User login
What is the role of the ObGyn in preventing and treating obesity?
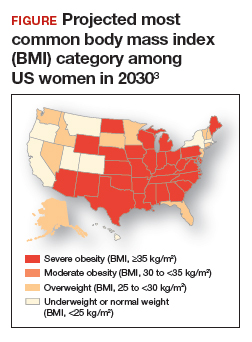
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3
As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Novel coronavirus may cause environmental contamination through fecal shedding
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
FROM JAMA
Implantable stimulator shows promise for chronic knee pain
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Best definition of malnutrition varies by cancer type
For patients undergoing major oncologic surgery, the best definition of malnutrition used to assess postoperative risk varies by cancer type, results of a retrospective study suggest.
The current, one-size-fits-all approach to nutritional status leads to both undertreatment and overtreatment of malnutrition, as well as inaccurate estimations of postoperative risk, reported lead study author Nicholas P. McKenna, MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Assessing nutritional status is important because it impacts preoperative planning, particularly with respect to the use of prehabilitation,” the investigators wrote. Their report is in the Journal of the American College of Surgeons. They noted that while prehabilitation has been shown to reduce postoperative risk among those who need it, identification of these patients is an area that needs improvement.
With this in mind, Dr. McKenna and colleagues analyzed 205,840 major oncologic operations, with data drawn from the American College of Surgeons National Surgical Quality Improvement (NSQIP) database.
The researchers evaluated patients’ nutritional status using three techniques: the NSQIP method, the European Society for Clinical Nutrition and Metabolism (ESPEN) definitions, and the World Health Organization body mass index (BMI) classification system.
Combining these three assessments led to seven hierarchical nutritional status categories:
- Severe malnutrition – BMI less than 18.5 kg/m2 and greater than 10% weight loss
- ESPEN 1 – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- ESPEN 2 – BMI less than 18.5 kg/m2
- NSQIP – BMI greater than 20 kg/m2 (if younger than 70 years) or 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- Mild malnutrition – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older)
- Obese – BMI at least 30 kg/m2
- No malnutrition.
The study’s primary outcomes were 30-day mortality and 30-day morbidity. The latter included a variety of complications, such as deep incisional surgical site infection, septic shock, and acute renal failure. Demographic and clinical factors were included in multivariate analyses.
Results
Most of the operations involved patients with colorectal cancer (74%), followed by pancreatic (10%), lung (9%), gastric (3%), esophageal (3%), and liver (2%) cancer.
Across all patients, 16% fell into one of five malnutrition categories: mild malnutrition (6%), NSQIP (6%), ESPEN 2 (2%), ESPEN 1 (1%), or severe malnutrition (0.6%). The remainder of patients were either obese (31%) or had normal nutritional status (54%).
Malnutrition was most common among patients with pancreatic cancer (28%) and least common among those with colorectal cancer (14%).
Aligning with previous research, this study showed that nutritional status was associated with postoperative risk. Mortality risk was highest among patients with severe malnutrition, and morbidity was most common in the severe and ESPEN 1 groups (P less than .0001 for both).
While the spectrum of classifications appeared accurate across the population, multivariable models for mortality and morbidity revealed an interaction between cancer type and malnutrition definition (P less than .0001 for both), which suggested the most accurate definition of malnutrition differed from one type of cancer to another.
Specifically, a classification of severe malnutrition was most predictive of mortality among patients with esophageal or colorectal cancer. ESPEN 1 was most predictive of mortality for patients with gastric or lung cancer, and NSQIP was most predictive for those with liver cancer.
For predicting morbidity, severe malnutrition was most accurate among patients with colorectal cancer, whereas ESPEN 1 was better suited for gastric and lung cancer.
Interpreting and applying the results
“The biggest takeaway is that the optimal definition of malnutrition varies by cancer type,” Dr. McKenna said in an interview.
He went on to explain that weight loss is a particularly important indicator of malnutrition for patients with esophageal or gastric cancer. “These are the cancers that more commonly undergo neoadjuvant chemotherapy,” he noted.
The other major finding, Dr. McKenna said, offers some perspective on short-term versus long-term risk.
“Most people consider obesity a negative prognostic factor,” he said. “But in terms of operative risk, it’s kind of a neutral effect. It doesn’t really affect the short-term outcomes of an operation.”
Still, Dr. McKenna warned that a visual assessment of patient body condition is not enough to predict postoperative risk. Instead, he recommended accurate height and weight measurements during annual and preoperative exams. He also noted that more patients are at risk than clinicians may suspect.
“Even definitions that didn’t previously exist, such as mild malnutrition, had a somewhat negative effect within colorectal cancer and esophageal cancer,” Dr. McKenna said. “So these are patients who previously probably would be considered pretty healthy, but there is probably some room to improve their nutritional status.”
While the study revealed that different types of cancer should have unique tools for measuring nutritional status, development of these systems will require more research concerning prehabilitation outcomes, according to Dr. McKenna. In the meantime, he highlighted a point of action in the clinic.
“We think, overall, especially with the rise of neoadjuvant chemotherapy upfront, before surgery, that identifying patients at risk before they start neoadjuvant chemotherapy is going to be important,” he said. “They are the ones who really need to be targeted.”
There was no external funding for this study, and the investigators reported no conflicts of interest.
SOURCE: McKenna NP et al. J Am Coll Surg. 2020 Feb 26. doi: 10.1016/j.jamcollsurg.2019.12.034.
For patients undergoing major oncologic surgery, the best definition of malnutrition used to assess postoperative risk varies by cancer type, results of a retrospective study suggest.
The current, one-size-fits-all approach to nutritional status leads to both undertreatment and overtreatment of malnutrition, as well as inaccurate estimations of postoperative risk, reported lead study author Nicholas P. McKenna, MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Assessing nutritional status is important because it impacts preoperative planning, particularly with respect to the use of prehabilitation,” the investigators wrote. Their report is in the Journal of the American College of Surgeons. They noted that while prehabilitation has been shown to reduce postoperative risk among those who need it, identification of these patients is an area that needs improvement.
With this in mind, Dr. McKenna and colleagues analyzed 205,840 major oncologic operations, with data drawn from the American College of Surgeons National Surgical Quality Improvement (NSQIP) database.
The researchers evaluated patients’ nutritional status using three techniques: the NSQIP method, the European Society for Clinical Nutrition and Metabolism (ESPEN) definitions, and the World Health Organization body mass index (BMI) classification system.
Combining these three assessments led to seven hierarchical nutritional status categories:
- Severe malnutrition – BMI less than 18.5 kg/m2 and greater than 10% weight loss
- ESPEN 1 – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- ESPEN 2 – BMI less than 18.5 kg/m2
- NSQIP – BMI greater than 20 kg/m2 (if younger than 70 years) or 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- Mild malnutrition – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older)
- Obese – BMI at least 30 kg/m2
- No malnutrition.
The study’s primary outcomes were 30-day mortality and 30-day morbidity. The latter included a variety of complications, such as deep incisional surgical site infection, septic shock, and acute renal failure. Demographic and clinical factors were included in multivariate analyses.
Results
Most of the operations involved patients with colorectal cancer (74%), followed by pancreatic (10%), lung (9%), gastric (3%), esophageal (3%), and liver (2%) cancer.
Across all patients, 16% fell into one of five malnutrition categories: mild malnutrition (6%), NSQIP (6%), ESPEN 2 (2%), ESPEN 1 (1%), or severe malnutrition (0.6%). The remainder of patients were either obese (31%) or had normal nutritional status (54%).
Malnutrition was most common among patients with pancreatic cancer (28%) and least common among those with colorectal cancer (14%).
Aligning with previous research, this study showed that nutritional status was associated with postoperative risk. Mortality risk was highest among patients with severe malnutrition, and morbidity was most common in the severe and ESPEN 1 groups (P less than .0001 for both).
While the spectrum of classifications appeared accurate across the population, multivariable models for mortality and morbidity revealed an interaction between cancer type and malnutrition definition (P less than .0001 for both), which suggested the most accurate definition of malnutrition differed from one type of cancer to another.
Specifically, a classification of severe malnutrition was most predictive of mortality among patients with esophageal or colorectal cancer. ESPEN 1 was most predictive of mortality for patients with gastric or lung cancer, and NSQIP was most predictive for those with liver cancer.
For predicting morbidity, severe malnutrition was most accurate among patients with colorectal cancer, whereas ESPEN 1 was better suited for gastric and lung cancer.
Interpreting and applying the results
“The biggest takeaway is that the optimal definition of malnutrition varies by cancer type,” Dr. McKenna said in an interview.
He went on to explain that weight loss is a particularly important indicator of malnutrition for patients with esophageal or gastric cancer. “These are the cancers that more commonly undergo neoadjuvant chemotherapy,” he noted.
The other major finding, Dr. McKenna said, offers some perspective on short-term versus long-term risk.
“Most people consider obesity a negative prognostic factor,” he said. “But in terms of operative risk, it’s kind of a neutral effect. It doesn’t really affect the short-term outcomes of an operation.”
Still, Dr. McKenna warned that a visual assessment of patient body condition is not enough to predict postoperative risk. Instead, he recommended accurate height and weight measurements during annual and preoperative exams. He also noted that more patients are at risk than clinicians may suspect.
“Even definitions that didn’t previously exist, such as mild malnutrition, had a somewhat negative effect within colorectal cancer and esophageal cancer,” Dr. McKenna said. “So these are patients who previously probably would be considered pretty healthy, but there is probably some room to improve their nutritional status.”
While the study revealed that different types of cancer should have unique tools for measuring nutritional status, development of these systems will require more research concerning prehabilitation outcomes, according to Dr. McKenna. In the meantime, he highlighted a point of action in the clinic.
“We think, overall, especially with the rise of neoadjuvant chemotherapy upfront, before surgery, that identifying patients at risk before they start neoadjuvant chemotherapy is going to be important,” he said. “They are the ones who really need to be targeted.”
There was no external funding for this study, and the investigators reported no conflicts of interest.
SOURCE: McKenna NP et al. J Am Coll Surg. 2020 Feb 26. doi: 10.1016/j.jamcollsurg.2019.12.034.
For patients undergoing major oncologic surgery, the best definition of malnutrition used to assess postoperative risk varies by cancer type, results of a retrospective study suggest.
The current, one-size-fits-all approach to nutritional status leads to both undertreatment and overtreatment of malnutrition, as well as inaccurate estimations of postoperative risk, reported lead study author Nicholas P. McKenna, MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Assessing nutritional status is important because it impacts preoperative planning, particularly with respect to the use of prehabilitation,” the investigators wrote. Their report is in the Journal of the American College of Surgeons. They noted that while prehabilitation has been shown to reduce postoperative risk among those who need it, identification of these patients is an area that needs improvement.
With this in mind, Dr. McKenna and colleagues analyzed 205,840 major oncologic operations, with data drawn from the American College of Surgeons National Surgical Quality Improvement (NSQIP) database.
The researchers evaluated patients’ nutritional status using three techniques: the NSQIP method, the European Society for Clinical Nutrition and Metabolism (ESPEN) definitions, and the World Health Organization body mass index (BMI) classification system.
Combining these three assessments led to seven hierarchical nutritional status categories:
- Severe malnutrition – BMI less than 18.5 kg/m2 and greater than 10% weight loss
- ESPEN 1 – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- ESPEN 2 – BMI less than 18.5 kg/m2
- NSQIP – BMI greater than 20 kg/m2 (if younger than 70 years) or 22 kg/m2 (if 70 years or older) plus greater than 10% weight loss
- Mild malnutrition – BMI 18.5-20 kg/m2 (if younger than 70 years) or less than 22 kg/m2 (if 70 years or older)
- Obese – BMI at least 30 kg/m2
- No malnutrition.
The study’s primary outcomes were 30-day mortality and 30-day morbidity. The latter included a variety of complications, such as deep incisional surgical site infection, septic shock, and acute renal failure. Demographic and clinical factors were included in multivariate analyses.
Results
Most of the operations involved patients with colorectal cancer (74%), followed by pancreatic (10%), lung (9%), gastric (3%), esophageal (3%), and liver (2%) cancer.
Across all patients, 16% fell into one of five malnutrition categories: mild malnutrition (6%), NSQIP (6%), ESPEN 2 (2%), ESPEN 1 (1%), or severe malnutrition (0.6%). The remainder of patients were either obese (31%) or had normal nutritional status (54%).
Malnutrition was most common among patients with pancreatic cancer (28%) and least common among those with colorectal cancer (14%).
Aligning with previous research, this study showed that nutritional status was associated with postoperative risk. Mortality risk was highest among patients with severe malnutrition, and morbidity was most common in the severe and ESPEN 1 groups (P less than .0001 for both).
While the spectrum of classifications appeared accurate across the population, multivariable models for mortality and morbidity revealed an interaction between cancer type and malnutrition definition (P less than .0001 for both), which suggested the most accurate definition of malnutrition differed from one type of cancer to another.
Specifically, a classification of severe malnutrition was most predictive of mortality among patients with esophageal or colorectal cancer. ESPEN 1 was most predictive of mortality for patients with gastric or lung cancer, and NSQIP was most predictive for those with liver cancer.
For predicting morbidity, severe malnutrition was most accurate among patients with colorectal cancer, whereas ESPEN 1 was better suited for gastric and lung cancer.
Interpreting and applying the results
“The biggest takeaway is that the optimal definition of malnutrition varies by cancer type,” Dr. McKenna said in an interview.
He went on to explain that weight loss is a particularly important indicator of malnutrition for patients with esophageal or gastric cancer. “These are the cancers that more commonly undergo neoadjuvant chemotherapy,” he noted.
The other major finding, Dr. McKenna said, offers some perspective on short-term versus long-term risk.
“Most people consider obesity a negative prognostic factor,” he said. “But in terms of operative risk, it’s kind of a neutral effect. It doesn’t really affect the short-term outcomes of an operation.”
Still, Dr. McKenna warned that a visual assessment of patient body condition is not enough to predict postoperative risk. Instead, he recommended accurate height and weight measurements during annual and preoperative exams. He also noted that more patients are at risk than clinicians may suspect.
“Even definitions that didn’t previously exist, such as mild malnutrition, had a somewhat negative effect within colorectal cancer and esophageal cancer,” Dr. McKenna said. “So these are patients who previously probably would be considered pretty healthy, but there is probably some room to improve their nutritional status.”
While the study revealed that different types of cancer should have unique tools for measuring nutritional status, development of these systems will require more research concerning prehabilitation outcomes, according to Dr. McKenna. In the meantime, he highlighted a point of action in the clinic.
“We think, overall, especially with the rise of neoadjuvant chemotherapy upfront, before surgery, that identifying patients at risk before they start neoadjuvant chemotherapy is going to be important,” he said. “They are the ones who really need to be targeted.”
There was no external funding for this study, and the investigators reported no conflicts of interest.
SOURCE: McKenna NP et al. J Am Coll Surg. 2020 Feb 26. doi: 10.1016/j.jamcollsurg.2019.12.034.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Telehealth seen as a key tool to help fight COVID-19
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
The possibilities of pembrolizumab plus chemo in breast cancer treatment
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
COVID-19 and public health preparedness in the United States
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Effective osteoarthritis therapy remains elusive
MAUI, HAWAII – Osteoarthritis therapy remains a barren landscape where conventional medicine has so little to offer that many affected patients eagerly embrace unproven Internet claims for costly out-of-pocket intra-articular injections of platelet-rich plasma, oxygen ozone, and mesenchymal stem cells, Eric M. Ruderman, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
He presented a whirlwind review of the evidence – or lack thereof – for a wide range of contemporary OA therapies, ranging from acupuncture to cupping, lateral wedge insoles, various substances for intra-articular injection, radiofrequency therapy, medical leeches, and several widely ballyhooed medications that are well along in the developmental pipeline but whose placebo-subtracted efficacy is actually quite modest.
“I’ve shown you the evidence, such as it is – it’s not very much. The question is, have we really moved the needle in this disease in the last 30 years? The answer is I’m not so sure we have,” concluded Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Several audience members were less restrained in their assessments.
“You have not moved the needle in osteoarthritis,” stated orthopedic surgeon William Bugbee, MD, chief of joint reconstruction at the Scripps Clinic in La Jolla, Calif., adding that he’s not much impressed by the long-term impact of intra-articular injections, be they of glucocorticoid, hyaluronic acid, or anything else being put into osteoarthritic joints.
“To me, and to most orthopedic surgeons, an injection is a handshake. It’s like: ‘How do you do? Let’s get to know each other.’ But you know where this interaction is going – it’s going to end up in surgery. But that’s okay. Surgery is great. Let’s face it: It’s the only disease-modifying treatment for OA,” Dr. Bugbee declared.
Roy Fleischmann, MD, rose from the audience to assert that “the biggest need in rheumatology right now is a medication for OA that actually works and is actually disease modifying.”
That’s not going to happen until clinical trialists and the pharmaceutical industry learn how to subgroup OA and separate inflammatory OA from noninflammatory OA. Lumping the two together tends to wash out any strong efficacy signal an investigational agent might have, added Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
Dr. Ruderman noted that his own analysis of the contemporary evidence base for OA pharmacotherapies reached conclusions generally similar to those contained in the new American College of Rheumatology/Arthritis Foundation “Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee”. The list of pharmacotherapies that were strongly recommended in the guidelines was slim and rather tired: oral NSAIDs, intra-articular glucocorticoid injections, and – for knee OA only – topical NSAIDs. The strength of those favorable recommendations was based more upon solid evidence of safety rather than impressive efficacy, in his view. And the guidelines’ list of therapies whose use was strongly discouraged was much longer.
A factor in the flimsy evidence base for OA treatment is the strikingly large across-the-board placebo effect. This was nicely captured in a meta-analysis of 215 randomized, controlled trials of various therapies totaling more than 41,000 participants. Drilling down into the data, the British and Chinese investigators concluded that on average 75% of the overall treatment effect seen in the trials was caused by the placebo effect and only 25% represented a treatment-specific effect. The “winner” was intra-articular glucocorticoid, where the placebo effect was a mere 47%.
In the OA pipeline
“There’s a lot of interest in DMOADs – disease-modifying osteoarthritis drugs – but not a lot of success so far,” Dr. Ruderman observed.
Case in point: intra-articular sprifermin, a recombinant human fibroblast growth factor 18. In a 549-patient, multicenter, dose-ranging study, the two highest doses achieved a modest yet statistically significant advantage over placebo in total femorotibial joint cartilage thickness on MRI at 2 years. However, the investigators added that the result was of “uncertain clinical importance,” given the lack of a difference from baseline in total Western Ontario and McMaster Universities OA Index score.
“I’m not sure this is going anywhere,” Dr. Ruderman commented.
Tanezumab, a novel subcutaneously injected monoclonal antibody directed against nerve growth factor, has drawn a lot of attention. In an initial multicenter, phase 3, randomized trial it showed what Dr. Ruderman termed “some modest benefit” on pain and function.
“Very much like everything else in OA, you see a huge placebo effect buried in there,” according to the rheumatologist.
This modest clinical benefit was accompanied by a safety signal at the higher 5-mg dose of tanezumab. Moreover, a second phase 3 trial, this one conducted in nearly 3,000 OA patients and presented at the 2019 annual meeting of the American College of Rheumatology, also raised significant safety concerns at the higher dose. And while the 2.5-mg dose was safer, it was disappointingly no more effective in terms of improvement in pain and function scores than diclofenac at 75 mg twice daily, Dr. Ruderman noted.
Dr. Fleischmann predicted that, given the enormous unmet need for new OA treatments, tanezumab at 2.5 mg will win regulatory approval, but it will be a costly niche drug reserved for the challenging subset of patients who can’t take an NSAID and are poor surgical candidates. (On March 2, 2020, Eli Lilly announced that the Food and Drug Administration had accepted its Biologics License Application for tanezumab for the treatment of chronic pain caused by moderate to severe OA.)
Intra-articular FX006, a microsphere-based, extended-release formulation of triamcinolone, outperformed conventional triamcinolone in a phase 3 clinical trial. However, the placebo-subtracted improvement in pain was modest.
“There’s some marginal benefit here, but over time I’m not sure this adds much to just straight-up triamcinolone,” Dr. Ruderman opined.
Welcome to the wild, wild West
Patients with knee OA ask Dr. Ruderman all the time about the intra-articular injections of platelet-rich plasma (PRP) or mesenchymal stem cells they’ve seen touted on the Internet. As an evidence-based physician, he’s not a fan. A meta-analysis of 14 controlled trials of PRP, none double blind, showed some benefit in terms of pain and function at 3, 6, and 12 months, with little risk of adverse events. However, PRP is being marketed with a hype and claimed efficacy out of all proportion to the actual evidence.
“It’s the wild, wild West out there,” the rheumatologist warned.
He cited a study involving a scripted survey of 179 U.S. clinics offering PRP. The mean price quoted for a unilateral knee injection in a hypothetical 52-year-old man with knee OA was $714, and it’s a cash business, since insurance companies won’t cover PRP. Out of 84 centers that were willing to share their claimed efficacy, 10 quoted 90%-100% rates of good results or symptomatic improvement, 27 claimed 80%-90% efficacy, and 29 quoted figures of 70%-80%, all of which are well above the success rates achieved in the flawed clinical trials.
As for mesenchymal stem cells, “if PRP is the wild, wild West, this is the surface of Mars,” Dr. Ruderman quipped.
These stem cell injections are neither FDA regulated nor approved. There are no barriers to setting up a mesenchymal stem cell injection center, and the number of such centers is skyrocketing. Anybody can set up a center, and there’s essentially no oversight.
“The evidence in this area is really terrible,” the rheumatologist said. He pointed to a meta-analysis of five trials, only two of which were rated by the researchers as having a low risk of bias. The conclusion: There was limited evidence of short-term benefit in pain and function, but no evidence of cartilage repair, which is the chief claimed benefit.
The same group of investigators who queried the PRP clinics also successfully contacted 273 of the proliferating U.S. centers offering direct-to-consumer mesenchymal stem cell therapy. The mean price quoted for a unilateral knee injection was a whopping $5,156, which – like PRP – isn’t covered by insurance. At the 36 clinics responding to a request for their efficacy rates, the mean claim was good results or symptomatic improvement in 82% of treated patients.
Dr. Bugbee didn’t endorse this intervention, which is increasingly popular among his fellow orthopedic surgeons.
“The regulatory pathway drives this. Mesenchymal stem cells are categorized as a minimally manipulated tissue, so the regulatory pathway is easy,” explained Dr. Bugbee. “I talk 9 out of 10 patients out of it because there’s no evidence of disease modification.”
On a brighter note
Radiofrequency ablation and neuromodulation procedures for treatment of symptomatic knee OA make no pretense of being disease modifying, but a new systematic review of 33 published studies deemed of moderate or high methodological quality totaling 1,512 patients documented improved pain, function, and disease-specific quality of life for 3-12 months with minimal complications.
Most of these procedures target the genicular nerve, the sensory nerve that innervates the knee.
“There’s some value here to this,” Dr. Ruderman said. “Our pain folks are actually pretty interested in this. At our center, they’re using this for patients with persistent knee pain after knee replacement, with some success. It’s not an unreasonable approach.”
As part of his examination of the evidence base for OA treatment, Dr. Ruderman looked into an intervention he wasn’t familiar with: acupuncture. He was pleasantly surprised.
“There’s quite a bit of data. There is decent evidence that acupuncture has significant benefit, at least for pain, and is certainly without significant side effects,” according to the rheumatologist.
He cited a review of a dozen systematic reviews of randomized, controlled trials of acupuncture for OA. The Chinese investigators rated the overall quality of the evidence as moderate to low, but with some studies being rated high quality. “That’s not as bad as some of the other stuff I looked at,” Dr. Ruderman said.
Acupuncture was found to be 2.4 times more effective than Western medicine for short-term pain relief, and 4.1 times better in terms of total efficacy, with a lower risk of adverse events than with Western medicine.
Dr. Ruderman reported serving as a consultant for and/or receiving research grants from more than a half-dozen pharmaceutical companies.
MAUI, HAWAII – Osteoarthritis therapy remains a barren landscape where conventional medicine has so little to offer that many affected patients eagerly embrace unproven Internet claims for costly out-of-pocket intra-articular injections of platelet-rich plasma, oxygen ozone, and mesenchymal stem cells, Eric M. Ruderman, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
He presented a whirlwind review of the evidence – or lack thereof – for a wide range of contemporary OA therapies, ranging from acupuncture to cupping, lateral wedge insoles, various substances for intra-articular injection, radiofrequency therapy, medical leeches, and several widely ballyhooed medications that are well along in the developmental pipeline but whose placebo-subtracted efficacy is actually quite modest.
“I’ve shown you the evidence, such as it is – it’s not very much. The question is, have we really moved the needle in this disease in the last 30 years? The answer is I’m not so sure we have,” concluded Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Several audience members were less restrained in their assessments.
“You have not moved the needle in osteoarthritis,” stated orthopedic surgeon William Bugbee, MD, chief of joint reconstruction at the Scripps Clinic in La Jolla, Calif., adding that he’s not much impressed by the long-term impact of intra-articular injections, be they of glucocorticoid, hyaluronic acid, or anything else being put into osteoarthritic joints.
“To me, and to most orthopedic surgeons, an injection is a handshake. It’s like: ‘How do you do? Let’s get to know each other.’ But you know where this interaction is going – it’s going to end up in surgery. But that’s okay. Surgery is great. Let’s face it: It’s the only disease-modifying treatment for OA,” Dr. Bugbee declared.
Roy Fleischmann, MD, rose from the audience to assert that “the biggest need in rheumatology right now is a medication for OA that actually works and is actually disease modifying.”
That’s not going to happen until clinical trialists and the pharmaceutical industry learn how to subgroup OA and separate inflammatory OA from noninflammatory OA. Lumping the two together tends to wash out any strong efficacy signal an investigational agent might have, added Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
Dr. Ruderman noted that his own analysis of the contemporary evidence base for OA pharmacotherapies reached conclusions generally similar to those contained in the new American College of Rheumatology/Arthritis Foundation “Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee”. The list of pharmacotherapies that were strongly recommended in the guidelines was slim and rather tired: oral NSAIDs, intra-articular glucocorticoid injections, and – for knee OA only – topical NSAIDs. The strength of those favorable recommendations was based more upon solid evidence of safety rather than impressive efficacy, in his view. And the guidelines’ list of therapies whose use was strongly discouraged was much longer.
A factor in the flimsy evidence base for OA treatment is the strikingly large across-the-board placebo effect. This was nicely captured in a meta-analysis of 215 randomized, controlled trials of various therapies totaling more than 41,000 participants. Drilling down into the data, the British and Chinese investigators concluded that on average 75% of the overall treatment effect seen in the trials was caused by the placebo effect and only 25% represented a treatment-specific effect. The “winner” was intra-articular glucocorticoid, where the placebo effect was a mere 47%.
In the OA pipeline
“There’s a lot of interest in DMOADs – disease-modifying osteoarthritis drugs – but not a lot of success so far,” Dr. Ruderman observed.
Case in point: intra-articular sprifermin, a recombinant human fibroblast growth factor 18. In a 549-patient, multicenter, dose-ranging study, the two highest doses achieved a modest yet statistically significant advantage over placebo in total femorotibial joint cartilage thickness on MRI at 2 years. However, the investigators added that the result was of “uncertain clinical importance,” given the lack of a difference from baseline in total Western Ontario and McMaster Universities OA Index score.
“I’m not sure this is going anywhere,” Dr. Ruderman commented.
Tanezumab, a novel subcutaneously injected monoclonal antibody directed against nerve growth factor, has drawn a lot of attention. In an initial multicenter, phase 3, randomized trial it showed what Dr. Ruderman termed “some modest benefit” on pain and function.
“Very much like everything else in OA, you see a huge placebo effect buried in there,” according to the rheumatologist.
This modest clinical benefit was accompanied by a safety signal at the higher 5-mg dose of tanezumab. Moreover, a second phase 3 trial, this one conducted in nearly 3,000 OA patients and presented at the 2019 annual meeting of the American College of Rheumatology, also raised significant safety concerns at the higher dose. And while the 2.5-mg dose was safer, it was disappointingly no more effective in terms of improvement in pain and function scores than diclofenac at 75 mg twice daily, Dr. Ruderman noted.
Dr. Fleischmann predicted that, given the enormous unmet need for new OA treatments, tanezumab at 2.5 mg will win regulatory approval, but it will be a costly niche drug reserved for the challenging subset of patients who can’t take an NSAID and are poor surgical candidates. (On March 2, 2020, Eli Lilly announced that the Food and Drug Administration had accepted its Biologics License Application for tanezumab for the treatment of chronic pain caused by moderate to severe OA.)
Intra-articular FX006, a microsphere-based, extended-release formulation of triamcinolone, outperformed conventional triamcinolone in a phase 3 clinical trial. However, the placebo-subtracted improvement in pain was modest.
“There’s some marginal benefit here, but over time I’m not sure this adds much to just straight-up triamcinolone,” Dr. Ruderman opined.
Welcome to the wild, wild West
Patients with knee OA ask Dr. Ruderman all the time about the intra-articular injections of platelet-rich plasma (PRP) or mesenchymal stem cells they’ve seen touted on the Internet. As an evidence-based physician, he’s not a fan. A meta-analysis of 14 controlled trials of PRP, none double blind, showed some benefit in terms of pain and function at 3, 6, and 12 months, with little risk of adverse events. However, PRP is being marketed with a hype and claimed efficacy out of all proportion to the actual evidence.
“It’s the wild, wild West out there,” the rheumatologist warned.
He cited a study involving a scripted survey of 179 U.S. clinics offering PRP. The mean price quoted for a unilateral knee injection in a hypothetical 52-year-old man with knee OA was $714, and it’s a cash business, since insurance companies won’t cover PRP. Out of 84 centers that were willing to share their claimed efficacy, 10 quoted 90%-100% rates of good results or symptomatic improvement, 27 claimed 80%-90% efficacy, and 29 quoted figures of 70%-80%, all of which are well above the success rates achieved in the flawed clinical trials.
As for mesenchymal stem cells, “if PRP is the wild, wild West, this is the surface of Mars,” Dr. Ruderman quipped.
These stem cell injections are neither FDA regulated nor approved. There are no barriers to setting up a mesenchymal stem cell injection center, and the number of such centers is skyrocketing. Anybody can set up a center, and there’s essentially no oversight.
“The evidence in this area is really terrible,” the rheumatologist said. He pointed to a meta-analysis of five trials, only two of which were rated by the researchers as having a low risk of bias. The conclusion: There was limited evidence of short-term benefit in pain and function, but no evidence of cartilage repair, which is the chief claimed benefit.
The same group of investigators who queried the PRP clinics also successfully contacted 273 of the proliferating U.S. centers offering direct-to-consumer mesenchymal stem cell therapy. The mean price quoted for a unilateral knee injection was a whopping $5,156, which – like PRP – isn’t covered by insurance. At the 36 clinics responding to a request for their efficacy rates, the mean claim was good results or symptomatic improvement in 82% of treated patients.
Dr. Bugbee didn’t endorse this intervention, which is increasingly popular among his fellow orthopedic surgeons.
“The regulatory pathway drives this. Mesenchymal stem cells are categorized as a minimally manipulated tissue, so the regulatory pathway is easy,” explained Dr. Bugbee. “I talk 9 out of 10 patients out of it because there’s no evidence of disease modification.”
On a brighter note
Radiofrequency ablation and neuromodulation procedures for treatment of symptomatic knee OA make no pretense of being disease modifying, but a new systematic review of 33 published studies deemed of moderate or high methodological quality totaling 1,512 patients documented improved pain, function, and disease-specific quality of life for 3-12 months with minimal complications.
Most of these procedures target the genicular nerve, the sensory nerve that innervates the knee.
“There’s some value here to this,” Dr. Ruderman said. “Our pain folks are actually pretty interested in this. At our center, they’re using this for patients with persistent knee pain after knee replacement, with some success. It’s not an unreasonable approach.”
As part of his examination of the evidence base for OA treatment, Dr. Ruderman looked into an intervention he wasn’t familiar with: acupuncture. He was pleasantly surprised.
“There’s quite a bit of data. There is decent evidence that acupuncture has significant benefit, at least for pain, and is certainly without significant side effects,” according to the rheumatologist.
He cited a review of a dozen systematic reviews of randomized, controlled trials of acupuncture for OA. The Chinese investigators rated the overall quality of the evidence as moderate to low, but with some studies being rated high quality. “That’s not as bad as some of the other stuff I looked at,” Dr. Ruderman said.
Acupuncture was found to be 2.4 times more effective than Western medicine for short-term pain relief, and 4.1 times better in terms of total efficacy, with a lower risk of adverse events than with Western medicine.
Dr. Ruderman reported serving as a consultant for and/or receiving research grants from more than a half-dozen pharmaceutical companies.
MAUI, HAWAII – Osteoarthritis therapy remains a barren landscape where conventional medicine has so little to offer that many affected patients eagerly embrace unproven Internet claims for costly out-of-pocket intra-articular injections of platelet-rich plasma, oxygen ozone, and mesenchymal stem cells, Eric M. Ruderman, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
He presented a whirlwind review of the evidence – or lack thereof – for a wide range of contemporary OA therapies, ranging from acupuncture to cupping, lateral wedge insoles, various substances for intra-articular injection, radiofrequency therapy, medical leeches, and several widely ballyhooed medications that are well along in the developmental pipeline but whose placebo-subtracted efficacy is actually quite modest.
“I’ve shown you the evidence, such as it is – it’s not very much. The question is, have we really moved the needle in this disease in the last 30 years? The answer is I’m not so sure we have,” concluded Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Several audience members were less restrained in their assessments.
“You have not moved the needle in osteoarthritis,” stated orthopedic surgeon William Bugbee, MD, chief of joint reconstruction at the Scripps Clinic in La Jolla, Calif., adding that he’s not much impressed by the long-term impact of intra-articular injections, be they of glucocorticoid, hyaluronic acid, or anything else being put into osteoarthritic joints.
“To me, and to most orthopedic surgeons, an injection is a handshake. It’s like: ‘How do you do? Let’s get to know each other.’ But you know where this interaction is going – it’s going to end up in surgery. But that’s okay. Surgery is great. Let’s face it: It’s the only disease-modifying treatment for OA,” Dr. Bugbee declared.
Roy Fleischmann, MD, rose from the audience to assert that “the biggest need in rheumatology right now is a medication for OA that actually works and is actually disease modifying.”
That’s not going to happen until clinical trialists and the pharmaceutical industry learn how to subgroup OA and separate inflammatory OA from noninflammatory OA. Lumping the two together tends to wash out any strong efficacy signal an investigational agent might have, added Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
Dr. Ruderman noted that his own analysis of the contemporary evidence base for OA pharmacotherapies reached conclusions generally similar to those contained in the new American College of Rheumatology/Arthritis Foundation “Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee”. The list of pharmacotherapies that were strongly recommended in the guidelines was slim and rather tired: oral NSAIDs, intra-articular glucocorticoid injections, and – for knee OA only – topical NSAIDs. The strength of those favorable recommendations was based more upon solid evidence of safety rather than impressive efficacy, in his view. And the guidelines’ list of therapies whose use was strongly discouraged was much longer.
A factor in the flimsy evidence base for OA treatment is the strikingly large across-the-board placebo effect. This was nicely captured in a meta-analysis of 215 randomized, controlled trials of various therapies totaling more than 41,000 participants. Drilling down into the data, the British and Chinese investigators concluded that on average 75% of the overall treatment effect seen in the trials was caused by the placebo effect and only 25% represented a treatment-specific effect. The “winner” was intra-articular glucocorticoid, where the placebo effect was a mere 47%.
In the OA pipeline
“There’s a lot of interest in DMOADs – disease-modifying osteoarthritis drugs – but not a lot of success so far,” Dr. Ruderman observed.
Case in point: intra-articular sprifermin, a recombinant human fibroblast growth factor 18. In a 549-patient, multicenter, dose-ranging study, the two highest doses achieved a modest yet statistically significant advantage over placebo in total femorotibial joint cartilage thickness on MRI at 2 years. However, the investigators added that the result was of “uncertain clinical importance,” given the lack of a difference from baseline in total Western Ontario and McMaster Universities OA Index score.
“I’m not sure this is going anywhere,” Dr. Ruderman commented.
Tanezumab, a novel subcutaneously injected monoclonal antibody directed against nerve growth factor, has drawn a lot of attention. In an initial multicenter, phase 3, randomized trial it showed what Dr. Ruderman termed “some modest benefit” on pain and function.
“Very much like everything else in OA, you see a huge placebo effect buried in there,” according to the rheumatologist.
This modest clinical benefit was accompanied by a safety signal at the higher 5-mg dose of tanezumab. Moreover, a second phase 3 trial, this one conducted in nearly 3,000 OA patients and presented at the 2019 annual meeting of the American College of Rheumatology, also raised significant safety concerns at the higher dose. And while the 2.5-mg dose was safer, it was disappointingly no more effective in terms of improvement in pain and function scores than diclofenac at 75 mg twice daily, Dr. Ruderman noted.
Dr. Fleischmann predicted that, given the enormous unmet need for new OA treatments, tanezumab at 2.5 mg will win regulatory approval, but it will be a costly niche drug reserved for the challenging subset of patients who can’t take an NSAID and are poor surgical candidates. (On March 2, 2020, Eli Lilly announced that the Food and Drug Administration had accepted its Biologics License Application for tanezumab for the treatment of chronic pain caused by moderate to severe OA.)
Intra-articular FX006, a microsphere-based, extended-release formulation of triamcinolone, outperformed conventional triamcinolone in a phase 3 clinical trial. However, the placebo-subtracted improvement in pain was modest.
“There’s some marginal benefit here, but over time I’m not sure this adds much to just straight-up triamcinolone,” Dr. Ruderman opined.
Welcome to the wild, wild West
Patients with knee OA ask Dr. Ruderman all the time about the intra-articular injections of platelet-rich plasma (PRP) or mesenchymal stem cells they’ve seen touted on the Internet. As an evidence-based physician, he’s not a fan. A meta-analysis of 14 controlled trials of PRP, none double blind, showed some benefit in terms of pain and function at 3, 6, and 12 months, with little risk of adverse events. However, PRP is being marketed with a hype and claimed efficacy out of all proportion to the actual evidence.
“It’s the wild, wild West out there,” the rheumatologist warned.
He cited a study involving a scripted survey of 179 U.S. clinics offering PRP. The mean price quoted for a unilateral knee injection in a hypothetical 52-year-old man with knee OA was $714, and it’s a cash business, since insurance companies won’t cover PRP. Out of 84 centers that were willing to share their claimed efficacy, 10 quoted 90%-100% rates of good results or symptomatic improvement, 27 claimed 80%-90% efficacy, and 29 quoted figures of 70%-80%, all of which are well above the success rates achieved in the flawed clinical trials.
As for mesenchymal stem cells, “if PRP is the wild, wild West, this is the surface of Mars,” Dr. Ruderman quipped.
These stem cell injections are neither FDA regulated nor approved. There are no barriers to setting up a mesenchymal stem cell injection center, and the number of such centers is skyrocketing. Anybody can set up a center, and there’s essentially no oversight.
“The evidence in this area is really terrible,” the rheumatologist said. He pointed to a meta-analysis of five trials, only two of which were rated by the researchers as having a low risk of bias. The conclusion: There was limited evidence of short-term benefit in pain and function, but no evidence of cartilage repair, which is the chief claimed benefit.
The same group of investigators who queried the PRP clinics also successfully contacted 273 of the proliferating U.S. centers offering direct-to-consumer mesenchymal stem cell therapy. The mean price quoted for a unilateral knee injection was a whopping $5,156, which – like PRP – isn’t covered by insurance. At the 36 clinics responding to a request for their efficacy rates, the mean claim was good results or symptomatic improvement in 82% of treated patients.
Dr. Bugbee didn’t endorse this intervention, which is increasingly popular among his fellow orthopedic surgeons.
“The regulatory pathway drives this. Mesenchymal stem cells are categorized as a minimally manipulated tissue, so the regulatory pathway is easy,” explained Dr. Bugbee. “I talk 9 out of 10 patients out of it because there’s no evidence of disease modification.”
On a brighter note
Radiofrequency ablation and neuromodulation procedures for treatment of symptomatic knee OA make no pretense of being disease modifying, but a new systematic review of 33 published studies deemed of moderate or high methodological quality totaling 1,512 patients documented improved pain, function, and disease-specific quality of life for 3-12 months with minimal complications.
Most of these procedures target the genicular nerve, the sensory nerve that innervates the knee.
“There’s some value here to this,” Dr. Ruderman said. “Our pain folks are actually pretty interested in this. At our center, they’re using this for patients with persistent knee pain after knee replacement, with some success. It’s not an unreasonable approach.”
As part of his examination of the evidence base for OA treatment, Dr. Ruderman looked into an intervention he wasn’t familiar with: acupuncture. He was pleasantly surprised.
“There’s quite a bit of data. There is decent evidence that acupuncture has significant benefit, at least for pain, and is certainly without significant side effects,” according to the rheumatologist.
He cited a review of a dozen systematic reviews of randomized, controlled trials of acupuncture for OA. The Chinese investigators rated the overall quality of the evidence as moderate to low, but with some studies being rated high quality. “That’s not as bad as some of the other stuff I looked at,” Dr. Ruderman said.
Acupuncture was found to be 2.4 times more effective than Western medicine for short-term pain relief, and 4.1 times better in terms of total efficacy, with a lower risk of adverse events than with Western medicine.
Dr. Ruderman reported serving as a consultant for and/or receiving research grants from more than a half-dozen pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2020
Short-course calcipotriol plus 5-FU for AKs: potential major public health impact
LAHAINA, HAWAII – The most intriguing recent development in the treatment of actinic keratosis is a study in which a short-course of topical field therapy with , with a resultant markedly reduced risk of developing squamous cell carcinoma within the next 3 years, Kishwer S. Nehal, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The annual cost of treating actinic keratoses (AKs) exceeds $1 billion in the United States, so the billion-dollar question in dermatology is, does AK reduction lead to long-term protection against cancer? And this 3-year study by investigators at Washington University in St. Louis and Massachusetts General Hospital in Boston suggests the answer may be yes – when topical immunotherapy is utilized to induce robust T cell immunity, said Dr. Nehal, director of Mohs micrographic and dermatologic surgery, and codirector of the multidisciplinary skin cancer management program at Memorial Sloan Kettering Cancer Center in New York.
The investigators previously conducted a randomized, double-blind clinical trial in which 130 participants with a substantial AK burden received a 4-day course of 0.005% calcipotriol ointment plus 5% 5-FU cream or Vaseline plus 5-FU. The combination therapy proved more effective than the comparator in eliminating AKs at 8 weeks of follow-up. Moreover, tissue analysis pointed to the mechanism of benefit: The combination treatment induced keratinocyte expression of thymic stromal lymphopoietin (TSLP) cytokine, which led to a powerful CD4+ T cell response against AKs.
The researchers’ follow-up study addressed two key questions: Whether this epidermal T cell immunity persists long term, and if it actually achieves a reduced risk of squamous cell carcinoma (SCC) over time. The answers were yes and yes.
Seventy of the original 130 patients were prospectively followed for 3 years. Only 2 of 30 (7%) in the short-course combination therapy group developed SCC in treated areas of the face and scalp, compared with 11 of 40 controls (28%), a statistically significant difference, which constitutes a 79% relative risk reduction. This chemopreventive effect was long lasting at the cellular level, as the combination therapy group still retained measurable T cell immunity in the skin at the 3-year mark. As expected, the topical therapy had no impact on the development of basal cell carcinoma. The question now becomes how long the chemopreventive effect extends beyond 3 years.
“These remarkable findings substantiate the use of immunotherapeutic agents with minimal side effects and high efficacy against precancerous lesions in order to reduce the risk of cancer development and recurrence, which may be broadly applicable to skin and internal malignancies,” the investigators wrote in the study (JCI Insight. 2019 Mar 21;4(6). pii: 125476. doi: 10.1172/jci.insight.125476). Dr. Nehal said that this study requires confirmation in light of its post hoc design and the relatively small numbers of patients and SCCs. But the potential public health implications are profound, since roughly 40 million Americans have AKs, subclinical AKs are 10 times more common than visible ones, AKs are known precursors for SCC, and high-risk SCC is the cause of roughly 10,000 deaths per year, an underappreciated mortality burden that’s actually comparable to that of melanoma.
While awaiting further studies, this short-course combination therapy also offers the ready appeal of a field therapy without much downtime due to treatment-induced inflammation. In the study, 4 days of combo therapy resulted in moderate inflammation which quickly resolved.
“Does treatment duration and severity affect patient compliance? I would argue that in 2020 it does. People have very active, busy lives and they don’t want a lot of downtime. I can tell you that in Manhattan they want no downtime,” said Dr. Nehal, professor of dermatology at Weill Cornell Medicine, New York.
Even though a traditional 4-week, twice-daily course of 5% 5-FU cream has been convincingly shown in a recent large randomized Dutch trial (N Engl J Med. 2019 Mar 7;380[10]:935-46) to be the most effective field therapy for eradicating AKs – outperforming in descending order of efficacy at 12 months of follow-up imiquimod (Zyclara), photodynamic therapy, and ingenol mebutate (Picato) – Dr. Nehal finds few takers for 5-FU. People balk at the downtime. Her female patients with a significant AK burden typically opt for photodynamic therapy because of the aesthetic side benefit and shorter downtime, while the men – even those who’ve already had a large SCC – are more likely to prefer a watch-and-wait approach, dealing with an SCC if and when it arises.
“I love the science behind using calcipotriol with 5-FU, but I wish it was more friendly to dermatologists,” said fellow panelist Paul Nghiem, MD, PhD. “Of all the things we should have a combination product for, the pharmaceutical industry should really prepare a [calcipotriol/5-FU] cream and market it with the improved efficacy data.”
He added that he has no use for the conventional 2- to 4-week, twice-daily 5-FU regimens employed by many dermatologists.
“I don’t understand this need to feel like you’ve got to treat patients until they look like they’ve fallen off a motorcycle at 50 mph. I just can’t see that,” said Dr. Nghiem, professor and chair of dermatology at the University of Washington, Seattle.
Instead, he routinely utilizes the nearly 3-decade-old Pearlman technique of weekly pulsed dosing of topical 5-FU (J Am Acad Dermatol. 1991 Oct;25[4]:665-7).
“I almost never even ask my patients, ‘Are you OK with having a bunch of downtime?’ I just say, ‘Treat with the 5-FU until you get some erythema, until you’re bothered by it, and then stop for a while,’ ” he explained. “I’ve treated many patients with that technique over the years. It might not be quite as effective, but I hardly have to do any treatment with liquid nitrogen.”
Session chair Ashfaq A. Marghoob, MD, is of a similar mind.
“I also don’t go to the point that you’re fire engine red. Once the irritation sets in, we stop,” said Dr. Marghoob, director of clinical dermatology, Memorial Sloan Kettering Skin Cancer Center Hauppauge (New York).
Neither Dr. Nehal nor Dr. Nghiem has used short-course calcipotriol plus 5-FU therapy. When they polled the large audience as to who has, only a few hands went up.
“I feel like residents who are keeping up with the literature are using it,” Dr. Nehal observed. “My fellows say, ‘Yup, that’s what we’re doing,’ but I’m not using it.”
“I use it,” volunteered fellow panelist Trilokraj Tejasvi, MBBS. “I was educated by one of my residents, and now I use it all the time, especially in my VA population. I’ve been using it for a year. Things get red, but it clears fast,” said Dr. Tejasvi, director of the cutaneous lymphoma program and director of teledermatology services at the University of Michigan, Ann Arbor, and chief of the dermatology service at Ann Arbor Veteran Affairs Hospital.
Dr. Nehal reported having no conflicts of interest regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The most intriguing recent development in the treatment of actinic keratosis is a study in which a short-course of topical field therapy with , with a resultant markedly reduced risk of developing squamous cell carcinoma within the next 3 years, Kishwer S. Nehal, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The annual cost of treating actinic keratoses (AKs) exceeds $1 billion in the United States, so the billion-dollar question in dermatology is, does AK reduction lead to long-term protection against cancer? And this 3-year study by investigators at Washington University in St. Louis and Massachusetts General Hospital in Boston suggests the answer may be yes – when topical immunotherapy is utilized to induce robust T cell immunity, said Dr. Nehal, director of Mohs micrographic and dermatologic surgery, and codirector of the multidisciplinary skin cancer management program at Memorial Sloan Kettering Cancer Center in New York.
The investigators previously conducted a randomized, double-blind clinical trial in which 130 participants with a substantial AK burden received a 4-day course of 0.005% calcipotriol ointment plus 5% 5-FU cream or Vaseline plus 5-FU. The combination therapy proved more effective than the comparator in eliminating AKs at 8 weeks of follow-up. Moreover, tissue analysis pointed to the mechanism of benefit: The combination treatment induced keratinocyte expression of thymic stromal lymphopoietin (TSLP) cytokine, which led to a powerful CD4+ T cell response against AKs.
The researchers’ follow-up study addressed two key questions: Whether this epidermal T cell immunity persists long term, and if it actually achieves a reduced risk of squamous cell carcinoma (SCC) over time. The answers were yes and yes.
Seventy of the original 130 patients were prospectively followed for 3 years. Only 2 of 30 (7%) in the short-course combination therapy group developed SCC in treated areas of the face and scalp, compared with 11 of 40 controls (28%), a statistically significant difference, which constitutes a 79% relative risk reduction. This chemopreventive effect was long lasting at the cellular level, as the combination therapy group still retained measurable T cell immunity in the skin at the 3-year mark. As expected, the topical therapy had no impact on the development of basal cell carcinoma. The question now becomes how long the chemopreventive effect extends beyond 3 years.
“These remarkable findings substantiate the use of immunotherapeutic agents with minimal side effects and high efficacy against precancerous lesions in order to reduce the risk of cancer development and recurrence, which may be broadly applicable to skin and internal malignancies,” the investigators wrote in the study (JCI Insight. 2019 Mar 21;4(6). pii: 125476. doi: 10.1172/jci.insight.125476). Dr. Nehal said that this study requires confirmation in light of its post hoc design and the relatively small numbers of patients and SCCs. But the potential public health implications are profound, since roughly 40 million Americans have AKs, subclinical AKs are 10 times more common than visible ones, AKs are known precursors for SCC, and high-risk SCC is the cause of roughly 10,000 deaths per year, an underappreciated mortality burden that’s actually comparable to that of melanoma.
While awaiting further studies, this short-course combination therapy also offers the ready appeal of a field therapy without much downtime due to treatment-induced inflammation. In the study, 4 days of combo therapy resulted in moderate inflammation which quickly resolved.
“Does treatment duration and severity affect patient compliance? I would argue that in 2020 it does. People have very active, busy lives and they don’t want a lot of downtime. I can tell you that in Manhattan they want no downtime,” said Dr. Nehal, professor of dermatology at Weill Cornell Medicine, New York.
Even though a traditional 4-week, twice-daily course of 5% 5-FU cream has been convincingly shown in a recent large randomized Dutch trial (N Engl J Med. 2019 Mar 7;380[10]:935-46) to be the most effective field therapy for eradicating AKs – outperforming in descending order of efficacy at 12 months of follow-up imiquimod (Zyclara), photodynamic therapy, and ingenol mebutate (Picato) – Dr. Nehal finds few takers for 5-FU. People balk at the downtime. Her female patients with a significant AK burden typically opt for photodynamic therapy because of the aesthetic side benefit and shorter downtime, while the men – even those who’ve already had a large SCC – are more likely to prefer a watch-and-wait approach, dealing with an SCC if and when it arises.
“I love the science behind using calcipotriol with 5-FU, but I wish it was more friendly to dermatologists,” said fellow panelist Paul Nghiem, MD, PhD. “Of all the things we should have a combination product for, the pharmaceutical industry should really prepare a [calcipotriol/5-FU] cream and market it with the improved efficacy data.”
He added that he has no use for the conventional 2- to 4-week, twice-daily 5-FU regimens employed by many dermatologists.
“I don’t understand this need to feel like you’ve got to treat patients until they look like they’ve fallen off a motorcycle at 50 mph. I just can’t see that,” said Dr. Nghiem, professor and chair of dermatology at the University of Washington, Seattle.
Instead, he routinely utilizes the nearly 3-decade-old Pearlman technique of weekly pulsed dosing of topical 5-FU (J Am Acad Dermatol. 1991 Oct;25[4]:665-7).
“I almost never even ask my patients, ‘Are you OK with having a bunch of downtime?’ I just say, ‘Treat with the 5-FU until you get some erythema, until you’re bothered by it, and then stop for a while,’ ” he explained. “I’ve treated many patients with that technique over the years. It might not be quite as effective, but I hardly have to do any treatment with liquid nitrogen.”
Session chair Ashfaq A. Marghoob, MD, is of a similar mind.
“I also don’t go to the point that you’re fire engine red. Once the irritation sets in, we stop,” said Dr. Marghoob, director of clinical dermatology, Memorial Sloan Kettering Skin Cancer Center Hauppauge (New York).
Neither Dr. Nehal nor Dr. Nghiem has used short-course calcipotriol plus 5-FU therapy. When they polled the large audience as to who has, only a few hands went up.
“I feel like residents who are keeping up with the literature are using it,” Dr. Nehal observed. “My fellows say, ‘Yup, that’s what we’re doing,’ but I’m not using it.”
“I use it,” volunteered fellow panelist Trilokraj Tejasvi, MBBS. “I was educated by one of my residents, and now I use it all the time, especially in my VA population. I’ve been using it for a year. Things get red, but it clears fast,” said Dr. Tejasvi, director of the cutaneous lymphoma program and director of teledermatology services at the University of Michigan, Ann Arbor, and chief of the dermatology service at Ann Arbor Veteran Affairs Hospital.
Dr. Nehal reported having no conflicts of interest regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The most intriguing recent development in the treatment of actinic keratosis is a study in which a short-course of topical field therapy with , with a resultant markedly reduced risk of developing squamous cell carcinoma within the next 3 years, Kishwer S. Nehal, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The annual cost of treating actinic keratoses (AKs) exceeds $1 billion in the United States, so the billion-dollar question in dermatology is, does AK reduction lead to long-term protection against cancer? And this 3-year study by investigators at Washington University in St. Louis and Massachusetts General Hospital in Boston suggests the answer may be yes – when topical immunotherapy is utilized to induce robust T cell immunity, said Dr. Nehal, director of Mohs micrographic and dermatologic surgery, and codirector of the multidisciplinary skin cancer management program at Memorial Sloan Kettering Cancer Center in New York.
The investigators previously conducted a randomized, double-blind clinical trial in which 130 participants with a substantial AK burden received a 4-day course of 0.005% calcipotriol ointment plus 5% 5-FU cream or Vaseline plus 5-FU. The combination therapy proved more effective than the comparator in eliminating AKs at 8 weeks of follow-up. Moreover, tissue analysis pointed to the mechanism of benefit: The combination treatment induced keratinocyte expression of thymic stromal lymphopoietin (TSLP) cytokine, which led to a powerful CD4+ T cell response against AKs.
The researchers’ follow-up study addressed two key questions: Whether this epidermal T cell immunity persists long term, and if it actually achieves a reduced risk of squamous cell carcinoma (SCC) over time. The answers were yes and yes.
Seventy of the original 130 patients were prospectively followed for 3 years. Only 2 of 30 (7%) in the short-course combination therapy group developed SCC in treated areas of the face and scalp, compared with 11 of 40 controls (28%), a statistically significant difference, which constitutes a 79% relative risk reduction. This chemopreventive effect was long lasting at the cellular level, as the combination therapy group still retained measurable T cell immunity in the skin at the 3-year mark. As expected, the topical therapy had no impact on the development of basal cell carcinoma. The question now becomes how long the chemopreventive effect extends beyond 3 years.
“These remarkable findings substantiate the use of immunotherapeutic agents with minimal side effects and high efficacy against precancerous lesions in order to reduce the risk of cancer development and recurrence, which may be broadly applicable to skin and internal malignancies,” the investigators wrote in the study (JCI Insight. 2019 Mar 21;4(6). pii: 125476. doi: 10.1172/jci.insight.125476). Dr. Nehal said that this study requires confirmation in light of its post hoc design and the relatively small numbers of patients and SCCs. But the potential public health implications are profound, since roughly 40 million Americans have AKs, subclinical AKs are 10 times more common than visible ones, AKs are known precursors for SCC, and high-risk SCC is the cause of roughly 10,000 deaths per year, an underappreciated mortality burden that’s actually comparable to that of melanoma.
While awaiting further studies, this short-course combination therapy also offers the ready appeal of a field therapy without much downtime due to treatment-induced inflammation. In the study, 4 days of combo therapy resulted in moderate inflammation which quickly resolved.
“Does treatment duration and severity affect patient compliance? I would argue that in 2020 it does. People have very active, busy lives and they don’t want a lot of downtime. I can tell you that in Manhattan they want no downtime,” said Dr. Nehal, professor of dermatology at Weill Cornell Medicine, New York.
Even though a traditional 4-week, twice-daily course of 5% 5-FU cream has been convincingly shown in a recent large randomized Dutch trial (N Engl J Med. 2019 Mar 7;380[10]:935-46) to be the most effective field therapy for eradicating AKs – outperforming in descending order of efficacy at 12 months of follow-up imiquimod (Zyclara), photodynamic therapy, and ingenol mebutate (Picato) – Dr. Nehal finds few takers for 5-FU. People balk at the downtime. Her female patients with a significant AK burden typically opt for photodynamic therapy because of the aesthetic side benefit and shorter downtime, while the men – even those who’ve already had a large SCC – are more likely to prefer a watch-and-wait approach, dealing with an SCC if and when it arises.
“I love the science behind using calcipotriol with 5-FU, but I wish it was more friendly to dermatologists,” said fellow panelist Paul Nghiem, MD, PhD. “Of all the things we should have a combination product for, the pharmaceutical industry should really prepare a [calcipotriol/5-FU] cream and market it with the improved efficacy data.”
He added that he has no use for the conventional 2- to 4-week, twice-daily 5-FU regimens employed by many dermatologists.
“I don’t understand this need to feel like you’ve got to treat patients until they look like they’ve fallen off a motorcycle at 50 mph. I just can’t see that,” said Dr. Nghiem, professor and chair of dermatology at the University of Washington, Seattle.
Instead, he routinely utilizes the nearly 3-decade-old Pearlman technique of weekly pulsed dosing of topical 5-FU (J Am Acad Dermatol. 1991 Oct;25[4]:665-7).
“I almost never even ask my patients, ‘Are you OK with having a bunch of downtime?’ I just say, ‘Treat with the 5-FU until you get some erythema, until you’re bothered by it, and then stop for a while,’ ” he explained. “I’ve treated many patients with that technique over the years. It might not be quite as effective, but I hardly have to do any treatment with liquid nitrogen.”
Session chair Ashfaq A. Marghoob, MD, is of a similar mind.
“I also don’t go to the point that you’re fire engine red. Once the irritation sets in, we stop,” said Dr. Marghoob, director of clinical dermatology, Memorial Sloan Kettering Skin Cancer Center Hauppauge (New York).
Neither Dr. Nehal nor Dr. Nghiem has used short-course calcipotriol plus 5-FU therapy. When they polled the large audience as to who has, only a few hands went up.
“I feel like residents who are keeping up with the literature are using it,” Dr. Nehal observed. “My fellows say, ‘Yup, that’s what we’re doing,’ but I’m not using it.”
“I use it,” volunteered fellow panelist Trilokraj Tejasvi, MBBS. “I was educated by one of my residents, and now I use it all the time, especially in my VA population. I’ve been using it for a year. Things get red, but it clears fast,” said Dr. Tejasvi, director of the cutaneous lymphoma program and director of teledermatology services at the University of Michigan, Ann Arbor, and chief of the dermatology service at Ann Arbor Veteran Affairs Hospital.
Dr. Nehal reported having no conflicts of interest regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
A Nervous Recipient of a “Tongue Lashing”
Self-injurious behaviors are common and can be either volitional or unintentional. Often people who perform these behaviors receive “tongue lashings” from family, friends, and loved ones. We recently treated a patient whose lesion in the oral cavity was thought to be caused by some form of self-injury, though the prognosis clearly depended on the true culprit. It is important for clinicians to identify the cause of the injury when encountering patients with oral cavity lesions.
Case Presentation
A 40-year-old white male with a medical history of bipolar disorder, posttraumatic stress disorder, polysubstance abuse, and recently diagnosed temporomandibular joint (TMJ) syndrome was seen in outpatient primary care for a bleeding lesion in his mouth for the past 3 weeks. The lesion was under the surface of his right tongue. He first noted the lesion after he had burned himself tasting some homemade rice pudding while under the influence of marijuana. The next day, an impression was taken of his mouth by a dental assistant who was fitting him for an oral appliance for his TMJ syndrome; according to his history, she did not perform a visual inspection of his mouth nor could he recall his last dental examination. He had neither lost weight nor experienced dysphagia. He was not taking any prescribed medications, had an 8 pack-year history of smoking cigarettes, and had smoked crack cocaine intermittently for several years. The also patient had chewed one-half tin per day of chewing tobacco for 5 years, though he had quit 7 years before presentation. He was consuming 6 alcoholic drinks daily and had no history of chewing betel nuts.
On physical examination, the patient seemed extremely anxious, but his vital signs were unremarkable. The nasal dorsum was straight, and the nares were widely patent. There were no suspicious cutaneous lesions noted of the face, head, trunk, or extremities. The salivary glands were soft and showed no lesions or masses within the parotid or submandibular glands bilaterally. There was no obvious obstruction of Stenson or Wharton ducts bilaterally. He had normal lips and oral competence. The dentition was noted to be fair.
A nonfriable, 1.5 cm-wide lesion was found on the ventral surface of the right tongue (Figure 1). The tongue was mobile. The mouth floor was soft and without evidence of masses or lesions. The tonsils, tonsillar pillars, palate, and base of tongue did not show any concerning lesions or masses. The neck revealed a nonenlarged thyroid and no lymphadenopathy. The remainder of the examination was unremarkable.
Diagnosis
Given his risk factors of alcohol use disorder and a history of both inhaled and chewing tobacco, oral squamous cell carcinoma (SCC) was considered. The differential diagnosis also included pyogenic granuloma, mucocele, sublingual fibroma, and metastasis to the oral soft tissue. Due to its implications with respect to morbidity and mortality, we thought it necessary to rule out SCC of the oral cavity. SCC comprises more than 90% of oral malignancies, and tobacco-related products, alcohol, and human papilloma virus are well-established risk factors.1
Pyogenic granuloma, also known as eruptive hemangioma and lobular capillary hemangioma, is a relatively common benign lesion of the skin and mucosal surfaces that often presents as a solitary, rapidly enlarging papule or nodule that is extremely friable.2 Interestingly, pyogenic granuloma is a misnomer, since it is neither infectious in origin nor granulomatous when visualized under the microscope and is thought to arise from an exuberant tissue response to localized irritation or trauma. An individual lesion can range in size from a few millimeters to a few centimeters and generally reaches its maximum size within a matter of weeks; they often arise at sites of minor trauma.3 While the pathogenesis of pyogenic granuloma has not been clearly established, it seems to be related to an imbalance of angiogenesis secondary to overexpression of vascular endothelial growth factor and basic fibroblast growth factor.4 While they can occur at any age, pyogenic granulomas are frequently seen in pediatric patients and during pregnancy.
A fibroma, also known as an irritation fibroma, is one of the more common fibrous tumorlike growths and is often caused by trauma or irritation. It usually presents as a smooth-surfaced, painless solid lesion, though it can be nodular and histopathologically shows collagen and connective tissue.5 While fibromas can occur anywhere in the oral cavity, they commonly arise on the buccal mucosa along the plane of occlusion between the maxillary and mandibular teeth.
Mucoceles are the most common benign lesions in the mouth and are commonly found on the lower lip and are mucus-filled cavities, arising from the accumulation of mucus from trauma or lip-biting and alteration of minor salivary glands.6 Our patient’s rapid evolution and history of trauma were consistent with a mucocele. Although the lower lip is the most common site of involvement, mucoceles also occur on the tongue, cheek, palate, and mouth floor.Metastases to the oral cavity are rare and comprise only 1% of all oral cavity malignancies.7 Although most commonly seen in the jaw, nearly one-third of oral cavity metastases are in the soft tissue.8 They generally occur late in the course of disease, and the time between appearance and death is usually short.8 Our patient’s lack of known primary malignancy and lack of weight loss rendered this diagnosis unlikely.
Other possibilities include peripheral giant cell granuloma, a reactive hyperplastic lesion of the oral cavity originating from the periosteum or periodontal membrane following local irritation or chronic trauma,9 and peripheral ossifying fibroma, a reactive soft tissue growth usually seen on the interdental papilla.10
Surgical excision was performed and revealed reactive epidermal hyperplasia, ulceration, granulation tissue formation, and marked inflammation with reactive changes. There was no evidence of malignancy and was interpreted as consistent with pyogenic granuloma (Figures 2 and 3) likely due to the trauma from the thermal burn or poor dentition.
Management
The patient was relieved to be informed of the diagnosis of an unusual presentation of pyogenic granuloma with no evidence of cancer. Current treatment strategies for pyogenic granuloma include surgical excision, shave excision with cautery, cryotherapy, sclerotherapy, carbon dioxide or pulsed dye laser, as well as expectant management. However, recurrence after initial treatment can occur, with lower recurrence rates occurring with surgical excision.11
Although we wouldn’t state that we gave the patient a “tongue-lashing,” we strongly advised him that he return to his dentist and abstain from tobacco products, alcohol, illicit drugs, and taste-testing scalding food directly from the pot.
1. Khot KP, Deshmane S, Choudhari S. Human papilloma virus in oral squamous cell carcinoma-the enigma unraveled. Clin J Dent Res. 2016;19(1):17-23.
2. Bolognia JL, Jorizzo JL, Rapini RP, eds. Neoplasms of the skin. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. St. Louis, MO: Mosby; 2007:1627-1901.
3. Tatusov M, Reddy S, Federman DG. Pyogenic granuloma: yet another motorcycle peril. Postgrad Med. 2012;124(6):124-126.
4. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71(5):701-709.
5. Krishnan V, Shunmugavelu K. A clinical challenging situation of intra oral fibroma mimicking pyogenic granuloma. J Pan African Med. 2015;22(1):263.
6. Nallasivam KU, Sudha BR. Oral mucocele: review of literature and a case report. J Pharm Bioallied Sci. 2015;7(suppl 2):S731-S733.
7. Zachariades N. Neoplasms metastatic to the mouth, jaws, and surrounding tissues. J Craniomaxillofac Surg. 1989;17(6):283-290.
8. Irani S. Metastasis to the oral soft tissues: a review of 412 cases. J Int Soc Prev Community Dent. 2016;6(5):393-401.
9. Shadman N, Ebrahimi SF, Jafari S, Eslami M. Peripheral giant cell granuloma: a review of 123 cases. Dent Res J (Isfahan). 2009;6(1):47-50.
10. Poonacha KS, Shigli AL, Shirol D. Peripheral ossifying fibroma: a clinical report. Contemp Clin Dent. 2010;1(1):54-56.
11. Gilmore A, Kelsberg G, Safranek G. Clinical inquiries. What’s the best treatment for pyogenic granuloma? J Fam Pract. 2010;59(1):40-42.
Self-injurious behaviors are common and can be either volitional or unintentional. Often people who perform these behaviors receive “tongue lashings” from family, friends, and loved ones. We recently treated a patient whose lesion in the oral cavity was thought to be caused by some form of self-injury, though the prognosis clearly depended on the true culprit. It is important for clinicians to identify the cause of the injury when encountering patients with oral cavity lesions.
Case Presentation
A 40-year-old white male with a medical history of bipolar disorder, posttraumatic stress disorder, polysubstance abuse, and recently diagnosed temporomandibular joint (TMJ) syndrome was seen in outpatient primary care for a bleeding lesion in his mouth for the past 3 weeks. The lesion was under the surface of his right tongue. He first noted the lesion after he had burned himself tasting some homemade rice pudding while under the influence of marijuana. The next day, an impression was taken of his mouth by a dental assistant who was fitting him for an oral appliance for his TMJ syndrome; according to his history, she did not perform a visual inspection of his mouth nor could he recall his last dental examination. He had neither lost weight nor experienced dysphagia. He was not taking any prescribed medications, had an 8 pack-year history of smoking cigarettes, and had smoked crack cocaine intermittently for several years. The also patient had chewed one-half tin per day of chewing tobacco for 5 years, though he had quit 7 years before presentation. He was consuming 6 alcoholic drinks daily and had no history of chewing betel nuts.
On physical examination, the patient seemed extremely anxious, but his vital signs were unremarkable. The nasal dorsum was straight, and the nares were widely patent. There were no suspicious cutaneous lesions noted of the face, head, trunk, or extremities. The salivary glands were soft and showed no lesions or masses within the parotid or submandibular glands bilaterally. There was no obvious obstruction of Stenson or Wharton ducts bilaterally. He had normal lips and oral competence. The dentition was noted to be fair.
A nonfriable, 1.5 cm-wide lesion was found on the ventral surface of the right tongue (Figure 1). The tongue was mobile. The mouth floor was soft and without evidence of masses or lesions. The tonsils, tonsillar pillars, palate, and base of tongue did not show any concerning lesions or masses. The neck revealed a nonenlarged thyroid and no lymphadenopathy. The remainder of the examination was unremarkable.
Diagnosis
Given his risk factors of alcohol use disorder and a history of both inhaled and chewing tobacco, oral squamous cell carcinoma (SCC) was considered. The differential diagnosis also included pyogenic granuloma, mucocele, sublingual fibroma, and metastasis to the oral soft tissue. Due to its implications with respect to morbidity and mortality, we thought it necessary to rule out SCC of the oral cavity. SCC comprises more than 90% of oral malignancies, and tobacco-related products, alcohol, and human papilloma virus are well-established risk factors.1
Pyogenic granuloma, also known as eruptive hemangioma and lobular capillary hemangioma, is a relatively common benign lesion of the skin and mucosal surfaces that often presents as a solitary, rapidly enlarging papule or nodule that is extremely friable.2 Interestingly, pyogenic granuloma is a misnomer, since it is neither infectious in origin nor granulomatous when visualized under the microscope and is thought to arise from an exuberant tissue response to localized irritation or trauma. An individual lesion can range in size from a few millimeters to a few centimeters and generally reaches its maximum size within a matter of weeks; they often arise at sites of minor trauma.3 While the pathogenesis of pyogenic granuloma has not been clearly established, it seems to be related to an imbalance of angiogenesis secondary to overexpression of vascular endothelial growth factor and basic fibroblast growth factor.4 While they can occur at any age, pyogenic granulomas are frequently seen in pediatric patients and during pregnancy.
A fibroma, also known as an irritation fibroma, is one of the more common fibrous tumorlike growths and is often caused by trauma or irritation. It usually presents as a smooth-surfaced, painless solid lesion, though it can be nodular and histopathologically shows collagen and connective tissue.5 While fibromas can occur anywhere in the oral cavity, they commonly arise on the buccal mucosa along the plane of occlusion between the maxillary and mandibular teeth.
Mucoceles are the most common benign lesions in the mouth and are commonly found on the lower lip and are mucus-filled cavities, arising from the accumulation of mucus from trauma or lip-biting and alteration of minor salivary glands.6 Our patient’s rapid evolution and history of trauma were consistent with a mucocele. Although the lower lip is the most common site of involvement, mucoceles also occur on the tongue, cheek, palate, and mouth floor.Metastases to the oral cavity are rare and comprise only 1% of all oral cavity malignancies.7 Although most commonly seen in the jaw, nearly one-third of oral cavity metastases are in the soft tissue.8 They generally occur late in the course of disease, and the time between appearance and death is usually short.8 Our patient’s lack of known primary malignancy and lack of weight loss rendered this diagnosis unlikely.
Other possibilities include peripheral giant cell granuloma, a reactive hyperplastic lesion of the oral cavity originating from the periosteum or periodontal membrane following local irritation or chronic trauma,9 and peripheral ossifying fibroma, a reactive soft tissue growth usually seen on the interdental papilla.10
Surgical excision was performed and revealed reactive epidermal hyperplasia, ulceration, granulation tissue formation, and marked inflammation with reactive changes. There was no evidence of malignancy and was interpreted as consistent with pyogenic granuloma (Figures 2 and 3) likely due to the trauma from the thermal burn or poor dentition.
Management
The patient was relieved to be informed of the diagnosis of an unusual presentation of pyogenic granuloma with no evidence of cancer. Current treatment strategies for pyogenic granuloma include surgical excision, shave excision with cautery, cryotherapy, sclerotherapy, carbon dioxide or pulsed dye laser, as well as expectant management. However, recurrence after initial treatment can occur, with lower recurrence rates occurring with surgical excision.11
Although we wouldn’t state that we gave the patient a “tongue-lashing,” we strongly advised him that he return to his dentist and abstain from tobacco products, alcohol, illicit drugs, and taste-testing scalding food directly from the pot.
Self-injurious behaviors are common and can be either volitional or unintentional. Often people who perform these behaviors receive “tongue lashings” from family, friends, and loved ones. We recently treated a patient whose lesion in the oral cavity was thought to be caused by some form of self-injury, though the prognosis clearly depended on the true culprit. It is important for clinicians to identify the cause of the injury when encountering patients with oral cavity lesions.
Case Presentation
A 40-year-old white male with a medical history of bipolar disorder, posttraumatic stress disorder, polysubstance abuse, and recently diagnosed temporomandibular joint (TMJ) syndrome was seen in outpatient primary care for a bleeding lesion in his mouth for the past 3 weeks. The lesion was under the surface of his right tongue. He first noted the lesion after he had burned himself tasting some homemade rice pudding while under the influence of marijuana. The next day, an impression was taken of his mouth by a dental assistant who was fitting him for an oral appliance for his TMJ syndrome; according to his history, she did not perform a visual inspection of his mouth nor could he recall his last dental examination. He had neither lost weight nor experienced dysphagia. He was not taking any prescribed medications, had an 8 pack-year history of smoking cigarettes, and had smoked crack cocaine intermittently for several years. The also patient had chewed one-half tin per day of chewing tobacco for 5 years, though he had quit 7 years before presentation. He was consuming 6 alcoholic drinks daily and had no history of chewing betel nuts.
On physical examination, the patient seemed extremely anxious, but his vital signs were unremarkable. The nasal dorsum was straight, and the nares were widely patent. There were no suspicious cutaneous lesions noted of the face, head, trunk, or extremities. The salivary glands were soft and showed no lesions or masses within the parotid or submandibular glands bilaterally. There was no obvious obstruction of Stenson or Wharton ducts bilaterally. He had normal lips and oral competence. The dentition was noted to be fair.
A nonfriable, 1.5 cm-wide lesion was found on the ventral surface of the right tongue (Figure 1). The tongue was mobile. The mouth floor was soft and without evidence of masses or lesions. The tonsils, tonsillar pillars, palate, and base of tongue did not show any concerning lesions or masses. The neck revealed a nonenlarged thyroid and no lymphadenopathy. The remainder of the examination was unremarkable.
Diagnosis
Given his risk factors of alcohol use disorder and a history of both inhaled and chewing tobacco, oral squamous cell carcinoma (SCC) was considered. The differential diagnosis also included pyogenic granuloma, mucocele, sublingual fibroma, and metastasis to the oral soft tissue. Due to its implications with respect to morbidity and mortality, we thought it necessary to rule out SCC of the oral cavity. SCC comprises more than 90% of oral malignancies, and tobacco-related products, alcohol, and human papilloma virus are well-established risk factors.1
Pyogenic granuloma, also known as eruptive hemangioma and lobular capillary hemangioma, is a relatively common benign lesion of the skin and mucosal surfaces that often presents as a solitary, rapidly enlarging papule or nodule that is extremely friable.2 Interestingly, pyogenic granuloma is a misnomer, since it is neither infectious in origin nor granulomatous when visualized under the microscope and is thought to arise from an exuberant tissue response to localized irritation or trauma. An individual lesion can range in size from a few millimeters to a few centimeters and generally reaches its maximum size within a matter of weeks; they often arise at sites of minor trauma.3 While the pathogenesis of pyogenic granuloma has not been clearly established, it seems to be related to an imbalance of angiogenesis secondary to overexpression of vascular endothelial growth factor and basic fibroblast growth factor.4 While they can occur at any age, pyogenic granulomas are frequently seen in pediatric patients and during pregnancy.
A fibroma, also known as an irritation fibroma, is one of the more common fibrous tumorlike growths and is often caused by trauma or irritation. It usually presents as a smooth-surfaced, painless solid lesion, though it can be nodular and histopathologically shows collagen and connective tissue.5 While fibromas can occur anywhere in the oral cavity, they commonly arise on the buccal mucosa along the plane of occlusion between the maxillary and mandibular teeth.
Mucoceles are the most common benign lesions in the mouth and are commonly found on the lower lip and are mucus-filled cavities, arising from the accumulation of mucus from trauma or lip-biting and alteration of minor salivary glands.6 Our patient’s rapid evolution and history of trauma were consistent with a mucocele. Although the lower lip is the most common site of involvement, mucoceles also occur on the tongue, cheek, palate, and mouth floor.Metastases to the oral cavity are rare and comprise only 1% of all oral cavity malignancies.7 Although most commonly seen in the jaw, nearly one-third of oral cavity metastases are in the soft tissue.8 They generally occur late in the course of disease, and the time between appearance and death is usually short.8 Our patient’s lack of known primary malignancy and lack of weight loss rendered this diagnosis unlikely.
Other possibilities include peripheral giant cell granuloma, a reactive hyperplastic lesion of the oral cavity originating from the periosteum or periodontal membrane following local irritation or chronic trauma,9 and peripheral ossifying fibroma, a reactive soft tissue growth usually seen on the interdental papilla.10
Surgical excision was performed and revealed reactive epidermal hyperplasia, ulceration, granulation tissue formation, and marked inflammation with reactive changes. There was no evidence of malignancy and was interpreted as consistent with pyogenic granuloma (Figures 2 and 3) likely due to the trauma from the thermal burn or poor dentition.
Management
The patient was relieved to be informed of the diagnosis of an unusual presentation of pyogenic granuloma with no evidence of cancer. Current treatment strategies for pyogenic granuloma include surgical excision, shave excision with cautery, cryotherapy, sclerotherapy, carbon dioxide or pulsed dye laser, as well as expectant management. However, recurrence after initial treatment can occur, with lower recurrence rates occurring with surgical excision.11
Although we wouldn’t state that we gave the patient a “tongue-lashing,” we strongly advised him that he return to his dentist and abstain from tobacco products, alcohol, illicit drugs, and taste-testing scalding food directly from the pot.
1. Khot KP, Deshmane S, Choudhari S. Human papilloma virus in oral squamous cell carcinoma-the enigma unraveled. Clin J Dent Res. 2016;19(1):17-23.
2. Bolognia JL, Jorizzo JL, Rapini RP, eds. Neoplasms of the skin. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. St. Louis, MO: Mosby; 2007:1627-1901.
3. Tatusov M, Reddy S, Federman DG. Pyogenic granuloma: yet another motorcycle peril. Postgrad Med. 2012;124(6):124-126.
4. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71(5):701-709.
5. Krishnan V, Shunmugavelu K. A clinical challenging situation of intra oral fibroma mimicking pyogenic granuloma. J Pan African Med. 2015;22(1):263.
6. Nallasivam KU, Sudha BR. Oral mucocele: review of literature and a case report. J Pharm Bioallied Sci. 2015;7(suppl 2):S731-S733.
7. Zachariades N. Neoplasms metastatic to the mouth, jaws, and surrounding tissues. J Craniomaxillofac Surg. 1989;17(6):283-290.
8. Irani S. Metastasis to the oral soft tissues: a review of 412 cases. J Int Soc Prev Community Dent. 2016;6(5):393-401.
9. Shadman N, Ebrahimi SF, Jafari S, Eslami M. Peripheral giant cell granuloma: a review of 123 cases. Dent Res J (Isfahan). 2009;6(1):47-50.
10. Poonacha KS, Shigli AL, Shirol D. Peripheral ossifying fibroma: a clinical report. Contemp Clin Dent. 2010;1(1):54-56.
11. Gilmore A, Kelsberg G, Safranek G. Clinical inquiries. What’s the best treatment for pyogenic granuloma? J Fam Pract. 2010;59(1):40-42.
1. Khot KP, Deshmane S, Choudhari S. Human papilloma virus in oral squamous cell carcinoma-the enigma unraveled. Clin J Dent Res. 2016;19(1):17-23.
2. Bolognia JL, Jorizzo JL, Rapini RP, eds. Neoplasms of the skin. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. St. Louis, MO: Mosby; 2007:1627-1901.
3. Tatusov M, Reddy S, Federman DG. Pyogenic granuloma: yet another motorcycle peril. Postgrad Med. 2012;124(6):124-126.
4. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71(5):701-709.
5. Krishnan V, Shunmugavelu K. A clinical challenging situation of intra oral fibroma mimicking pyogenic granuloma. J Pan African Med. 2015;22(1):263.
6. Nallasivam KU, Sudha BR. Oral mucocele: review of literature and a case report. J Pharm Bioallied Sci. 2015;7(suppl 2):S731-S733.
7. Zachariades N. Neoplasms metastatic to the mouth, jaws, and surrounding tissues. J Craniomaxillofac Surg. 1989;17(6):283-290.
8. Irani S. Metastasis to the oral soft tissues: a review of 412 cases. J Int Soc Prev Community Dent. 2016;6(5):393-401.
9. Shadman N, Ebrahimi SF, Jafari S, Eslami M. Peripheral giant cell granuloma: a review of 123 cases. Dent Res J (Isfahan). 2009;6(1):47-50.
10. Poonacha KS, Shigli AL, Shirol D. Peripheral ossifying fibroma: a clinical report. Contemp Clin Dent. 2010;1(1):54-56.
11. Gilmore A, Kelsberg G, Safranek G. Clinical inquiries. What’s the best treatment for pyogenic granuloma? J Fam Pract. 2010;59(1):40-42.