPV. NIPPV included continuous positive airway pressure or bilevel ventilation. The data were extracted independently by 2 investigators, with discrepancies resolved by consensus. The authors don't report if the decision to include or exclude studies was similarly done independently. Additionally, they don't say if they looked for unpublished studies. The eligible studies included more than 1300 patients with cardiogenic pulmonary edema. NIPPV was more effective than standard care in preventing in‐hospital mortality (11.7% vs 21.3%; number needed to treat [NNT] =11; 95% CI, 7 ‐ 21), with no difference between continuous positive airway pressure and bilevel ventilation. Similarly, patients receiving NIPPV required mechanical ventilation less frequently (11.9% vs 28.1%; NNT = 7; 5 ‐ 9), with no difference in outcomes between continuous positive airway pressure and bilevel ventilation. The data were fairly consistent across the studies. Since there is a possibility of publication bias in favor of positive results, the results of a mega‐trial (if one occurs) may not look this good.
-
CLINICAL QUESTION: Is noninvasive positive pressure ventilation effective in managing patients with acute cardiogenic pulmonary edema?
-
BOTTOM LINE: Patients with acute cardiogenic pulmonary edema treated with noninvasive positive pressure ventilation (NIPPV) are less likely than those receiving standard care to die in the hospital or to require mechanical ventilation. (LOE = 1a)
-
REFERENCE: Peter JV, Moran JL, Phillips‐Hughes J, Graham P, Bersten AD. Effect of non‐invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta‐analysis. Lancet 2006;367:1155‐1163.
-
STUDY DESIGN: Meta‐analysis (randomized controlled trials)
-
SETTING: Inpatient (any location)
-
SYNOPSIS: This team systematically reviewed multiple databases, using a sensible search strategy,q to find 23 small randomized controlled trials of NIPPV. NIPPV included continuous positive airway pressure or bilevel ventilation. The data were extracted independently by 2 investigators, with discrepancies resolved by consensus. The authors don't report if the decision to include or exclude studies was similarly done independently. Additionally, they don't say if they looked for unpublished studies. The eligible studies included more than 1300 patients with cardiogenic pulmonary edema. NIPPV was more effective than standard care in preventing in‐hospital mortality (11.7% vs 21.3%; number needed to treat [NNT] =11; 95% CI, 7 ‐ 21), with no difference between continuous positive airway pressure and bilevel ventilation. Similarly, patients receiving NIPPV required mechanical ventilation less frequently (11.9% vs 28.1%; NNT = 7; 5 ‐ 9), with no difference in outcomes between continuous positive airway pressure and bilevel ventilation. The data were fairly consistent across the studies. Since there is a possibility of publication bias in favor of positive results, the results of a mega‐trial (if one occurs) may not look this good.