User login
How to nearly eliminate CLABSIs in children’s hospitals
SEATTLE – Levine Children’s Hospital, in Charlotte, N.C., dropped its central line–associated bloodstream infection rate from 1.13 per 1,000 line days to 0.67 in just a few months, with a mix of common sense steps and public accountability.
Levine Children’s was at about the 50th percentile for CLABSIs, compared with other children’s hospitals, but dropped to the 10th percentile after the changes. There were 21 CLABSIs in 2017, but only 12 in 2018. The hospital went 6 straight months without a CLABSI after the changes were made. The efforts saved about $300,000 and 63 patient days.
“We really had great success,” said Kayla S. Koch, MD, a pediatric hospitalist at Levine Children’s, who presented the findings at Pediatric Hospital Medicine.
Hospital units had been working to reduce CLABSIs, but they were each doing their own thing. “Many of our units were already dabbling, so we just sort of brought them together. We standardized the process and got everyone on the same page,” said copresenter Ketan P. Nadkarni, MD, also a pediatric hospitalist at Levine Children’s.
It wasn’t hard to get buy-in. “I don’t think the units were aware that everyone was doing it differently,” and were on board once the problem was explained. Also, using the same approach throughout the hospital made it easier for nurses and physicians moving between units, he said.
Each morning, the nurse supervisor and patient nurse would partner up at the bedside to check that central venous lines were set up correctly. They examined the alcohol disinfectant caps to make sure they were clean; determined that children were getting chlorhexidine gluconate baths; checked the dressings for bleeding and soiling; noted in the electronic medical record why the patient had a central line; and discussed with hospitalists if it were still needed. Problems were addressed immediately.
These quality processes were all tracked on wall racks placed in plain sight on each unit, including the neonatal and pediatric ICUs. Each central line patient had a card that listed what needed to be done, with a green stripe on one side and a red stripe on the other. If everything was done right, the green side faced out; if even one thing was done wrong, the red side was displayed, for all to see. It brought accountability to the process, the presenters said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The wall rack also had the central line audit schedule, plus diagrams that showed every failed item, the reason for it, and the unit’s compliance rate. Anyone walking by could see at a glance how the unit was doing that day and overall.
The number of dressing options was reduced from 10 to 2, a SorbaView SHIELD and a Tegaderm-like dressing, which made it easier to standardize the efforts. A protocol also was put in place to reinforce oozing dressings, instead of automatically changing them. “We were doing too many changes,” Dr. Koch said.
Compliance with the bundle was almost 90%. Staff “really got into it, and it was great to see,” she said.
The “initial success was almost unexpected, and so dramatic.” The goal now is to sustain the improvements, and roll them out to radiology and other places were central lines are placed, Dr. Nadkarni said.
There was no external funding, and the investigators had no disclosures.
SEATTLE – Levine Children’s Hospital, in Charlotte, N.C., dropped its central line–associated bloodstream infection rate from 1.13 per 1,000 line days to 0.67 in just a few months, with a mix of common sense steps and public accountability.
Levine Children’s was at about the 50th percentile for CLABSIs, compared with other children’s hospitals, but dropped to the 10th percentile after the changes. There were 21 CLABSIs in 2017, but only 12 in 2018. The hospital went 6 straight months without a CLABSI after the changes were made. The efforts saved about $300,000 and 63 patient days.
“We really had great success,” said Kayla S. Koch, MD, a pediatric hospitalist at Levine Children’s, who presented the findings at Pediatric Hospital Medicine.
Hospital units had been working to reduce CLABSIs, but they were each doing their own thing. “Many of our units were already dabbling, so we just sort of brought them together. We standardized the process and got everyone on the same page,” said copresenter Ketan P. Nadkarni, MD, also a pediatric hospitalist at Levine Children’s.
It wasn’t hard to get buy-in. “I don’t think the units were aware that everyone was doing it differently,” and were on board once the problem was explained. Also, using the same approach throughout the hospital made it easier for nurses and physicians moving between units, he said.
Each morning, the nurse supervisor and patient nurse would partner up at the bedside to check that central venous lines were set up correctly. They examined the alcohol disinfectant caps to make sure they were clean; determined that children were getting chlorhexidine gluconate baths; checked the dressings for bleeding and soiling; noted in the electronic medical record why the patient had a central line; and discussed with hospitalists if it were still needed. Problems were addressed immediately.
These quality processes were all tracked on wall racks placed in plain sight on each unit, including the neonatal and pediatric ICUs. Each central line patient had a card that listed what needed to be done, with a green stripe on one side and a red stripe on the other. If everything was done right, the green side faced out; if even one thing was done wrong, the red side was displayed, for all to see. It brought accountability to the process, the presenters said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The wall rack also had the central line audit schedule, plus diagrams that showed every failed item, the reason for it, and the unit’s compliance rate. Anyone walking by could see at a glance how the unit was doing that day and overall.
The number of dressing options was reduced from 10 to 2, a SorbaView SHIELD and a Tegaderm-like dressing, which made it easier to standardize the efforts. A protocol also was put in place to reinforce oozing dressings, instead of automatically changing them. “We were doing too many changes,” Dr. Koch said.
Compliance with the bundle was almost 90%. Staff “really got into it, and it was great to see,” she said.
The “initial success was almost unexpected, and so dramatic.” The goal now is to sustain the improvements, and roll them out to radiology and other places were central lines are placed, Dr. Nadkarni said.
There was no external funding, and the investigators had no disclosures.
SEATTLE – Levine Children’s Hospital, in Charlotte, N.C., dropped its central line–associated bloodstream infection rate from 1.13 per 1,000 line days to 0.67 in just a few months, with a mix of common sense steps and public accountability.
Levine Children’s was at about the 50th percentile for CLABSIs, compared with other children’s hospitals, but dropped to the 10th percentile after the changes. There were 21 CLABSIs in 2017, but only 12 in 2018. The hospital went 6 straight months without a CLABSI after the changes were made. The efforts saved about $300,000 and 63 patient days.
“We really had great success,” said Kayla S. Koch, MD, a pediatric hospitalist at Levine Children’s, who presented the findings at Pediatric Hospital Medicine.
Hospital units had been working to reduce CLABSIs, but they were each doing their own thing. “Many of our units were already dabbling, so we just sort of brought them together. We standardized the process and got everyone on the same page,” said copresenter Ketan P. Nadkarni, MD, also a pediatric hospitalist at Levine Children’s.
It wasn’t hard to get buy-in. “I don’t think the units were aware that everyone was doing it differently,” and were on board once the problem was explained. Also, using the same approach throughout the hospital made it easier for nurses and physicians moving between units, he said.
Each morning, the nurse supervisor and patient nurse would partner up at the bedside to check that central venous lines were set up correctly. They examined the alcohol disinfectant caps to make sure they were clean; determined that children were getting chlorhexidine gluconate baths; checked the dressings for bleeding and soiling; noted in the electronic medical record why the patient had a central line; and discussed with hospitalists if it were still needed. Problems were addressed immediately.
These quality processes were all tracked on wall racks placed in plain sight on each unit, including the neonatal and pediatric ICUs. Each central line patient had a card that listed what needed to be done, with a green stripe on one side and a red stripe on the other. If everything was done right, the green side faced out; if even one thing was done wrong, the red side was displayed, for all to see. It brought accountability to the process, the presenters said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The wall rack also had the central line audit schedule, plus diagrams that showed every failed item, the reason for it, and the unit’s compliance rate. Anyone walking by could see at a glance how the unit was doing that day and overall.
The number of dressing options was reduced from 10 to 2, a SorbaView SHIELD and a Tegaderm-like dressing, which made it easier to standardize the efforts. A protocol also was put in place to reinforce oozing dressings, instead of automatically changing them. “We were doing too many changes,” Dr. Koch said.
Compliance with the bundle was almost 90%. Staff “really got into it, and it was great to see,” she said.
The “initial success was almost unexpected, and so dramatic.” The goal now is to sustain the improvements, and roll them out to radiology and other places were central lines are placed, Dr. Nadkarni said.
There was no external funding, and the investigators had no disclosures.
REPORTING FROM PHM 2019
Recalling a medical education
As I look back, there have been many changes during my 25 years of clinical practice. I always assumed there would be advancements in medical research during my career. I expected those advancements to produce progress rather than a random walk.
One area of positive change has been the recommendations for safe sleep practices for young infants. The Back to Sleep program of the mid 1990s reversed prior advice. It recommended that babies should sleep on their backs to avoid accidental suffocation. Prior advice had been that they should sleep on their stomachs to avoid aspiration. The new advice cut infant deaths by 50%.
Over the years, treatment of gastroesophageal reflux has significantly changed. Polysomnograms are ordered much less frequently. Medications to reduce stomach acid have been associated with side effects and now are discouraged. Raising the head of the crib was common advice in 2000s that was contradicted in the 2010s. For 2 decades I wrote orders in the hospital to elevate the head of the crib. More frequently, the nurses did it without my orders whenever they found a spitty baby.
In May 2019, there was a product recall of inclined infant sleepers. The Fisher-Price Rock ‘n Play was one product recalled; 4.7 million of these were sold in the United States in the past 10 years. Because they are used only by infants, and because there are about 4 million births per year in the United States, there are enough of these items stored in basements and garages for every infant to have one.
Investigative reporting by the Washington Post yielded an article highly critical of the product and the way it was originally created and designed. There is outrage in the author’s description of events. Because I have degrees in both engineering and pediatric medicine, I reviewed his assertions and tried to compare his ideal of the medical research world with my reality.
There are 3,600 infant deaths per year in the United States attributed to SUID/SIDS (sudden unexplained infant death/sudden infant death syndrome). From that perspective, I don’t know what 30 deaths in a decade associated with the sleeper really means. There is a high potential for recall bias and confirmation bias. It doesn’t surprise me that there was a delay in assigning blame to an ubiquitous consumer product. The article assumes that medical opinion is monolithic and synchronized rather than undergoing a diffusion of innovation, as described by Everett M. Rogers. Sorting out who knew what and when they knew it will take the courts many years.
Some of my columns earlier this year have appraised medical information in social media, and particularly on Facebook, as being harmfully unreliable.
An example of the unreliability of modern medical research was documented in an article in Hospital Pediatrics in July 2019.
The authors were performing a meta-analysis to determine whether the use of respiratory viral (RV) detection tests are helpful in reducing length of stay or reducing unnecessary antibiotic use. To me, that is a much simpler issue, scientifically, than safe sleep practices. The authors found 23 relevant studies that met their criteria for inclusion. Their overall conclusion was that the quality of the studies, the heterogeneity of the studies, and the statistically significant but contradictory results between the studies made it impossible to prove RV testing is beneficial. However, as I read the article, they cannot – for a litany of reasons – rule out such a benefit. Twenty three published articles in total yielded no reliable medical knowledge.
RV testing already has been widely adopted, particularly in emergency rooms. It is expensive. Clinical guidelines discourage RV testing but those guidelines are based on RV testing in the 2003-2006 time frame, which is obsolete technology. The author of the article on the infant sleepers expressed shock at what he considered to be inadequate medical research supporting the development of the inclined infant sleeper. RV testing is a product in widespread use, with lots of research, and has no better proof of efficacy or safety.
I expected, when I first started practice, that when I was older and grayer I would look back and recall many advances. I anticipated my recall would be of fond memories and of many patients helped. What I didn’t expect was so much of the advice that I provided to be wrong. Perhaps my medical education and parts of the academic research system should be subject to a product recall.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
As I look back, there have been many changes during my 25 years of clinical practice. I always assumed there would be advancements in medical research during my career. I expected those advancements to produce progress rather than a random walk.
One area of positive change has been the recommendations for safe sleep practices for young infants. The Back to Sleep program of the mid 1990s reversed prior advice. It recommended that babies should sleep on their backs to avoid accidental suffocation. Prior advice had been that they should sleep on their stomachs to avoid aspiration. The new advice cut infant deaths by 50%.
Over the years, treatment of gastroesophageal reflux has significantly changed. Polysomnograms are ordered much less frequently. Medications to reduce stomach acid have been associated with side effects and now are discouraged. Raising the head of the crib was common advice in 2000s that was contradicted in the 2010s. For 2 decades I wrote orders in the hospital to elevate the head of the crib. More frequently, the nurses did it without my orders whenever they found a spitty baby.
In May 2019, there was a product recall of inclined infant sleepers. The Fisher-Price Rock ‘n Play was one product recalled; 4.7 million of these were sold in the United States in the past 10 years. Because they are used only by infants, and because there are about 4 million births per year in the United States, there are enough of these items stored in basements and garages for every infant to have one.
Investigative reporting by the Washington Post yielded an article highly critical of the product and the way it was originally created and designed. There is outrage in the author’s description of events. Because I have degrees in both engineering and pediatric medicine, I reviewed his assertions and tried to compare his ideal of the medical research world with my reality.
There are 3,600 infant deaths per year in the United States attributed to SUID/SIDS (sudden unexplained infant death/sudden infant death syndrome). From that perspective, I don’t know what 30 deaths in a decade associated with the sleeper really means. There is a high potential for recall bias and confirmation bias. It doesn’t surprise me that there was a delay in assigning blame to an ubiquitous consumer product. The article assumes that medical opinion is monolithic and synchronized rather than undergoing a diffusion of innovation, as described by Everett M. Rogers. Sorting out who knew what and when they knew it will take the courts many years.
Some of my columns earlier this year have appraised medical information in social media, and particularly on Facebook, as being harmfully unreliable.
An example of the unreliability of modern medical research was documented in an article in Hospital Pediatrics in July 2019.
The authors were performing a meta-analysis to determine whether the use of respiratory viral (RV) detection tests are helpful in reducing length of stay or reducing unnecessary antibiotic use. To me, that is a much simpler issue, scientifically, than safe sleep practices. The authors found 23 relevant studies that met their criteria for inclusion. Their overall conclusion was that the quality of the studies, the heterogeneity of the studies, and the statistically significant but contradictory results between the studies made it impossible to prove RV testing is beneficial. However, as I read the article, they cannot – for a litany of reasons – rule out such a benefit. Twenty three published articles in total yielded no reliable medical knowledge.
RV testing already has been widely adopted, particularly in emergency rooms. It is expensive. Clinical guidelines discourage RV testing but those guidelines are based on RV testing in the 2003-2006 time frame, which is obsolete technology. The author of the article on the infant sleepers expressed shock at what he considered to be inadequate medical research supporting the development of the inclined infant sleeper. RV testing is a product in widespread use, with lots of research, and has no better proof of efficacy or safety.
I expected, when I first started practice, that when I was older and grayer I would look back and recall many advances. I anticipated my recall would be of fond memories and of many patients helped. What I didn’t expect was so much of the advice that I provided to be wrong. Perhaps my medical education and parts of the academic research system should be subject to a product recall.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
As I look back, there have been many changes during my 25 years of clinical practice. I always assumed there would be advancements in medical research during my career. I expected those advancements to produce progress rather than a random walk.
One area of positive change has been the recommendations for safe sleep practices for young infants. The Back to Sleep program of the mid 1990s reversed prior advice. It recommended that babies should sleep on their backs to avoid accidental suffocation. Prior advice had been that they should sleep on their stomachs to avoid aspiration. The new advice cut infant deaths by 50%.
Over the years, treatment of gastroesophageal reflux has significantly changed. Polysomnograms are ordered much less frequently. Medications to reduce stomach acid have been associated with side effects and now are discouraged. Raising the head of the crib was common advice in 2000s that was contradicted in the 2010s. For 2 decades I wrote orders in the hospital to elevate the head of the crib. More frequently, the nurses did it without my orders whenever they found a spitty baby.
In May 2019, there was a product recall of inclined infant sleepers. The Fisher-Price Rock ‘n Play was one product recalled; 4.7 million of these were sold in the United States in the past 10 years. Because they are used only by infants, and because there are about 4 million births per year in the United States, there are enough of these items stored in basements and garages for every infant to have one.
Investigative reporting by the Washington Post yielded an article highly critical of the product and the way it was originally created and designed. There is outrage in the author’s description of events. Because I have degrees in both engineering and pediatric medicine, I reviewed his assertions and tried to compare his ideal of the medical research world with my reality.
There are 3,600 infant deaths per year in the United States attributed to SUID/SIDS (sudden unexplained infant death/sudden infant death syndrome). From that perspective, I don’t know what 30 deaths in a decade associated with the sleeper really means. There is a high potential for recall bias and confirmation bias. It doesn’t surprise me that there was a delay in assigning blame to an ubiquitous consumer product. The article assumes that medical opinion is monolithic and synchronized rather than undergoing a diffusion of innovation, as described by Everett M. Rogers. Sorting out who knew what and when they knew it will take the courts many years.
Some of my columns earlier this year have appraised medical information in social media, and particularly on Facebook, as being harmfully unreliable.
An example of the unreliability of modern medical research was documented in an article in Hospital Pediatrics in July 2019.
The authors were performing a meta-analysis to determine whether the use of respiratory viral (RV) detection tests are helpful in reducing length of stay or reducing unnecessary antibiotic use. To me, that is a much simpler issue, scientifically, than safe sleep practices. The authors found 23 relevant studies that met their criteria for inclusion. Their overall conclusion was that the quality of the studies, the heterogeneity of the studies, and the statistically significant but contradictory results between the studies made it impossible to prove RV testing is beneficial. However, as I read the article, they cannot – for a litany of reasons – rule out such a benefit. Twenty three published articles in total yielded no reliable medical knowledge.
RV testing already has been widely adopted, particularly in emergency rooms. It is expensive. Clinical guidelines discourage RV testing but those guidelines are based on RV testing in the 2003-2006 time frame, which is obsolete technology. The author of the article on the infant sleepers expressed shock at what he considered to be inadequate medical research supporting the development of the inclined infant sleeper. RV testing is a product in widespread use, with lots of research, and has no better proof of efficacy or safety.
I expected, when I first started practice, that when I was older and grayer I would look back and recall many advances. I anticipated my recall would be of fond memories and of many patients helped. What I didn’t expect was so much of the advice that I provided to be wrong. Perhaps my medical education and parts of the academic research system should be subject to a product recall.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
Regulations and the death of common sense
I jumped in the cab and told the driver to take me straight to hell. “Oh,” he said. “You mean LaGuardia?” He was correct, of course.
It takes me just as long to get to LaGuardia from a hotel in midtown Manhattan as it does for me to fly from Cincinnati to New York. And once you are at the airport, it’s a hot mess. I hear that the terminal update at LaGuardia will be finished in 4 years. This is going to cost $8 billion, plus another $2 billion for an elevated train that connects to the railroad and subway.
Cincinnati built itself a streetcar to nowhere after the city council made a field trip to Portlandia. It was fueled by $45 million in federal “stimulus” grants, disrupted downtown for 9 years instead of 3, and cost $145 million instead of $110.
No one rides the streetcar. It is regularly delayed by people parking on the tracks, and collisions with cars happen regularly. In fact, checking to see if anyone buys a ticket was determined to not be cost effective. The city government cannot close the streetcar for 20 years because the city would otherwise have to give back the $45 million grant used to build it.
How do such boondoggles happen? It was all explained in a book given to me by a new friend in New York. “The Death of Common Sense,” by Philip K. Howard, spells it out.
If you want to fix a problem in any city you must run a gauntlet of meetings and meet regulations, many of which have nothing to do with engineering, quality, or safety. Expect action or approval to take years.
The “you can’t be too careful” movement has assumed a life of its own.
Of course, the same process is true in medicine, only more so! Human lives are at stake, so absolutely no chances can be taken. Medicine is not engineering. And the science of medicine is often so inexact that no one knows when they are taking a chance, or what is the right or wrong thing to do. The paperwork and rules become enormous. The regulations proliferate.
The resulting health care administration costs account for about 25% of health care dollars.
In one recent “you can’t be too careful” moment, I had a Joint Commission inspector tell me he was concerned about patients falling off our power tables when we perform procedures under local anesthesia. Now this has never happened in the last 30 years, but you can’t be too careful! I jokingly suggested we consider giant Velcro straps for the tables, and added that they would be particularly useful for the front office staff chairs. The inspector got excited. He thought giant Velcro straps were a great idea. I am now searching online for giant Velcro straps.
Several years ago, I had a clinical lab improvement inspection and everything was perfect. The inspectors could not find anything wrong, but they had allocated a half day for the inspection. They cast about, and finally insisted I buy a red stamper to indicate on the Mohs maps that the case was clear. I pointed out that a straight line though the map indicated the same thing, and even showed them the colored key codes on the back. No, we must have a red stamp! Now we stamp all the maps, sometimes several times! You can’t be too careful! To head off our next “what can we find” moment, we make sure we leave an expired bottle of stain or tissue dye in the back of the cabinet for the inspectors to find.
Pathologists are expected to report melanomas to the state, but we found out that they were behind in their reporting. So we thought we might help them out with the reporting. What were we thinking!? Upon investigation we obtained an online form that is almost incomprehensible and takes at least an hour to fill out. The form must be submitted online and completed in its entirety. There is a 4-hour webinar to help teach you how to fill it out. I called the state health department to ask for help, I was directed to the webinar, and was told in no uncertain terms that it is serious crime not to report melanoma. Thanks! I will be sure to tell the pathologist.
So avoid LaGuardia Airport for at least 4 more years, come ride the Cincinnati streetcar where you really don’t need a ticket, always leave something small for the inspector to find (mum’s the word), and let me know if you find any giant Velcro straps for sale online!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I jumped in the cab and told the driver to take me straight to hell. “Oh,” he said. “You mean LaGuardia?” He was correct, of course.
It takes me just as long to get to LaGuardia from a hotel in midtown Manhattan as it does for me to fly from Cincinnati to New York. And once you are at the airport, it’s a hot mess. I hear that the terminal update at LaGuardia will be finished in 4 years. This is going to cost $8 billion, plus another $2 billion for an elevated train that connects to the railroad and subway.
Cincinnati built itself a streetcar to nowhere after the city council made a field trip to Portlandia. It was fueled by $45 million in federal “stimulus” grants, disrupted downtown for 9 years instead of 3, and cost $145 million instead of $110.
No one rides the streetcar. It is regularly delayed by people parking on the tracks, and collisions with cars happen regularly. In fact, checking to see if anyone buys a ticket was determined to not be cost effective. The city government cannot close the streetcar for 20 years because the city would otherwise have to give back the $45 million grant used to build it.
How do such boondoggles happen? It was all explained in a book given to me by a new friend in New York. “The Death of Common Sense,” by Philip K. Howard, spells it out.
If you want to fix a problem in any city you must run a gauntlet of meetings and meet regulations, many of which have nothing to do with engineering, quality, or safety. Expect action or approval to take years.
The “you can’t be too careful” movement has assumed a life of its own.
Of course, the same process is true in medicine, only more so! Human lives are at stake, so absolutely no chances can be taken. Medicine is not engineering. And the science of medicine is often so inexact that no one knows when they are taking a chance, or what is the right or wrong thing to do. The paperwork and rules become enormous. The regulations proliferate.
The resulting health care administration costs account for about 25% of health care dollars.
In one recent “you can’t be too careful” moment, I had a Joint Commission inspector tell me he was concerned about patients falling off our power tables when we perform procedures under local anesthesia. Now this has never happened in the last 30 years, but you can’t be too careful! I jokingly suggested we consider giant Velcro straps for the tables, and added that they would be particularly useful for the front office staff chairs. The inspector got excited. He thought giant Velcro straps were a great idea. I am now searching online for giant Velcro straps.
Several years ago, I had a clinical lab improvement inspection and everything was perfect. The inspectors could not find anything wrong, but they had allocated a half day for the inspection. They cast about, and finally insisted I buy a red stamper to indicate on the Mohs maps that the case was clear. I pointed out that a straight line though the map indicated the same thing, and even showed them the colored key codes on the back. No, we must have a red stamp! Now we stamp all the maps, sometimes several times! You can’t be too careful! To head off our next “what can we find” moment, we make sure we leave an expired bottle of stain or tissue dye in the back of the cabinet for the inspectors to find.
Pathologists are expected to report melanomas to the state, but we found out that they were behind in their reporting. So we thought we might help them out with the reporting. What were we thinking!? Upon investigation we obtained an online form that is almost incomprehensible and takes at least an hour to fill out. The form must be submitted online and completed in its entirety. There is a 4-hour webinar to help teach you how to fill it out. I called the state health department to ask for help, I was directed to the webinar, and was told in no uncertain terms that it is serious crime not to report melanoma. Thanks! I will be sure to tell the pathologist.
So avoid LaGuardia Airport for at least 4 more years, come ride the Cincinnati streetcar where you really don’t need a ticket, always leave something small for the inspector to find (mum’s the word), and let me know if you find any giant Velcro straps for sale online!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I jumped in the cab and told the driver to take me straight to hell. “Oh,” he said. “You mean LaGuardia?” He was correct, of course.
It takes me just as long to get to LaGuardia from a hotel in midtown Manhattan as it does for me to fly from Cincinnati to New York. And once you are at the airport, it’s a hot mess. I hear that the terminal update at LaGuardia will be finished in 4 years. This is going to cost $8 billion, plus another $2 billion for an elevated train that connects to the railroad and subway.
Cincinnati built itself a streetcar to nowhere after the city council made a field trip to Portlandia. It was fueled by $45 million in federal “stimulus” grants, disrupted downtown for 9 years instead of 3, and cost $145 million instead of $110.
No one rides the streetcar. It is regularly delayed by people parking on the tracks, and collisions with cars happen regularly. In fact, checking to see if anyone buys a ticket was determined to not be cost effective. The city government cannot close the streetcar for 20 years because the city would otherwise have to give back the $45 million grant used to build it.
How do such boondoggles happen? It was all explained in a book given to me by a new friend in New York. “The Death of Common Sense,” by Philip K. Howard, spells it out.
If you want to fix a problem in any city you must run a gauntlet of meetings and meet regulations, many of which have nothing to do with engineering, quality, or safety. Expect action or approval to take years.
The “you can’t be too careful” movement has assumed a life of its own.
Of course, the same process is true in medicine, only more so! Human lives are at stake, so absolutely no chances can be taken. Medicine is not engineering. And the science of medicine is often so inexact that no one knows when they are taking a chance, or what is the right or wrong thing to do. The paperwork and rules become enormous. The regulations proliferate.
The resulting health care administration costs account for about 25% of health care dollars.
In one recent “you can’t be too careful” moment, I had a Joint Commission inspector tell me he was concerned about patients falling off our power tables when we perform procedures under local anesthesia. Now this has never happened in the last 30 years, but you can’t be too careful! I jokingly suggested we consider giant Velcro straps for the tables, and added that they would be particularly useful for the front office staff chairs. The inspector got excited. He thought giant Velcro straps were a great idea. I am now searching online for giant Velcro straps.
Several years ago, I had a clinical lab improvement inspection and everything was perfect. The inspectors could not find anything wrong, but they had allocated a half day for the inspection. They cast about, and finally insisted I buy a red stamper to indicate on the Mohs maps that the case was clear. I pointed out that a straight line though the map indicated the same thing, and even showed them the colored key codes on the back. No, we must have a red stamp! Now we stamp all the maps, sometimes several times! You can’t be too careful! To head off our next “what can we find” moment, we make sure we leave an expired bottle of stain or tissue dye in the back of the cabinet for the inspectors to find.
Pathologists are expected to report melanomas to the state, but we found out that they were behind in their reporting. So we thought we might help them out with the reporting. What were we thinking!? Upon investigation we obtained an online form that is almost incomprehensible and takes at least an hour to fill out. The form must be submitted online and completed in its entirety. There is a 4-hour webinar to help teach you how to fill it out. I called the state health department to ask for help, I was directed to the webinar, and was told in no uncertain terms that it is serious crime not to report melanoma. Thanks! I will be sure to tell the pathologist.
So avoid LaGuardia Airport for at least 4 more years, come ride the Cincinnati streetcar where you really don’t need a ticket, always leave something small for the inspector to find (mum’s the word), and let me know if you find any giant Velcro straps for sale online!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Diabetic neuropathy: Often silent, often dangerous
SAN DIEGO – Neuropathy can blur the seriousness of injuries, especially in patients with diabetes, and that can lead to severe consequences such as falls, foot ulcers, gangrene, and amputations, said Lucia M. Novak, MSN, ANP-BC, BC-ADM, CDTC, in a presentation on assessing and treating neuropathies at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
As many as half of the cases of diabetic neuropathy may have no symptoms, and more than two-thirds of cases of diabetic neuropathy, even some with obvious symptoms, are ignored or missed by clinicians, said Ms. Novak, director of the Riverside Diabetes Center and adjunct assistant professor at the Uniformed Services University of the Health Sciences, both in Bethesda, Md.
At the same time, she said, diabetic neuropathy is very common. It affects an estimated 10%-15% of newly diagnosed patients with type 2 diabetes, 50% of patients with type 2 disease after 10 years, and as many as 30% of patients with prediabetes.
The condition is less common in type 1 diabetes, affecting an estimated 20% of patients after 20 years, she said.
“All we can do in our patients with type 2 diabetes and diabetic neuropathy, is to slow down the progression, although improving glycemic control can prevent it in type 1,” she said, citing findings suggesting that
A 2012 report analyzed research into the effect of glycemic control on neuropathy in diabetes and found a pair of studies that reported a 60%-70% reduction of risk in patients with type 1 diabetes who received regular insulin dosing. However, the evidence for type 2 diabetes was not as definitive, and analysis of findings from eight randomized, controlled trials in patients with type 2 diabetes supported a relatively small reduction in the development of neuropathy in patients with type 2 diabetes who were receiving enhanced glycemic control (Lancet Neurol. 2012;11[6]:521-34).
Ms. Novak focused mainly on peripheral neuropathy, which is believed to account for 50%-75% of all neuropathy in patients with diabetes. She emphasized the importance of screening because it is crucial for preventing foot ulcers, which affect more than a third of patients with diabetes over their lifetimes.
She recommended following the American Diabetes Association’s 2017 position statement on diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54), beginning with performing a visual examination of the feet at every visit.
Comprehensive screening
In patients with type 1 diabetes, there should be an annual comprehensive screening beginning within 5 years of diagnosis. Patients with type 2 disease should be screened at diagnosis and then annually, as outlined in the ADA statement.
The comprehensive exam involves using tools, such as tuning forks and monofilaments, to test sensation. Different tools are required to test both small and large fibers in the foot, Ms. Novak said, and doing both kinds of testing greatly increases the likelihood of detecting neuropathy.
Check for pulse, bone deformities, dry skin
In addition, “you’ll be feeling for their pulses, looking for bony deformities, and looking at anything is going on between the toes [to make sure] the skin is intact,” she said.
Patients with diabetic neuropathy often have dry skin, she said, so make sure they’re moisturizing. “Look at the condition of their shoes,” she added, “which will tell you how they walk.”
Ill-fitting shoes are a common cause of foot ulcers, said Ms. Novak, who noted that some patients refuse to wear unattractive diabetic shoes and prefer to wear more fashionable – and dangerous – tight-fitting shoes.
Treatment options
Glycemic control makes a difference, especially for patients with type 1, as does control of risk factors, such as obesity. But diabetic neuropathy cannot be reversed.
Pain can be managed with a range of medications. “We can’t cure the neuropathy, we can at least help patients with the symptoms so that they can have a good night’s sleep,” she said.
Ms. Novak also suggested passing on the following snippets of advice to patients:
- Do not walk barefoot.
- Check your feet every day.
- Moisturize your skin, and always dry thoroughly between your toes.
- Seek medical attention if your nails cut into your skin or you develop a callus or areas of redness/warmth.
Global Academy and this news organization are owned by the same parent company. Ms. Novak reported relationships with Nova Nordisk, Sanofi, Janssen, and AstraZeneca.
SAN DIEGO – Neuropathy can blur the seriousness of injuries, especially in patients with diabetes, and that can lead to severe consequences such as falls, foot ulcers, gangrene, and amputations, said Lucia M. Novak, MSN, ANP-BC, BC-ADM, CDTC, in a presentation on assessing and treating neuropathies at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
As many as half of the cases of diabetic neuropathy may have no symptoms, and more than two-thirds of cases of diabetic neuropathy, even some with obvious symptoms, are ignored or missed by clinicians, said Ms. Novak, director of the Riverside Diabetes Center and adjunct assistant professor at the Uniformed Services University of the Health Sciences, both in Bethesda, Md.
At the same time, she said, diabetic neuropathy is very common. It affects an estimated 10%-15% of newly diagnosed patients with type 2 diabetes, 50% of patients with type 2 disease after 10 years, and as many as 30% of patients with prediabetes.
The condition is less common in type 1 diabetes, affecting an estimated 20% of patients after 20 years, she said.
“All we can do in our patients with type 2 diabetes and diabetic neuropathy, is to slow down the progression, although improving glycemic control can prevent it in type 1,” she said, citing findings suggesting that
A 2012 report analyzed research into the effect of glycemic control on neuropathy in diabetes and found a pair of studies that reported a 60%-70% reduction of risk in patients with type 1 diabetes who received regular insulin dosing. However, the evidence for type 2 diabetes was not as definitive, and analysis of findings from eight randomized, controlled trials in patients with type 2 diabetes supported a relatively small reduction in the development of neuropathy in patients with type 2 diabetes who were receiving enhanced glycemic control (Lancet Neurol. 2012;11[6]:521-34).
Ms. Novak focused mainly on peripheral neuropathy, which is believed to account for 50%-75% of all neuropathy in patients with diabetes. She emphasized the importance of screening because it is crucial for preventing foot ulcers, which affect more than a third of patients with diabetes over their lifetimes.
She recommended following the American Diabetes Association’s 2017 position statement on diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54), beginning with performing a visual examination of the feet at every visit.
Comprehensive screening
In patients with type 1 diabetes, there should be an annual comprehensive screening beginning within 5 years of diagnosis. Patients with type 2 disease should be screened at diagnosis and then annually, as outlined in the ADA statement.
The comprehensive exam involves using tools, such as tuning forks and monofilaments, to test sensation. Different tools are required to test both small and large fibers in the foot, Ms. Novak said, and doing both kinds of testing greatly increases the likelihood of detecting neuropathy.
Check for pulse, bone deformities, dry skin
In addition, “you’ll be feeling for their pulses, looking for bony deformities, and looking at anything is going on between the toes [to make sure] the skin is intact,” she said.
Patients with diabetic neuropathy often have dry skin, she said, so make sure they’re moisturizing. “Look at the condition of their shoes,” she added, “which will tell you how they walk.”
Ill-fitting shoes are a common cause of foot ulcers, said Ms. Novak, who noted that some patients refuse to wear unattractive diabetic shoes and prefer to wear more fashionable – and dangerous – tight-fitting shoes.
Treatment options
Glycemic control makes a difference, especially for patients with type 1, as does control of risk factors, such as obesity. But diabetic neuropathy cannot be reversed.
Pain can be managed with a range of medications. “We can’t cure the neuropathy, we can at least help patients with the symptoms so that they can have a good night’s sleep,” she said.
Ms. Novak also suggested passing on the following snippets of advice to patients:
- Do not walk barefoot.
- Check your feet every day.
- Moisturize your skin, and always dry thoroughly between your toes.
- Seek medical attention if your nails cut into your skin or you develop a callus or areas of redness/warmth.
Global Academy and this news organization are owned by the same parent company. Ms. Novak reported relationships with Nova Nordisk, Sanofi, Janssen, and AstraZeneca.
SAN DIEGO – Neuropathy can blur the seriousness of injuries, especially in patients with diabetes, and that can lead to severe consequences such as falls, foot ulcers, gangrene, and amputations, said Lucia M. Novak, MSN, ANP-BC, BC-ADM, CDTC, in a presentation on assessing and treating neuropathies at the Metabolic & Endocrine Disease Summit, sponsored by Global Academy for Medical Education.
As many as half of the cases of diabetic neuropathy may have no symptoms, and more than two-thirds of cases of diabetic neuropathy, even some with obvious symptoms, are ignored or missed by clinicians, said Ms. Novak, director of the Riverside Diabetes Center and adjunct assistant professor at the Uniformed Services University of the Health Sciences, both in Bethesda, Md.
At the same time, she said, diabetic neuropathy is very common. It affects an estimated 10%-15% of newly diagnosed patients with type 2 diabetes, 50% of patients with type 2 disease after 10 years, and as many as 30% of patients with prediabetes.
The condition is less common in type 1 diabetes, affecting an estimated 20% of patients after 20 years, she said.
“All we can do in our patients with type 2 diabetes and diabetic neuropathy, is to slow down the progression, although improving glycemic control can prevent it in type 1,” she said, citing findings suggesting that
A 2012 report analyzed research into the effect of glycemic control on neuropathy in diabetes and found a pair of studies that reported a 60%-70% reduction of risk in patients with type 1 diabetes who received regular insulin dosing. However, the evidence for type 2 diabetes was not as definitive, and analysis of findings from eight randomized, controlled trials in patients with type 2 diabetes supported a relatively small reduction in the development of neuropathy in patients with type 2 diabetes who were receiving enhanced glycemic control (Lancet Neurol. 2012;11[6]:521-34).
Ms. Novak focused mainly on peripheral neuropathy, which is believed to account for 50%-75% of all neuropathy in patients with diabetes. She emphasized the importance of screening because it is crucial for preventing foot ulcers, which affect more than a third of patients with diabetes over their lifetimes.
She recommended following the American Diabetes Association’s 2017 position statement on diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54), beginning with performing a visual examination of the feet at every visit.
Comprehensive screening
In patients with type 1 diabetes, there should be an annual comprehensive screening beginning within 5 years of diagnosis. Patients with type 2 disease should be screened at diagnosis and then annually, as outlined in the ADA statement.
The comprehensive exam involves using tools, such as tuning forks and monofilaments, to test sensation. Different tools are required to test both small and large fibers in the foot, Ms. Novak said, and doing both kinds of testing greatly increases the likelihood of detecting neuropathy.
Check for pulse, bone deformities, dry skin
In addition, “you’ll be feeling for their pulses, looking for bony deformities, and looking at anything is going on between the toes [to make sure] the skin is intact,” she said.
Patients with diabetic neuropathy often have dry skin, she said, so make sure they’re moisturizing. “Look at the condition of their shoes,” she added, “which will tell you how they walk.”
Ill-fitting shoes are a common cause of foot ulcers, said Ms. Novak, who noted that some patients refuse to wear unattractive diabetic shoes and prefer to wear more fashionable – and dangerous – tight-fitting shoes.
Treatment options
Glycemic control makes a difference, especially for patients with type 1, as does control of risk factors, such as obesity. But diabetic neuropathy cannot be reversed.
Pain can be managed with a range of medications. “We can’t cure the neuropathy, we can at least help patients with the symptoms so that they can have a good night’s sleep,” she said.
Ms. Novak also suggested passing on the following snippets of advice to patients:
- Do not walk barefoot.
- Check your feet every day.
- Moisturize your skin, and always dry thoroughly between your toes.
- Seek medical attention if your nails cut into your skin or you develop a callus or areas of redness/warmth.
Global Academy and this news organization are owned by the same parent company. Ms. Novak reported relationships with Nova Nordisk, Sanofi, Janssen, and AstraZeneca.
EXPERT ANALYSIS FROM MEDS 2019
Zanubrutinib may be poised to challenge ibrutinib for CLL
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
FROM BLOOD
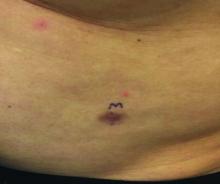
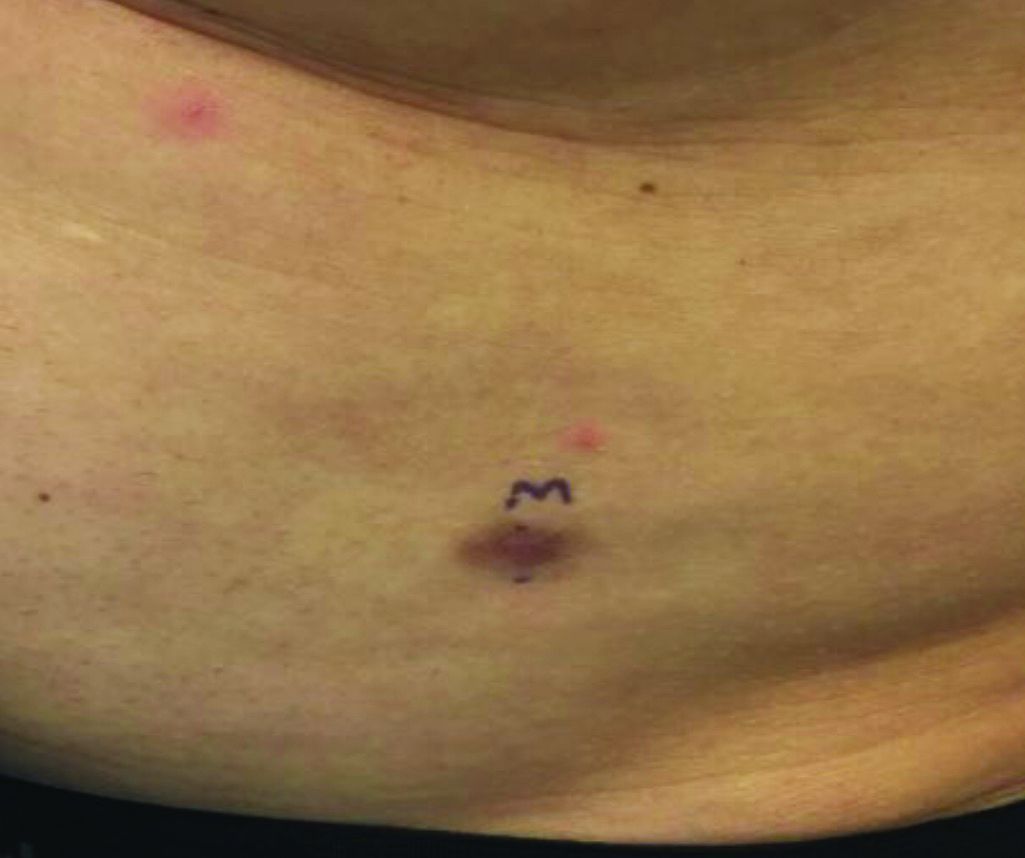
An asymptomatic reddish-brown plaque in a healthy adult man
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Lower BMD found in patients with severe hemophilia A
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
FROM HAEMOPHILIA
Diagnosing and managing diabetes and depression
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
EXPERT ANALYSIS FROM MEDS 2019
Evinacumab shows promise for HoFH in top line results
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
Boiling Points
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.
EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.
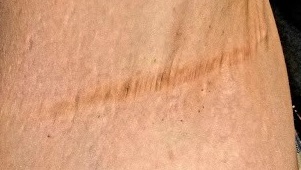
Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.

EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.

Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.

EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.

Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.