User login
Timing of adjuvant treatment impacts pancreatic cancer survival
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
FROM JAMA NETWORK OPEN
Tamoxifen benefit in lower-risk breast cancer varies by intrinsic subtype
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
FROM JAMA ONCOLOGY
Safety of ondansetron for nausea and vomiting of pregnancy
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
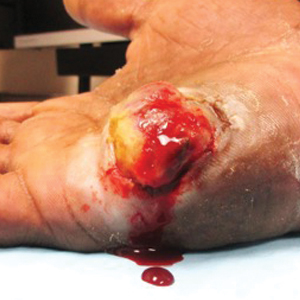
Well-Circumscribed Tumor on the Hand
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
A 52-year-old man presented to the dermatology clinic with a 2×3-cm, fungating, dome-shaped, ulcerative, moist, well-circumscribed tumor with peripheral maceration on the volar aspect of the right hand of 3 months’ duration. The tumor was malodorous, painful, and hemorrhaged easily with minimal trauma. The patient’s medical history was notable for human immunodeficiency virus and latent syphilis, with elevated rapid plasma reagin titers and a positive Treponema palladium antibody on chemiluminescent immunoassay, that was refractory to 3 treatments with penicillin. The patient was not on antiretroviral therapy. He had a CD4+ lymphocyte count of 980 cells/µL (reference range, 359–1519 cells/µL) and a viral load of 8560 copies/mL (reference range, <200 copies/mL). No other skin or systemic concerns were noted, and the patient denied any recent travel, exposure to animals, or constitutional symptoms. A deep shave biopsy of the lesion was performed.
Ketogenic diets are what’s cooking for drug-refractory epilepsy
BANGKOK – For a form of epilepsy treatment that’s been around since the 1920s, ketogenic diet therapy has lately been the focus of a surprising wealth of clinical research and development, Suvasini Sharma, MD, observed at the International Epilepsy Congress.
This high-fat, low-carbohydrate diet is now well established as a valid and effective treatment option for children and adults with drug-refractory epilepsy who aren’t candidates for surgery. That’s about a third of all epilepsy patients. And as the recently overhauled pediatric ketogenic diet therapy (KDT) best practice consensus guidelines emphasize, KDT should be strongly considered after two antiepileptic drugs have failed, and even earlier for several epilepsy syndromes, noted Dr. Sharma, a pediatric neurologist at Lady Hardinge Medical College and Kalawati Saran Children’s Hospital in New Delhi, and a coauthor of the updated guidelines.
“The consensus guidelines recommend that you start thinking about the diet early, without waiting for every drug to fail,” she said at the congress, sponsored by the International League Against Epilepsy.
Among the KDT-related topics she highlighted were the recently revised best practice consensus guidelines; an expanding role for KDT in infants, critical care settings, and in epileptic encephalopathies; mounting evidence that KDT provides additional benefits beyond seizure control; and promising new alternative diet therapies. She also described the challenges of using KDT in a low-resource nation such as India, where most of the 1.3 billion people shop in markets where food isn’t packaged with the nutritional content labels essential to traditional KDTs, and low literacy is common.
KDT best practice guidelines
The latest guidelines, which include the details of standardized KDT protocols as well as a summary of recent translational research into mechanisms of action, replace the previous 10-year-old version. Flexibility is now the watchword. While the classic KDT was started as an inpatient intervention involving several days of fasting followed by multiday gradual reintroduction of calories, that approach is now deemed optional (Epilepsia Open. 2018 May 21;3[2]:175-92).
“By and large, the trend now is going to nonfasting initiation on an outpatient basis, but with more stringent monitoring,” according to Dr. Sharma.
The guidelines note that while the research literature shows that, on average, KDT results in about a 50% chance of at least a 50% reduction in seizure frequency in patients with drug-refractory epilepsy, there are a dozen specific conditions with 70% or greater responder rates: infantile spasms, tuberous sclerosis, epilepsy with myoclonic-atonic seizures, Dravet syndrome, glucose transporter 1 deficiency syndrome (Glut 1DS), pyruvate dehydrogenase deficiency (PDHD), febrile infection-related epilepsy syndrome (FIRES), super-refractory status epilepticus (SRSE), Ohtahara syndrome, complex I mitochondrial disorders, Angelman syndrome, and children with gastrostomy tubes. For Glut1DS and PDHD, KDTs should be considered the treatment of first choice.
Traditionally, KDTs weren’t recommended for children younger than age 2 years. There were concerns that maintaining ketosis and meeting growth requirements were contradictory goals. That’s no longer believed to be so. Indeed, current evidence shows that KDT is highly effective and well tolerated in infants with refractory epilepsy. European guidelines address patient selection, pre-KDT counseling, preferred methods of initiation and KDT discontinuation, and other key issues (Eur J Paediatr Neurol. 2016 Nov;20[6]:798-809).
The guidelines recognize four major, well-studied types of KDT: the classic long-chain triglyceride-centric diet; the medium-chain triglyceride diet; the more user-friendly modified Atkins diet; and low glycemic index therapy. Except in children younger than 2 years old, who should be started on the classic KDT, the consensus panel recommended that the specific KDT selected should be based on the family and child situation and the expertise at the local KDT center. Perceived differences in efficacy between the diets aren’t supported by persuasive evidence.
KDT benefits beyond seizure control
“Most of us who work in the diet scene are aware that patients often report increased alertness, and sometimes improved cognition,” said Dr. Sharma.
That subjective experience is now supported by evidence from a randomized, controlled trial. Dutch investigators who randomized 50 drug-refractory pediatric epilepsy patients to KDT or usual care documented a positive impact of the diet therapy on cognitive activation, mood, and anxious behavior (Epilepsy Behav. 2016 Jul;60:153-7).
More recently, a systematic review showed that while subjective assessments support claims of improved alertness, attention, and global cognition in patients on KDT for refractory epilepsy, structured neuropsychologic testing confirms the enhanced alertness but without significantly improved global cognition. The investigators reported that the improvements were unrelated to decreases in medication, the type of KDT or age at its introduction, or sleep improvement. Rather, the benefits appeared to be due to a combination of seizure reduction and direct effects of KDT on cognition (Epilepsy Behav. 2018 Oct;87:69-77).
There is also encouraging preliminary evidence of a possible protective effect of KDT against sudden unexpected death in epilepsy (SUDEP) in a mouse model (Epilepsia. 2016 Aug;57[8]:e178-82. doi: 10.1111/epi.13444).
The use of KDT in critical care settings
Investigators from the pediatric Status Epilepticus Research Group (pSERG) reported that 10 of 14 patients with convulsive refractory status epilepticus achieved EEG seizure resolution within 7 days after starting KDT. Moreover, 11 patients were able to be weaned off their continuous infusions within 14 days of starting KDT. Treatment-emergent gastroparesis and hypertriglyceridemia occurred in three patients (Epilepsy Res. 2018 Aug;144:1-6).
“It was reasonably well tolerated, but they started it quite late – a median of 13 days after onset of refractory status epilepticus. It should come much earlier on our list of therapies. We shouldn’t be waiting 2 weeks before going to the ketogenic diet, because we can diagnose refractory status epilepticus within 48 hours after arrival in the ICU most of the time,” Dr. Sharma said.
Austrian investigators have pioneered the use of intravenous KDT as a bridge when oral therapy is temporarily impossible because of status epilepticus, surgery, or other reasons. They reported that parental KDT with fat intake of 3.5-4 g/kg per day was safe and effective in their series of 17 young children with epilepsy (Epilepsia Open. 2017 Nov 16;3[1]:30-9).
The future: nonketogenic diet therapies
KDT in its various forms is just too demanding and restrictive for some patients. Nonketotic alternatives are being explored.
Triheptanoin is a synthetic medium-chain triglyceride in the form of an edible, odorless, tasteless oil. Its mechanism of action is by anaplerosis: that is, energy generation via replenishment of the tricarboxylic acid cycle. After demonstration of neuroprotective and anticonvulsant effects in several mouse models, Australian investigators conducted a pilot study of 30- to 100-mL/day of oral triheptanoin as add-on therapy in 12 children with drug-refractory epilepsy. Eight of the 12 took triheptanoin for longer than 12 weeks, and 5 of those 8 experienced a sustained greater than 50% reduction in seizure frequency, including 1 who remained seizure free for 30 weeks. Seven children had diarrhea or other GI side effects (Eur J Paediatr Neurol. 2018 Nov;22[6]:1074-80).
Parisian investigators have developed a nonketotic, palatable combination of amino acids, carbohydrates, and fatty acids with a low ratio of fat to protein-plus-carbohydrates that provided potent protection against seizures in a mouse model. This suggests that the traditional 4:1 ratio sought in KDT isn’t necessary for robust seizure reduction (Sci Rep. 2017 Jul 14;7[1]:5496).
“This is probably going to be the future of nutritional therapy in epilepsy,” Dr. Sharma predicted.
She reported having no financial conflicts regarding her presentation.
BANGKOK – For a form of epilepsy treatment that’s been around since the 1920s, ketogenic diet therapy has lately been the focus of a surprising wealth of clinical research and development, Suvasini Sharma, MD, observed at the International Epilepsy Congress.
This high-fat, low-carbohydrate diet is now well established as a valid and effective treatment option for children and adults with drug-refractory epilepsy who aren’t candidates for surgery. That’s about a third of all epilepsy patients. And as the recently overhauled pediatric ketogenic diet therapy (KDT) best practice consensus guidelines emphasize, KDT should be strongly considered after two antiepileptic drugs have failed, and even earlier for several epilepsy syndromes, noted Dr. Sharma, a pediatric neurologist at Lady Hardinge Medical College and Kalawati Saran Children’s Hospital in New Delhi, and a coauthor of the updated guidelines.
“The consensus guidelines recommend that you start thinking about the diet early, without waiting for every drug to fail,” she said at the congress, sponsored by the International League Against Epilepsy.
Among the KDT-related topics she highlighted were the recently revised best practice consensus guidelines; an expanding role for KDT in infants, critical care settings, and in epileptic encephalopathies; mounting evidence that KDT provides additional benefits beyond seizure control; and promising new alternative diet therapies. She also described the challenges of using KDT in a low-resource nation such as India, where most of the 1.3 billion people shop in markets where food isn’t packaged with the nutritional content labels essential to traditional KDTs, and low literacy is common.
KDT best practice guidelines
The latest guidelines, which include the details of standardized KDT protocols as well as a summary of recent translational research into mechanisms of action, replace the previous 10-year-old version. Flexibility is now the watchword. While the classic KDT was started as an inpatient intervention involving several days of fasting followed by multiday gradual reintroduction of calories, that approach is now deemed optional (Epilepsia Open. 2018 May 21;3[2]:175-92).
“By and large, the trend now is going to nonfasting initiation on an outpatient basis, but with more stringent monitoring,” according to Dr. Sharma.
The guidelines note that while the research literature shows that, on average, KDT results in about a 50% chance of at least a 50% reduction in seizure frequency in patients with drug-refractory epilepsy, there are a dozen specific conditions with 70% or greater responder rates: infantile spasms, tuberous sclerosis, epilepsy with myoclonic-atonic seizures, Dravet syndrome, glucose transporter 1 deficiency syndrome (Glut 1DS), pyruvate dehydrogenase deficiency (PDHD), febrile infection-related epilepsy syndrome (FIRES), super-refractory status epilepticus (SRSE), Ohtahara syndrome, complex I mitochondrial disorders, Angelman syndrome, and children with gastrostomy tubes. For Glut1DS and PDHD, KDTs should be considered the treatment of first choice.
Traditionally, KDTs weren’t recommended for children younger than age 2 years. There were concerns that maintaining ketosis and meeting growth requirements were contradictory goals. That’s no longer believed to be so. Indeed, current evidence shows that KDT is highly effective and well tolerated in infants with refractory epilepsy. European guidelines address patient selection, pre-KDT counseling, preferred methods of initiation and KDT discontinuation, and other key issues (Eur J Paediatr Neurol. 2016 Nov;20[6]:798-809).
The guidelines recognize four major, well-studied types of KDT: the classic long-chain triglyceride-centric diet; the medium-chain triglyceride diet; the more user-friendly modified Atkins diet; and low glycemic index therapy. Except in children younger than 2 years old, who should be started on the classic KDT, the consensus panel recommended that the specific KDT selected should be based on the family and child situation and the expertise at the local KDT center. Perceived differences in efficacy between the diets aren’t supported by persuasive evidence.
KDT benefits beyond seizure control
“Most of us who work in the diet scene are aware that patients often report increased alertness, and sometimes improved cognition,” said Dr. Sharma.
That subjective experience is now supported by evidence from a randomized, controlled trial. Dutch investigators who randomized 50 drug-refractory pediatric epilepsy patients to KDT or usual care documented a positive impact of the diet therapy on cognitive activation, mood, and anxious behavior (Epilepsy Behav. 2016 Jul;60:153-7).
More recently, a systematic review showed that while subjective assessments support claims of improved alertness, attention, and global cognition in patients on KDT for refractory epilepsy, structured neuropsychologic testing confirms the enhanced alertness but without significantly improved global cognition. The investigators reported that the improvements were unrelated to decreases in medication, the type of KDT or age at its introduction, or sleep improvement. Rather, the benefits appeared to be due to a combination of seizure reduction and direct effects of KDT on cognition (Epilepsy Behav. 2018 Oct;87:69-77).
There is also encouraging preliminary evidence of a possible protective effect of KDT against sudden unexpected death in epilepsy (SUDEP) in a mouse model (Epilepsia. 2016 Aug;57[8]:e178-82. doi: 10.1111/epi.13444).
The use of KDT in critical care settings
Investigators from the pediatric Status Epilepticus Research Group (pSERG) reported that 10 of 14 patients with convulsive refractory status epilepticus achieved EEG seizure resolution within 7 days after starting KDT. Moreover, 11 patients were able to be weaned off their continuous infusions within 14 days of starting KDT. Treatment-emergent gastroparesis and hypertriglyceridemia occurred in three patients (Epilepsy Res. 2018 Aug;144:1-6).
“It was reasonably well tolerated, but they started it quite late – a median of 13 days after onset of refractory status epilepticus. It should come much earlier on our list of therapies. We shouldn’t be waiting 2 weeks before going to the ketogenic diet, because we can diagnose refractory status epilepticus within 48 hours after arrival in the ICU most of the time,” Dr. Sharma said.
Austrian investigators have pioneered the use of intravenous KDT as a bridge when oral therapy is temporarily impossible because of status epilepticus, surgery, or other reasons. They reported that parental KDT with fat intake of 3.5-4 g/kg per day was safe and effective in their series of 17 young children with epilepsy (Epilepsia Open. 2017 Nov 16;3[1]:30-9).
The future: nonketogenic diet therapies
KDT in its various forms is just too demanding and restrictive for some patients. Nonketotic alternatives are being explored.
Triheptanoin is a synthetic medium-chain triglyceride in the form of an edible, odorless, tasteless oil. Its mechanism of action is by anaplerosis: that is, energy generation via replenishment of the tricarboxylic acid cycle. After demonstration of neuroprotective and anticonvulsant effects in several mouse models, Australian investigators conducted a pilot study of 30- to 100-mL/day of oral triheptanoin as add-on therapy in 12 children with drug-refractory epilepsy. Eight of the 12 took triheptanoin for longer than 12 weeks, and 5 of those 8 experienced a sustained greater than 50% reduction in seizure frequency, including 1 who remained seizure free for 30 weeks. Seven children had diarrhea or other GI side effects (Eur J Paediatr Neurol. 2018 Nov;22[6]:1074-80).
Parisian investigators have developed a nonketotic, palatable combination of amino acids, carbohydrates, and fatty acids with a low ratio of fat to protein-plus-carbohydrates that provided potent protection against seizures in a mouse model. This suggests that the traditional 4:1 ratio sought in KDT isn’t necessary for robust seizure reduction (Sci Rep. 2017 Jul 14;7[1]:5496).
“This is probably going to be the future of nutritional therapy in epilepsy,” Dr. Sharma predicted.
She reported having no financial conflicts regarding her presentation.
BANGKOK – For a form of epilepsy treatment that’s been around since the 1920s, ketogenic diet therapy has lately been the focus of a surprising wealth of clinical research and development, Suvasini Sharma, MD, observed at the International Epilepsy Congress.
This high-fat, low-carbohydrate diet is now well established as a valid and effective treatment option for children and adults with drug-refractory epilepsy who aren’t candidates for surgery. That’s about a third of all epilepsy patients. And as the recently overhauled pediatric ketogenic diet therapy (KDT) best practice consensus guidelines emphasize, KDT should be strongly considered after two antiepileptic drugs have failed, and even earlier for several epilepsy syndromes, noted Dr. Sharma, a pediatric neurologist at Lady Hardinge Medical College and Kalawati Saran Children’s Hospital in New Delhi, and a coauthor of the updated guidelines.
“The consensus guidelines recommend that you start thinking about the diet early, without waiting for every drug to fail,” she said at the congress, sponsored by the International League Against Epilepsy.
Among the KDT-related topics she highlighted were the recently revised best practice consensus guidelines; an expanding role for KDT in infants, critical care settings, and in epileptic encephalopathies; mounting evidence that KDT provides additional benefits beyond seizure control; and promising new alternative diet therapies. She also described the challenges of using KDT in a low-resource nation such as India, where most of the 1.3 billion people shop in markets where food isn’t packaged with the nutritional content labels essential to traditional KDTs, and low literacy is common.
KDT best practice guidelines
The latest guidelines, which include the details of standardized KDT protocols as well as a summary of recent translational research into mechanisms of action, replace the previous 10-year-old version. Flexibility is now the watchword. While the classic KDT was started as an inpatient intervention involving several days of fasting followed by multiday gradual reintroduction of calories, that approach is now deemed optional (Epilepsia Open. 2018 May 21;3[2]:175-92).
“By and large, the trend now is going to nonfasting initiation on an outpatient basis, but with more stringent monitoring,” according to Dr. Sharma.
The guidelines note that while the research literature shows that, on average, KDT results in about a 50% chance of at least a 50% reduction in seizure frequency in patients with drug-refractory epilepsy, there are a dozen specific conditions with 70% or greater responder rates: infantile spasms, tuberous sclerosis, epilepsy with myoclonic-atonic seizures, Dravet syndrome, glucose transporter 1 deficiency syndrome (Glut 1DS), pyruvate dehydrogenase deficiency (PDHD), febrile infection-related epilepsy syndrome (FIRES), super-refractory status epilepticus (SRSE), Ohtahara syndrome, complex I mitochondrial disorders, Angelman syndrome, and children with gastrostomy tubes. For Glut1DS and PDHD, KDTs should be considered the treatment of first choice.
Traditionally, KDTs weren’t recommended for children younger than age 2 years. There were concerns that maintaining ketosis and meeting growth requirements were contradictory goals. That’s no longer believed to be so. Indeed, current evidence shows that KDT is highly effective and well tolerated in infants with refractory epilepsy. European guidelines address patient selection, pre-KDT counseling, preferred methods of initiation and KDT discontinuation, and other key issues (Eur J Paediatr Neurol. 2016 Nov;20[6]:798-809).
The guidelines recognize four major, well-studied types of KDT: the classic long-chain triglyceride-centric diet; the medium-chain triglyceride diet; the more user-friendly modified Atkins diet; and low glycemic index therapy. Except in children younger than 2 years old, who should be started on the classic KDT, the consensus panel recommended that the specific KDT selected should be based on the family and child situation and the expertise at the local KDT center. Perceived differences in efficacy between the diets aren’t supported by persuasive evidence.
KDT benefits beyond seizure control
“Most of us who work in the diet scene are aware that patients often report increased alertness, and sometimes improved cognition,” said Dr. Sharma.
That subjective experience is now supported by evidence from a randomized, controlled trial. Dutch investigators who randomized 50 drug-refractory pediatric epilepsy patients to KDT or usual care documented a positive impact of the diet therapy on cognitive activation, mood, and anxious behavior (Epilepsy Behav. 2016 Jul;60:153-7).
More recently, a systematic review showed that while subjective assessments support claims of improved alertness, attention, and global cognition in patients on KDT for refractory epilepsy, structured neuropsychologic testing confirms the enhanced alertness but without significantly improved global cognition. The investigators reported that the improvements were unrelated to decreases in medication, the type of KDT or age at its introduction, or sleep improvement. Rather, the benefits appeared to be due to a combination of seizure reduction and direct effects of KDT on cognition (Epilepsy Behav. 2018 Oct;87:69-77).
There is also encouraging preliminary evidence of a possible protective effect of KDT against sudden unexpected death in epilepsy (SUDEP) in a mouse model (Epilepsia. 2016 Aug;57[8]:e178-82. doi: 10.1111/epi.13444).
The use of KDT in critical care settings
Investigators from the pediatric Status Epilepticus Research Group (pSERG) reported that 10 of 14 patients with convulsive refractory status epilepticus achieved EEG seizure resolution within 7 days after starting KDT. Moreover, 11 patients were able to be weaned off their continuous infusions within 14 days of starting KDT. Treatment-emergent gastroparesis and hypertriglyceridemia occurred in three patients (Epilepsy Res. 2018 Aug;144:1-6).
“It was reasonably well tolerated, but they started it quite late – a median of 13 days after onset of refractory status epilepticus. It should come much earlier on our list of therapies. We shouldn’t be waiting 2 weeks before going to the ketogenic diet, because we can diagnose refractory status epilepticus within 48 hours after arrival in the ICU most of the time,” Dr. Sharma said.
Austrian investigators have pioneered the use of intravenous KDT as a bridge when oral therapy is temporarily impossible because of status epilepticus, surgery, or other reasons. They reported that parental KDT with fat intake of 3.5-4 g/kg per day was safe and effective in their series of 17 young children with epilepsy (Epilepsia Open. 2017 Nov 16;3[1]:30-9).
The future: nonketogenic diet therapies
KDT in its various forms is just too demanding and restrictive for some patients. Nonketotic alternatives are being explored.
Triheptanoin is a synthetic medium-chain triglyceride in the form of an edible, odorless, tasteless oil. Its mechanism of action is by anaplerosis: that is, energy generation via replenishment of the tricarboxylic acid cycle. After demonstration of neuroprotective and anticonvulsant effects in several mouse models, Australian investigators conducted a pilot study of 30- to 100-mL/day of oral triheptanoin as add-on therapy in 12 children with drug-refractory epilepsy. Eight of the 12 took triheptanoin for longer than 12 weeks, and 5 of those 8 experienced a sustained greater than 50% reduction in seizure frequency, including 1 who remained seizure free for 30 weeks. Seven children had diarrhea or other GI side effects (Eur J Paediatr Neurol. 2018 Nov;22[6]:1074-80).
Parisian investigators have developed a nonketotic, palatable combination of amino acids, carbohydrates, and fatty acids with a low ratio of fat to protein-plus-carbohydrates that provided potent protection against seizures in a mouse model. This suggests that the traditional 4:1 ratio sought in KDT isn’t necessary for robust seizure reduction (Sci Rep. 2017 Jul 14;7[1]:5496).
“This is probably going to be the future of nutritional therapy in epilepsy,” Dr. Sharma predicted.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM IEC 2019
Asthma hospitalization in kids linked with doubled migraine incidence
when compared with a similar pediatric population without asthma. The finding is based on an analysis of more than 11 million U.S. pediatric hospitalizations over the course of a decade.
Among children and adolescents aged 3-21 years who were hospitalized for asthma, migraine rates were significantly higher among girls, adolescents, and whites, compared with boys, children aged 12 years or younger, and nonwhites, respectively, in a trio of adjusted analyses, Riddhiben S. Patel, MD, and associates reported in a poster at the annual meeting of the American Headache Society.
“Our hope is that, by establishing an association between childhood asthma and migraine, [these children] may be more easily screened for, diagnosed, and treated early by providers,” wrote Dr. Patel, a pediatric neurologist and headache specialist at the University of Mississippi, Jackson, and associates.
Their analysis used administrative billing data collected by the Kids’ Inpatient Database, maintained by the U.S. Healthcare Cost and Utilization Project. The project includes a representative national sample of about 3 million pediatric hospital discharges every 3 years. The study used data from 11,483,103 hospitalizations of children and adolescents aged 3-21 years during 2003, 2006, 2009, and 2012, and found an overall hospitalization rate of 0.8% billed for migraine. For patients also hospitalized with a billing code for asthma, the rate jumped to 1.36%, a 120% statistically significant relative increase in migraine hospitalizations after adjustment for baseline demographic differences, the researchers said.
Among the children and adolescents hospitalized with an asthma billing code, the relative rate of also having a billing code for migraine after adjustment was a statistically significant 80% higher in girls, compared with boys, a statistically significant 7% higher in adolescents, compared with children 12 years or younger, and was significantly reduced by a relative 45% rate in nonwhites, compared with whites.
The mechanisms behind these associations are not known, but could involve mast-cell degranulation, autonomic dysfunction, or shared genetic or environmental etiologic factors, the authors said.
Dr. Patel reported no relevant disclosures.
SOURCE: Patel RS et al. Headache. 2019 June;59[S1]:1-208, Abstract P78.
when compared with a similar pediatric population without asthma. The finding is based on an analysis of more than 11 million U.S. pediatric hospitalizations over the course of a decade.
Among children and adolescents aged 3-21 years who were hospitalized for asthma, migraine rates were significantly higher among girls, adolescents, and whites, compared with boys, children aged 12 years or younger, and nonwhites, respectively, in a trio of adjusted analyses, Riddhiben S. Patel, MD, and associates reported in a poster at the annual meeting of the American Headache Society.
“Our hope is that, by establishing an association between childhood asthma and migraine, [these children] may be more easily screened for, diagnosed, and treated early by providers,” wrote Dr. Patel, a pediatric neurologist and headache specialist at the University of Mississippi, Jackson, and associates.
Their analysis used administrative billing data collected by the Kids’ Inpatient Database, maintained by the U.S. Healthcare Cost and Utilization Project. The project includes a representative national sample of about 3 million pediatric hospital discharges every 3 years. The study used data from 11,483,103 hospitalizations of children and adolescents aged 3-21 years during 2003, 2006, 2009, and 2012, and found an overall hospitalization rate of 0.8% billed for migraine. For patients also hospitalized with a billing code for asthma, the rate jumped to 1.36%, a 120% statistically significant relative increase in migraine hospitalizations after adjustment for baseline demographic differences, the researchers said.
Among the children and adolescents hospitalized with an asthma billing code, the relative rate of also having a billing code for migraine after adjustment was a statistically significant 80% higher in girls, compared with boys, a statistically significant 7% higher in adolescents, compared with children 12 years or younger, and was significantly reduced by a relative 45% rate in nonwhites, compared with whites.
The mechanisms behind these associations are not known, but could involve mast-cell degranulation, autonomic dysfunction, or shared genetic or environmental etiologic factors, the authors said.
Dr. Patel reported no relevant disclosures.
SOURCE: Patel RS et al. Headache. 2019 June;59[S1]:1-208, Abstract P78.
when compared with a similar pediatric population without asthma. The finding is based on an analysis of more than 11 million U.S. pediatric hospitalizations over the course of a decade.
Among children and adolescents aged 3-21 years who were hospitalized for asthma, migraine rates were significantly higher among girls, adolescents, and whites, compared with boys, children aged 12 years or younger, and nonwhites, respectively, in a trio of adjusted analyses, Riddhiben S. Patel, MD, and associates reported in a poster at the annual meeting of the American Headache Society.
“Our hope is that, by establishing an association between childhood asthma and migraine, [these children] may be more easily screened for, diagnosed, and treated early by providers,” wrote Dr. Patel, a pediatric neurologist and headache specialist at the University of Mississippi, Jackson, and associates.
Their analysis used administrative billing data collected by the Kids’ Inpatient Database, maintained by the U.S. Healthcare Cost and Utilization Project. The project includes a representative national sample of about 3 million pediatric hospital discharges every 3 years. The study used data from 11,483,103 hospitalizations of children and adolescents aged 3-21 years during 2003, 2006, 2009, and 2012, and found an overall hospitalization rate of 0.8% billed for migraine. For patients also hospitalized with a billing code for asthma, the rate jumped to 1.36%, a 120% statistically significant relative increase in migraine hospitalizations after adjustment for baseline demographic differences, the researchers said.
Among the children and adolescents hospitalized with an asthma billing code, the relative rate of also having a billing code for migraine after adjustment was a statistically significant 80% higher in girls, compared with boys, a statistically significant 7% higher in adolescents, compared with children 12 years or younger, and was significantly reduced by a relative 45% rate in nonwhites, compared with whites.
The mechanisms behind these associations are not known, but could involve mast-cell degranulation, autonomic dysfunction, or shared genetic or environmental etiologic factors, the authors said.
Dr. Patel reported no relevant disclosures.
SOURCE: Patel RS et al. Headache. 2019 June;59[S1]:1-208, Abstract P78.
REPORTING FROM AHS 2019
Criteria found largely interchangeable for classifying radiographic axSpA
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
FROM ANNALS OF THE RHEUMATIC DISEASES
Fatal Drug-Resistant Invasive Pulmonary Aspergillus fumigatus in a 56-Year-Old Immunosuppressed Man (FULL)
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
Nicotinamide-containing products gaining interest for aging, dermatologic disorders
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Botulinum toxin injections: Err on the side of undercorrecting in first-time users
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
EXPERT ANALYSIS FROM SUMMER AAD 2019