User login
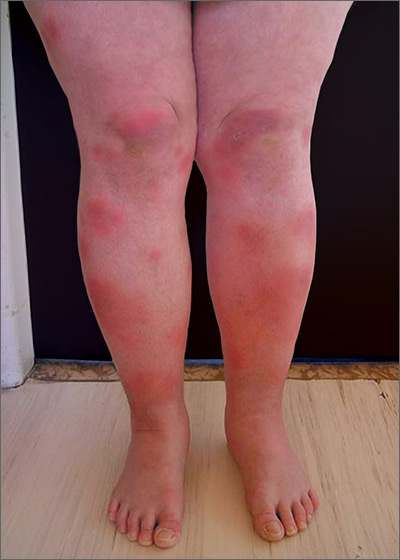
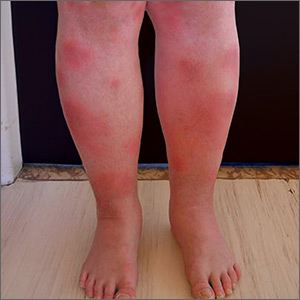
Tender swellings on legs
Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Maternal factors impact childhood skin microbiota
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Age, skin site, and maternal factors including delivery method and breastfeeding impact the bacterial makeup of children’s skin.
Major finding: The most common bacteria at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%).
Study details: The data come from 158 children aged 10 years and younger and included 474 skin samples.
Disclosures: The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
Source: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Hematuria is highly prevalent in pediatric hemophilia A and B
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
FROM HAEMOPHILIA
Two genetic variants modify risk of Alzheimer’s disease
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Two variants of MS4A are associated with the risk of Alzheimer’s disease.
Major finding: The rs1582763 SNP is associated with reduced risk for Alzheimer’s disease, and rs6591561 is associated with increased risk of Alzheimer’s disease.
Study details: A genome-wide association study of 813 participants in the Alzheimer’s Disease Neuroimaging Initiative.
Disclosures: Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
Source: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
Concomitant emicizumab, ITI shows promise in severe hemophilia A
Concomitant emicizumab prophylaxis and immune tolerance induction (ITI) may be suitable for bleeding prevention in pediatric patients with severe hemophilia A and inhibitors, according to a retrospective analysis.
“The primary objective of this study [was] to review a case series of pediatric patients with hemophilia A and inhibitors at our institution who have received emicizumab concurrently with ITI,” wrote Glaivy Batsuli, MD, of Emory University in Atlanta, and her colleagues. The results of the study were reported in Haemophilia.
The case series included seven pediatric patients with severe hemophilia A who received concurrent emicizumab for bleeding prevention and ITI for inhibitor eradication. Data were collected from electronic medical records at a single hemophilia treatment center from Aug. 1 to Dec. 1, 2018.
The researchers included male patients from 0 to 21 years old who had titres greater than 0.6 chromogenic Bethesda units (CBU) per mL on two instances more than 2 weeks apart.
The treatment protocol, termed the “Atlanta Protocol,” used a novel dosing regimen, established using provider consensus, for the concomitant use of ITI and emicizumab.
After analysis, the researchers found that three patients attained a negative inhibitor titer of less than 0.6 CBU/mL, and two patients had a normal factor VIII recovery of greater than or equal to 66%.
In total, nine bleeding events were observed in four patients; however, no thrombotic events were reported. All patients continued on concomitant therapy at the point of data analysis.
The researchers reported that six patients used three different recombinant factor VIII products at 100 IU/kg, three times each week. The remaining patient used a plasma‐derived factor VIII product at an initial dose of 50 IU/kg, three times each week.
The small sample size, retrospective design, and short follow‐up period were key limitations of the study.
“Prospective studies will be necessary to compare treatment outcomes of ITI and emicizumab to other ITI regimens and to investigate whether emicizumab modifies the immunologic response to FVIII,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bayer, Bioverativ, CSL Behring, Catalyst Biosciences, Genentech, HEMA Biologics, Novo Nordisk, Octapharma, and several others.
SOURCE: Batsuli G et al. Haemophilia. 2019 Aug 2. doi: 10.1111/hae.13819.
Concomitant emicizumab prophylaxis and immune tolerance induction (ITI) may be suitable for bleeding prevention in pediatric patients with severe hemophilia A and inhibitors, according to a retrospective analysis.
“The primary objective of this study [was] to review a case series of pediatric patients with hemophilia A and inhibitors at our institution who have received emicizumab concurrently with ITI,” wrote Glaivy Batsuli, MD, of Emory University in Atlanta, and her colleagues. The results of the study were reported in Haemophilia.
The case series included seven pediatric patients with severe hemophilia A who received concurrent emicizumab for bleeding prevention and ITI for inhibitor eradication. Data were collected from electronic medical records at a single hemophilia treatment center from Aug. 1 to Dec. 1, 2018.
The researchers included male patients from 0 to 21 years old who had titres greater than 0.6 chromogenic Bethesda units (CBU) per mL on two instances more than 2 weeks apart.
The treatment protocol, termed the “Atlanta Protocol,” used a novel dosing regimen, established using provider consensus, for the concomitant use of ITI and emicizumab.
After analysis, the researchers found that three patients attained a negative inhibitor titer of less than 0.6 CBU/mL, and two patients had a normal factor VIII recovery of greater than or equal to 66%.
In total, nine bleeding events were observed in four patients; however, no thrombotic events were reported. All patients continued on concomitant therapy at the point of data analysis.
The researchers reported that six patients used three different recombinant factor VIII products at 100 IU/kg, three times each week. The remaining patient used a plasma‐derived factor VIII product at an initial dose of 50 IU/kg, three times each week.
The small sample size, retrospective design, and short follow‐up period were key limitations of the study.
“Prospective studies will be necessary to compare treatment outcomes of ITI and emicizumab to other ITI regimens and to investigate whether emicizumab modifies the immunologic response to FVIII,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bayer, Bioverativ, CSL Behring, Catalyst Biosciences, Genentech, HEMA Biologics, Novo Nordisk, Octapharma, and several others.
SOURCE: Batsuli G et al. Haemophilia. 2019 Aug 2. doi: 10.1111/hae.13819.
Concomitant emicizumab prophylaxis and immune tolerance induction (ITI) may be suitable for bleeding prevention in pediatric patients with severe hemophilia A and inhibitors, according to a retrospective analysis.
“The primary objective of this study [was] to review a case series of pediatric patients with hemophilia A and inhibitors at our institution who have received emicizumab concurrently with ITI,” wrote Glaivy Batsuli, MD, of Emory University in Atlanta, and her colleagues. The results of the study were reported in Haemophilia.
The case series included seven pediatric patients with severe hemophilia A who received concurrent emicizumab for bleeding prevention and ITI for inhibitor eradication. Data were collected from electronic medical records at a single hemophilia treatment center from Aug. 1 to Dec. 1, 2018.
The researchers included male patients from 0 to 21 years old who had titres greater than 0.6 chromogenic Bethesda units (CBU) per mL on two instances more than 2 weeks apart.
The treatment protocol, termed the “Atlanta Protocol,” used a novel dosing regimen, established using provider consensus, for the concomitant use of ITI and emicizumab.
After analysis, the researchers found that three patients attained a negative inhibitor titer of less than 0.6 CBU/mL, and two patients had a normal factor VIII recovery of greater than or equal to 66%.
In total, nine bleeding events were observed in four patients; however, no thrombotic events were reported. All patients continued on concomitant therapy at the point of data analysis.
The researchers reported that six patients used three different recombinant factor VIII products at 100 IU/kg, three times each week. The remaining patient used a plasma‐derived factor VIII product at an initial dose of 50 IU/kg, three times each week.
The small sample size, retrospective design, and short follow‐up period were key limitations of the study.
“Prospective studies will be necessary to compare treatment outcomes of ITI and emicizumab to other ITI regimens and to investigate whether emicizumab modifies the immunologic response to FVIII,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bayer, Bioverativ, CSL Behring, Catalyst Biosciences, Genentech, HEMA Biologics, Novo Nordisk, Octapharma, and several others.
SOURCE: Batsuli G et al. Haemophilia. 2019 Aug 2. doi: 10.1111/hae.13819.
FROM HAEMOPHILIA
Dabrafenib plus trametinib yields long-term benefit in melanoma patients
Dabrafenib plus trametinib treatment was associated with a 5-year overall survival rate of 34% in patients with melanoma harboring a BRAF V600E or V600K mutation, according to a combined analysis of two trials.
The 5-year progression-free survival rate was 19% in the long-term, pooled analysis of the COMBI-d and COMBI-v trials, which included at total of 563 patients with previously untreated, unresectable or metastatic melanoma who received combined treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib.
Previously reported 5-year progression-free survival rates for patients treated with anti–programmed death-1 checkpoint inhibitors, either nivolumab or pembrolizumab, “appear to be similar” to these results for dabrafenib plus trametinib, investigators said in a report on the analysis appearing in the New England Journal of Medicine.
To date, however, 5-year survival data have not been reported for other BRAF-targeted therapies, according to the investigators, who were led by Caroline Robert, MD, PhD, of Institut Gustave Roussy and Paris-Sud-Paris-Saclay University, Villejuif, France.
“These data will be critical to assess the potential of therapy to exert long-term disease control through analysis of survival plateaus and to understand factors predictive of long-term survival,” Dr. Robert and coauthors wrote in their report.
A total of 211 patients in the COMBI-d trial were randomly allocated to receive the combination of dabrafenib plus trametinib, while in COMBI-v, 352 received this combination therapy, according to investigators.
Notably, the survival curves for dabrafenib plus trametinib appear to plateau starting at 3 years, investigators reported. In a previously published report on pooled COMBI-d and COMBI-v data, the 3-year progression-free survival rate was 23%, and the 3-year overall survival rate was 44%.
In this more recent analysis, progression-free survival rates were 21% at 4 years and 19% at 5 years, while overall survival rates were 37% at 4 years and 34% at 5 years.
“This finding suggests stabilization of rates of progression-free survival and overall survival over time in this population,” Dr. Robert and colleagues wrote.
Survival rates were higher in patients with normal lactate dehydrogenase (LDH) levels at baseline, and they were especially high in those with normal LDH and three or fewer disease sites at baseline, according to the report. Specifically, the reported 5-year rates of progression-free and overall survival were 31% and 55%, respectively.
Other factors associated with prolonged progression-free survival included female sex, older age, better performance status, and BRAF V600E genotype, according to results of a multivariate analysis that investigators said confirmed findings from the previously reported 3-year data.
The study was supported by GlaxoSmithKline and Novartis. Dr. Robert provided disclosures related to BMS, Pierre Fabre, Novartis, Amgen, Merck, Roche, MSD, and Sanofi.
SOURCE: Robert C et al. N Engl J Med. 2019 Aug 15. doi: 10.1056/NEJMoa1904059
Dabrafenib plus trametinib treatment was associated with a 5-year overall survival rate of 34% in patients with melanoma harboring a BRAF V600E or V600K mutation, according to a combined analysis of two trials.
The 5-year progression-free survival rate was 19% in the long-term, pooled analysis of the COMBI-d and COMBI-v trials, which included at total of 563 patients with previously untreated, unresectable or metastatic melanoma who received combined treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib.
Previously reported 5-year progression-free survival rates for patients treated with anti–programmed death-1 checkpoint inhibitors, either nivolumab or pembrolizumab, “appear to be similar” to these results for dabrafenib plus trametinib, investigators said in a report on the analysis appearing in the New England Journal of Medicine.
To date, however, 5-year survival data have not been reported for other BRAF-targeted therapies, according to the investigators, who were led by Caroline Robert, MD, PhD, of Institut Gustave Roussy and Paris-Sud-Paris-Saclay University, Villejuif, France.
“These data will be critical to assess the potential of therapy to exert long-term disease control through analysis of survival plateaus and to understand factors predictive of long-term survival,” Dr. Robert and coauthors wrote in their report.
A total of 211 patients in the COMBI-d trial were randomly allocated to receive the combination of dabrafenib plus trametinib, while in COMBI-v, 352 received this combination therapy, according to investigators.
Notably, the survival curves for dabrafenib plus trametinib appear to plateau starting at 3 years, investigators reported. In a previously published report on pooled COMBI-d and COMBI-v data, the 3-year progression-free survival rate was 23%, and the 3-year overall survival rate was 44%.
In this more recent analysis, progression-free survival rates were 21% at 4 years and 19% at 5 years, while overall survival rates were 37% at 4 years and 34% at 5 years.
“This finding suggests stabilization of rates of progression-free survival and overall survival over time in this population,” Dr. Robert and colleagues wrote.
Survival rates were higher in patients with normal lactate dehydrogenase (LDH) levels at baseline, and they were especially high in those with normal LDH and three or fewer disease sites at baseline, according to the report. Specifically, the reported 5-year rates of progression-free and overall survival were 31% and 55%, respectively.
Other factors associated with prolonged progression-free survival included female sex, older age, better performance status, and BRAF V600E genotype, according to results of a multivariate analysis that investigators said confirmed findings from the previously reported 3-year data.
The study was supported by GlaxoSmithKline and Novartis. Dr. Robert provided disclosures related to BMS, Pierre Fabre, Novartis, Amgen, Merck, Roche, MSD, and Sanofi.
SOURCE: Robert C et al. N Engl J Med. 2019 Aug 15. doi: 10.1056/NEJMoa1904059
Dabrafenib plus trametinib treatment was associated with a 5-year overall survival rate of 34% in patients with melanoma harboring a BRAF V600E or V600K mutation, according to a combined analysis of two trials.
The 5-year progression-free survival rate was 19% in the long-term, pooled analysis of the COMBI-d and COMBI-v trials, which included at total of 563 patients with previously untreated, unresectable or metastatic melanoma who received combined treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib.
Previously reported 5-year progression-free survival rates for patients treated with anti–programmed death-1 checkpoint inhibitors, either nivolumab or pembrolizumab, “appear to be similar” to these results for dabrafenib plus trametinib, investigators said in a report on the analysis appearing in the New England Journal of Medicine.
To date, however, 5-year survival data have not been reported for other BRAF-targeted therapies, according to the investigators, who were led by Caroline Robert, MD, PhD, of Institut Gustave Roussy and Paris-Sud-Paris-Saclay University, Villejuif, France.
“These data will be critical to assess the potential of therapy to exert long-term disease control through analysis of survival plateaus and to understand factors predictive of long-term survival,” Dr. Robert and coauthors wrote in their report.
A total of 211 patients in the COMBI-d trial were randomly allocated to receive the combination of dabrafenib plus trametinib, while in COMBI-v, 352 received this combination therapy, according to investigators.
Notably, the survival curves for dabrafenib plus trametinib appear to plateau starting at 3 years, investigators reported. In a previously published report on pooled COMBI-d and COMBI-v data, the 3-year progression-free survival rate was 23%, and the 3-year overall survival rate was 44%.
In this more recent analysis, progression-free survival rates were 21% at 4 years and 19% at 5 years, while overall survival rates were 37% at 4 years and 34% at 5 years.
“This finding suggests stabilization of rates of progression-free survival and overall survival over time in this population,” Dr. Robert and colleagues wrote.
Survival rates were higher in patients with normal lactate dehydrogenase (LDH) levels at baseline, and they were especially high in those with normal LDH and three or fewer disease sites at baseline, according to the report. Specifically, the reported 5-year rates of progression-free and overall survival were 31% and 55%, respectively.
Other factors associated with prolonged progression-free survival included female sex, older age, better performance status, and BRAF V600E genotype, according to results of a multivariate analysis that investigators said confirmed findings from the previously reported 3-year data.
The study was supported by GlaxoSmithKline and Novartis. Dr. Robert provided disclosures related to BMS, Pierre Fabre, Novartis, Amgen, Merck, Roche, MSD, and Sanofi.
SOURCE: Robert C et al. N Engl J Med. 2019 Aug 15. doi: 10.1056/NEJMoa1904059
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A long-term survival benefit was seen in about a third of patients with metastatic or unresectable melanoma who underwent first-line treatment with dabrafenib and trametinib.
Major finding: The 5-year rates of progression-free survival and overall survival were 19% and 34%, respectively.
Study details: Pooled analysis including 563 patients randomly allocated to the combination treatment in two randomized trials (COMBI-d and COMBI-v).
Disclosures: The study was supported by GlaxoSmithKline and Novartis. The first author provided disclosures related to BMS, Pierre Fabre, Novartis, Amgen, Merck, Roche, MSD, and Sanofi.
Source: Robert C et al. N Engl J Med. 2019 Aug 15. doi: 10.1056/NEJMoa1904059
FDA approves drug combo to treat highly resistant TB
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
NEWS FROM THE FDA
Pigmented Mass on the Shoulder
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
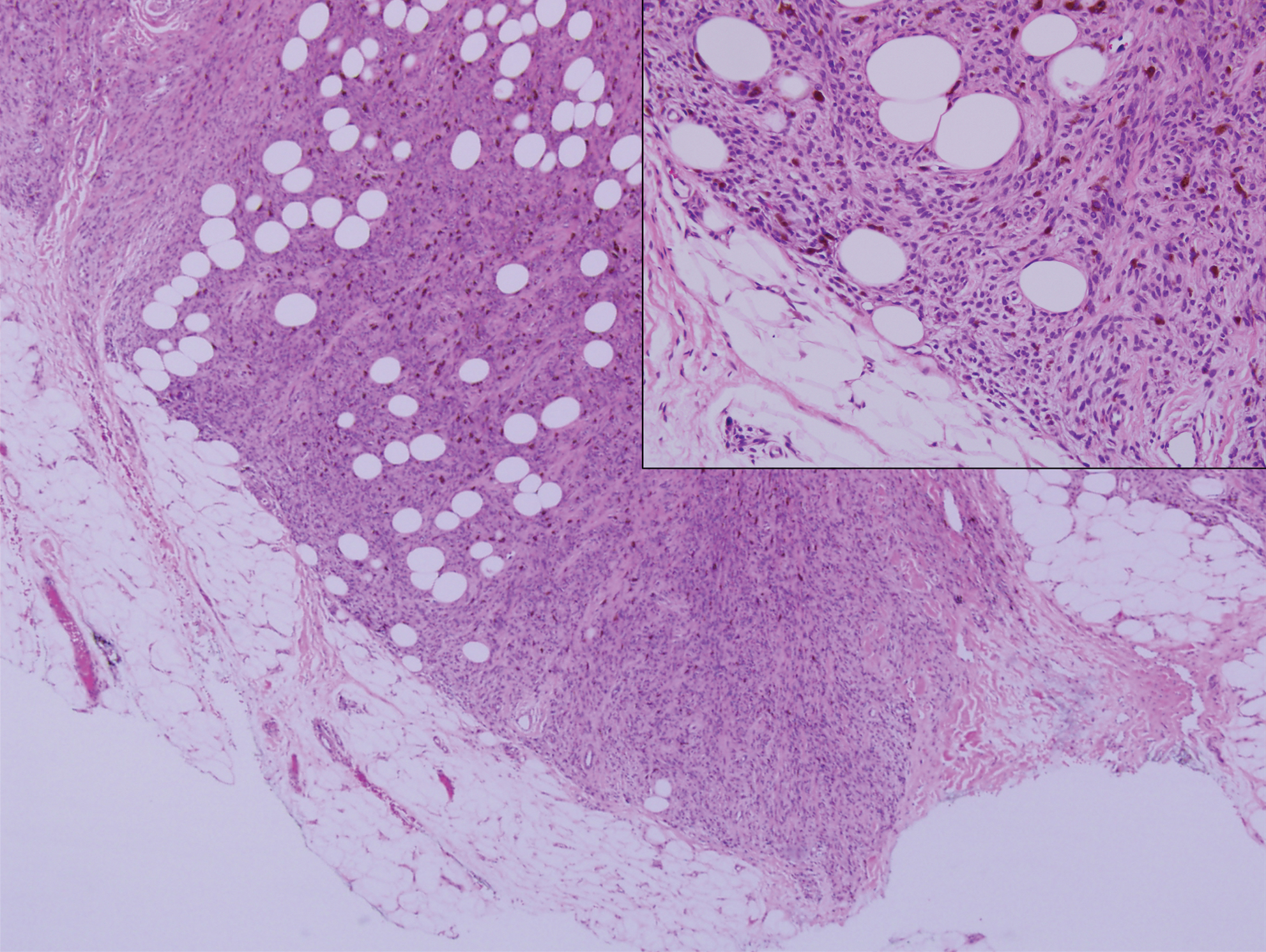
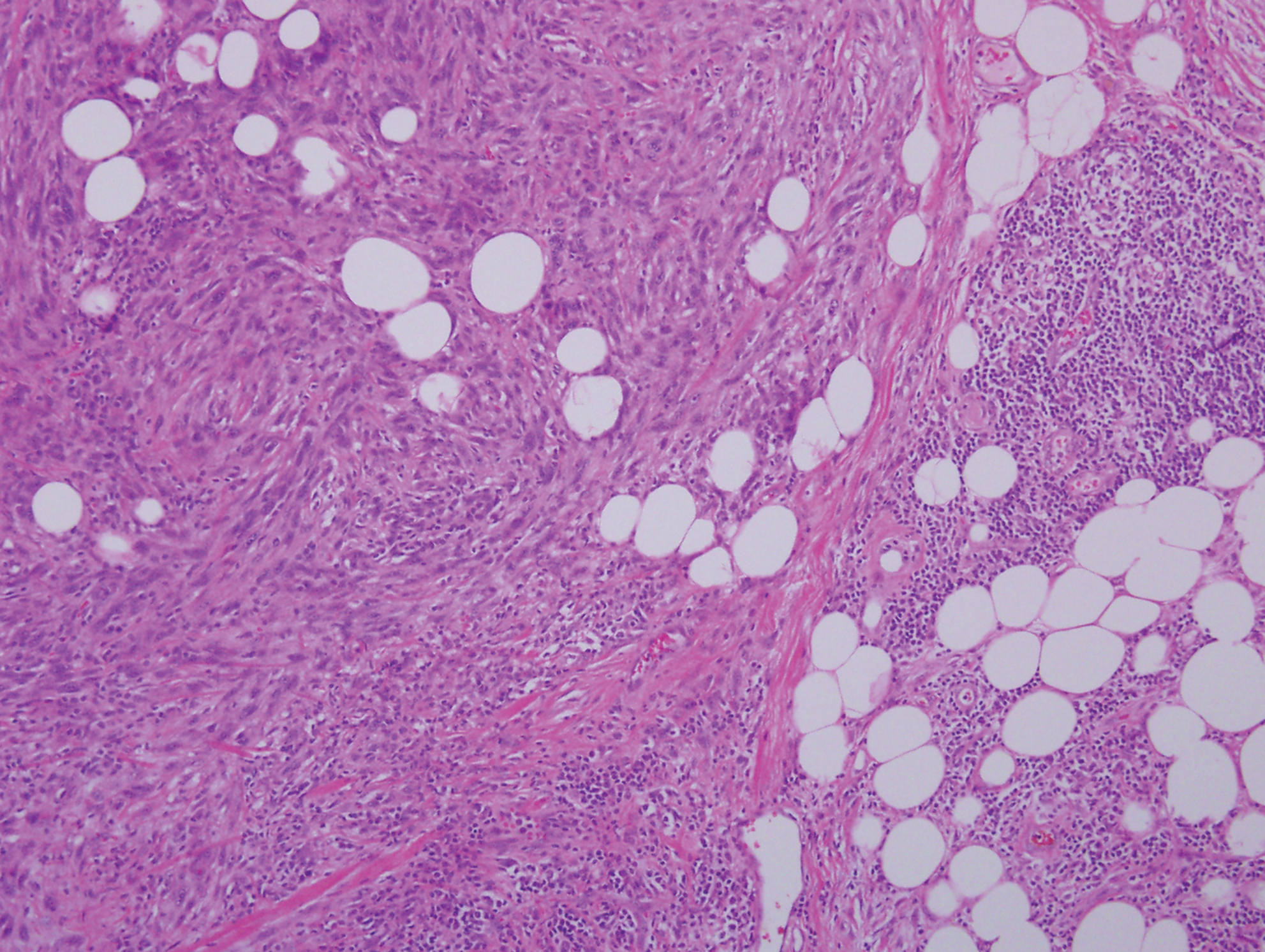
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
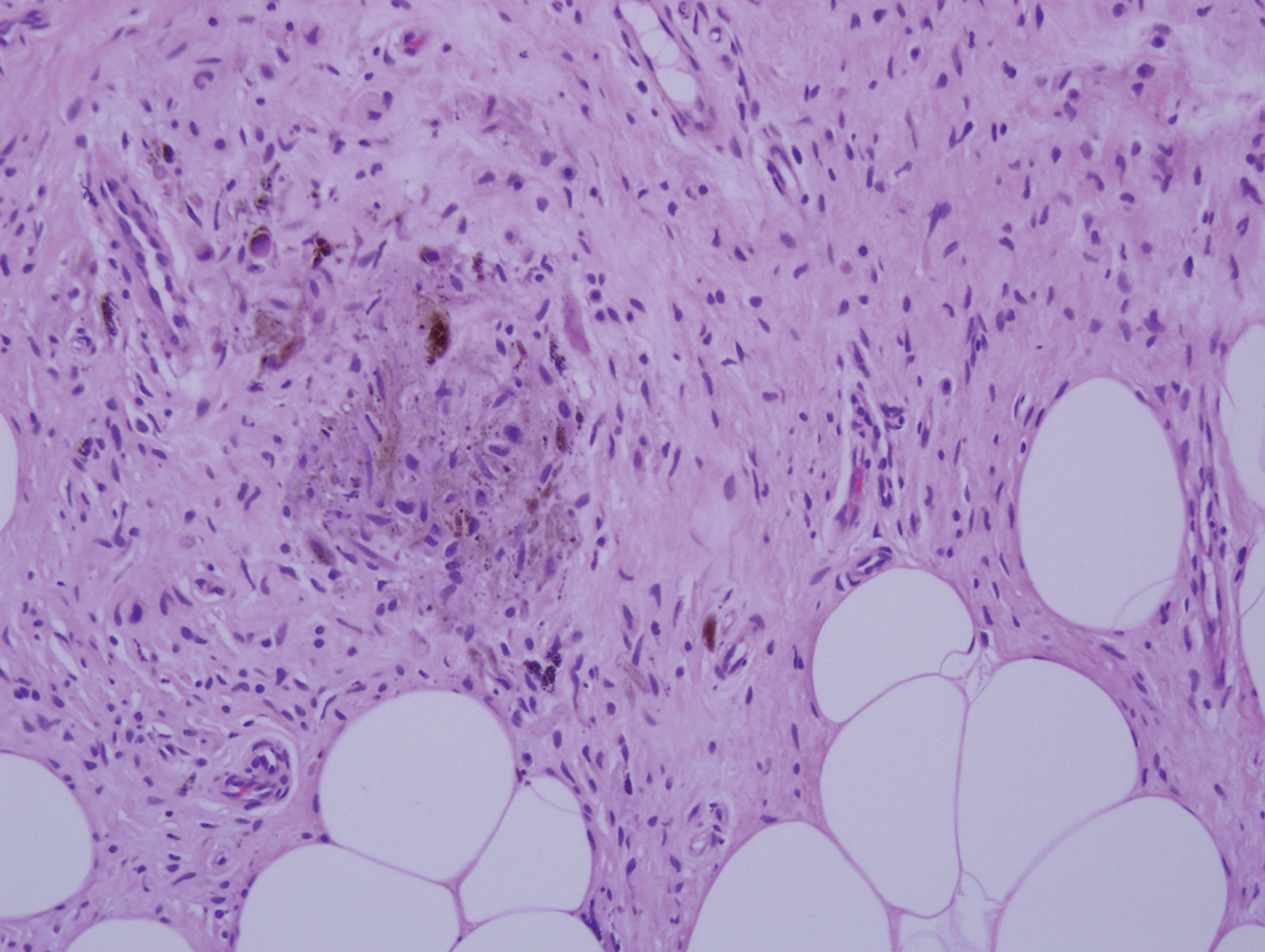
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
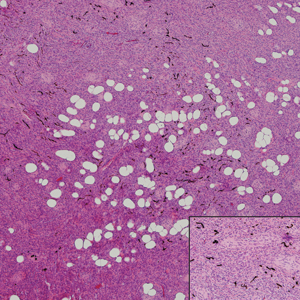
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
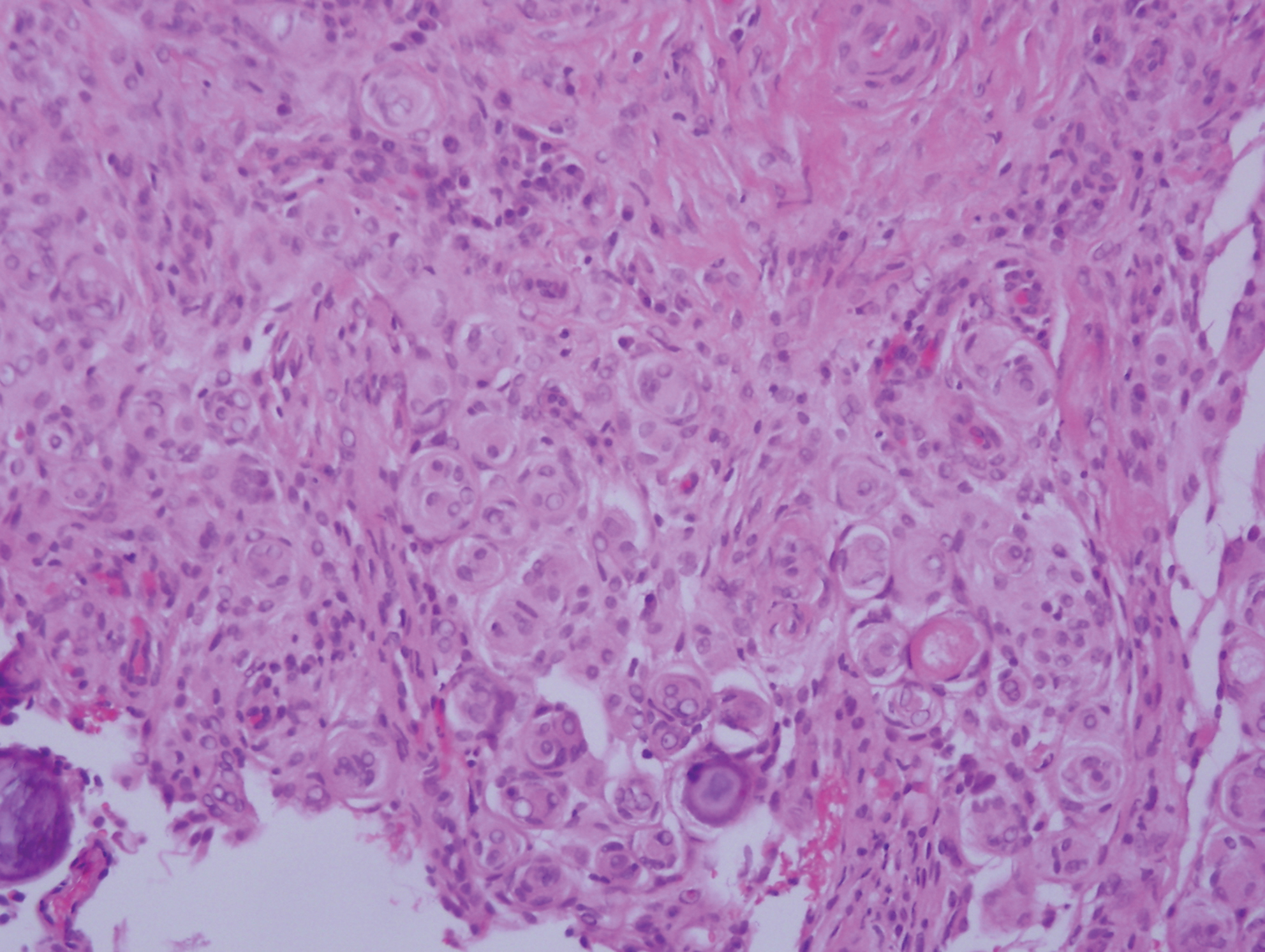
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
A 37-year-old woman presented with an asymptomatic, indurated, pigmented, subcutaneous nodule on the right shoulder of more than 3 years' duration. The lesion had gradually increased in size with no associated symptoms. The patient had a history of endometrial adenocarcinoma and papillary thyroid carcinoma, which had been treated by hysterectomy-oophorectomy and right thyroidectomy, respectively. She had no other notable systemic abnormalities, and there was no family history of genetic disease or cancer. Physical examination demonstrated a 1.2×1.8-cm nontender, pigmented, subcutaneous nodule with a rough surface and indistinct borders. An excisional biopsy was performed.
Self-reported adherence fails to line up with objective measures in hemophilia
Considerable differences may exist between objective and subjective measures of adherence to prophylaxis in patients with hemophilia, according to a cross-sectional study.
The results highlight the effect of social desirability bias in self-reported measures of adherence and differences in conceptual understanding of adherence between hemophilia experts and patients.
Vanessa Giroto Guedes, MPH, of the Federal University of Rio de Janeiro and colleagues studied 29 male patients with hemophilia who received prophylactic treatment between August 2015 and January 2016. The study was conducted at two hemophilia treatment centers in São Paulo.
Self-perceived adherence, measured using the estimated number of clotting factor concentrate doses missed over the previous dispensing interval, was compared with an objective estimate of adherence, measured using the number of vials returned by study participants. The findings were published in Haemophilia.
Patient interviews were conducted during regularly scheduled visits to the treatment facility. The team collected self-perceived adherence data using a 5-point Likert scale, scored from very poor to very good adherence.
After analysis, the researchers found no significant correlation between the objective categorization of adherence and self-perceived extent of adherence (correlation coefficient, 0.10; 95% confidence interval, –0.28 to 0.46; P = .61).
Additionally, there was no significant correlation between the categorization of adherence measured using the proportion of missed doses evaluated objectively and using participants’ self‐reports (correlation coefficient, 0.32; 95% CI, –0.01 to 0.59; P = .11).
“Participants’ self-reported perception of adherence was almost three times more likely to be rated as very good or good than it was for the objective assessment of adherence to be classified as adherent or suboptimally adherent,” the researchers wrote.
No funding sources were reported. One coauthor reported providing consultancy services for manufacturers of therapies for hemophilia. The others reported no conflicts of interest.
SOURCE: Guedes VG et al. Haemophilia. 2019 Jul 19. doi: 10.1111/hae.13811.
Considerable differences may exist between objective and subjective measures of adherence to prophylaxis in patients with hemophilia, according to a cross-sectional study.
The results highlight the effect of social desirability bias in self-reported measures of adherence and differences in conceptual understanding of adherence between hemophilia experts and patients.
Vanessa Giroto Guedes, MPH, of the Federal University of Rio de Janeiro and colleagues studied 29 male patients with hemophilia who received prophylactic treatment between August 2015 and January 2016. The study was conducted at two hemophilia treatment centers in São Paulo.
Self-perceived adherence, measured using the estimated number of clotting factor concentrate doses missed over the previous dispensing interval, was compared with an objective estimate of adherence, measured using the number of vials returned by study participants. The findings were published in Haemophilia.
Patient interviews were conducted during regularly scheduled visits to the treatment facility. The team collected self-perceived adherence data using a 5-point Likert scale, scored from very poor to very good adherence.
After analysis, the researchers found no significant correlation between the objective categorization of adherence and self-perceived extent of adherence (correlation coefficient, 0.10; 95% confidence interval, –0.28 to 0.46; P = .61).
Additionally, there was no significant correlation between the categorization of adherence measured using the proportion of missed doses evaluated objectively and using participants’ self‐reports (correlation coefficient, 0.32; 95% CI, –0.01 to 0.59; P = .11).
“Participants’ self-reported perception of adherence was almost three times more likely to be rated as very good or good than it was for the objective assessment of adherence to be classified as adherent or suboptimally adherent,” the researchers wrote.
No funding sources were reported. One coauthor reported providing consultancy services for manufacturers of therapies for hemophilia. The others reported no conflicts of interest.
SOURCE: Guedes VG et al. Haemophilia. 2019 Jul 19. doi: 10.1111/hae.13811.
Considerable differences may exist between objective and subjective measures of adherence to prophylaxis in patients with hemophilia, according to a cross-sectional study.
The results highlight the effect of social desirability bias in self-reported measures of adherence and differences in conceptual understanding of adherence between hemophilia experts and patients.
Vanessa Giroto Guedes, MPH, of the Federal University of Rio de Janeiro and colleagues studied 29 male patients with hemophilia who received prophylactic treatment between August 2015 and January 2016. The study was conducted at two hemophilia treatment centers in São Paulo.
Self-perceived adherence, measured using the estimated number of clotting factor concentrate doses missed over the previous dispensing interval, was compared with an objective estimate of adherence, measured using the number of vials returned by study participants. The findings were published in Haemophilia.
Patient interviews were conducted during regularly scheduled visits to the treatment facility. The team collected self-perceived adherence data using a 5-point Likert scale, scored from very poor to very good adherence.
After analysis, the researchers found no significant correlation between the objective categorization of adherence and self-perceived extent of adherence (correlation coefficient, 0.10; 95% confidence interval, –0.28 to 0.46; P = .61).
Additionally, there was no significant correlation between the categorization of adherence measured using the proportion of missed doses evaluated objectively and using participants’ self‐reports (correlation coefficient, 0.32; 95% CI, –0.01 to 0.59; P = .11).
“Participants’ self-reported perception of adherence was almost three times more likely to be rated as very good or good than it was for the objective assessment of adherence to be classified as adherent or suboptimally adherent,” the researchers wrote.
No funding sources were reported. One coauthor reported providing consultancy services for manufacturers of therapies for hemophilia. The others reported no conflicts of interest.
SOURCE: Guedes VG et al. Haemophilia. 2019 Jul 19. doi: 10.1111/hae.13811.
FROM HAEMOPHILIA
Drug-inducible gene therapy unlocks IL-12 for glioblastoma
For patients with recurrent, high-grade glioblastoma, localized, drug-inducible gene therapy could unlock the anticancer potential of interleukin-12, based on a phase 1 trial.
In 31 patients who had their tumors excised, intraoperative site injection with an IL-12 vector followed by postoperative administration of veledimex, an oral activator of the transgene, increased IL-12 levels in the brain and appeared to improve overall survival, reported E. Antonio Chiocca, MD, PhD, Harvey W. Cushing Professor of Neurosurgery at Harvard Medical School, Boston, and colleagues. Although some serious adverse events were encountered, the investigators noted that these were less common with lower doses of veledimex and were reversible upon discontinuation. These findings mark a turning point in IL-12 cancer research, which previously encountered prohibitive safety obstacles.
“There was interest in the use of recombinant IL-12 in humans with cancer, and clinical trials of systemic IL-12 were undertaken but had to be stopped because the cytokine, administered as a recombinant soluble protein, was poorly tolerated,” the investigators wrote in Science Translational Medicine.
To overcome this issue, a novel treatment approach was developed. “With the objective of minimizing systemic toxicity, a ligand-inducible expression switch [RheoSwitch Therapeutic System] was developed to locally control production of IL-12 in the tumor microenvironment. In this system, transcription of the IL-12 transgene occurs only in the presence of the activator ligand, veledimex,” they noted.
The primary aim of the study was to evaluate safety and determine the optimal dose of veledimex; four dose levels were tested: 10, 20, 30, and 40 mg. Survival outcomes also were reported.
The protocol-defined maximum tolerated dose was not reached; however, the 20-mg dose was chosen, based on observed tolerability. At this dose level, the most common grade 3 or higher adverse events were lymphopenia (20.3%), thrombocytopenia (13.3%), and hyponatremia (13.3%). Specifically for grade 3 or higher neurologic adverse events, headache was most common, occurring in 13.3% of patients. Grade 2 cytokine release syndrome occurred in about one-fourth of patients (26.7%), whereas grade 3 cytokine release syndrome occurred about half as frequently (13.3%). All adverse events, including cytokine release syndrome, were reversible upon discontinuation of veledimex.
After a mean follow-up of 13.1 months, the median overall survival among patients receiving the 20-mg dose was 12.7 months. The investigators pointed out that this compared favorably with historical controls, who had a weighted median overall survival of 8.1 months. Those who received 30- or 40-mg doses had the poorest survival, which the investigators attributed to intolerability and other subgroup factors.
Data analysis also revealed a negative correlation between dexamethasone use and survival. Among patients in the 20-mg veledimex group who received 20 mg or less of dexamethasone during active veledimex dosing, median overall survival was extended to 17.8 months. The investigators speculated that this was because of reduced immune suppression, although dexamethasone could have induced cytochrome P450 3A4, which may have increased elimination of veledimex.
“In summary, this phase 1 trial reports the use of a transcriptional switch to safely control dosing of [IL-12], highlighting that this can be accomplished across the [blood-brain barrier] to remodel the tumor microenvironment with an influx of activated immune cells,” the investigators wrote.
They noted that this strategy could potentially be applied to other types of cancer, particularly those that are immunologically cold. “These data contribute to our understanding of IL-12 as a ‘master regulator’ of the immune system and highlight that even the transient production of this cytokine may function as a match to turn tumors from cold to hot.”
The study was funded by Ziopharm Oncology and the National Institutes of Health. The investigators reported additional relationships with Advantagene, Stemgen, Sigilon Therapeutics, and others.
SOURCE: Chiocca EA et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aaw5680.
For patients with recurrent, high-grade glioblastoma, localized, drug-inducible gene therapy could unlock the anticancer potential of interleukin-12, based on a phase 1 trial.
In 31 patients who had their tumors excised, intraoperative site injection with an IL-12 vector followed by postoperative administration of veledimex, an oral activator of the transgene, increased IL-12 levels in the brain and appeared to improve overall survival, reported E. Antonio Chiocca, MD, PhD, Harvey W. Cushing Professor of Neurosurgery at Harvard Medical School, Boston, and colleagues. Although some serious adverse events were encountered, the investigators noted that these were less common with lower doses of veledimex and were reversible upon discontinuation. These findings mark a turning point in IL-12 cancer research, which previously encountered prohibitive safety obstacles.
“There was interest in the use of recombinant IL-12 in humans with cancer, and clinical trials of systemic IL-12 were undertaken but had to be stopped because the cytokine, administered as a recombinant soluble protein, was poorly tolerated,” the investigators wrote in Science Translational Medicine.
To overcome this issue, a novel treatment approach was developed. “With the objective of minimizing systemic toxicity, a ligand-inducible expression switch [RheoSwitch Therapeutic System] was developed to locally control production of IL-12 in the tumor microenvironment. In this system, transcription of the IL-12 transgene occurs only in the presence of the activator ligand, veledimex,” they noted.
The primary aim of the study was to evaluate safety and determine the optimal dose of veledimex; four dose levels were tested: 10, 20, 30, and 40 mg. Survival outcomes also were reported.
The protocol-defined maximum tolerated dose was not reached; however, the 20-mg dose was chosen, based on observed tolerability. At this dose level, the most common grade 3 or higher adverse events were lymphopenia (20.3%), thrombocytopenia (13.3%), and hyponatremia (13.3%). Specifically for grade 3 or higher neurologic adverse events, headache was most common, occurring in 13.3% of patients. Grade 2 cytokine release syndrome occurred in about one-fourth of patients (26.7%), whereas grade 3 cytokine release syndrome occurred about half as frequently (13.3%). All adverse events, including cytokine release syndrome, were reversible upon discontinuation of veledimex.
After a mean follow-up of 13.1 months, the median overall survival among patients receiving the 20-mg dose was 12.7 months. The investigators pointed out that this compared favorably with historical controls, who had a weighted median overall survival of 8.1 months. Those who received 30- or 40-mg doses had the poorest survival, which the investigators attributed to intolerability and other subgroup factors.
Data analysis also revealed a negative correlation between dexamethasone use and survival. Among patients in the 20-mg veledimex group who received 20 mg or less of dexamethasone during active veledimex dosing, median overall survival was extended to 17.8 months. The investigators speculated that this was because of reduced immune suppression, although dexamethasone could have induced cytochrome P450 3A4, which may have increased elimination of veledimex.
“In summary, this phase 1 trial reports the use of a transcriptional switch to safely control dosing of [IL-12], highlighting that this can be accomplished across the [blood-brain barrier] to remodel the tumor microenvironment with an influx of activated immune cells,” the investigators wrote.
They noted that this strategy could potentially be applied to other types of cancer, particularly those that are immunologically cold. “These data contribute to our understanding of IL-12 as a ‘master regulator’ of the immune system and highlight that even the transient production of this cytokine may function as a match to turn tumors from cold to hot.”
The study was funded by Ziopharm Oncology and the National Institutes of Health. The investigators reported additional relationships with Advantagene, Stemgen, Sigilon Therapeutics, and others.
SOURCE: Chiocca EA et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aaw5680.
For patients with recurrent, high-grade glioblastoma, localized, drug-inducible gene therapy could unlock the anticancer potential of interleukin-12, based on a phase 1 trial.
In 31 patients who had their tumors excised, intraoperative site injection with an IL-12 vector followed by postoperative administration of veledimex, an oral activator of the transgene, increased IL-12 levels in the brain and appeared to improve overall survival, reported E. Antonio Chiocca, MD, PhD, Harvey W. Cushing Professor of Neurosurgery at Harvard Medical School, Boston, and colleagues. Although some serious adverse events were encountered, the investigators noted that these were less common with lower doses of veledimex and were reversible upon discontinuation. These findings mark a turning point in IL-12 cancer research, which previously encountered prohibitive safety obstacles.
“There was interest in the use of recombinant IL-12 in humans with cancer, and clinical trials of systemic IL-12 were undertaken but had to be stopped because the cytokine, administered as a recombinant soluble protein, was poorly tolerated,” the investigators wrote in Science Translational Medicine.
To overcome this issue, a novel treatment approach was developed. “With the objective of minimizing systemic toxicity, a ligand-inducible expression switch [RheoSwitch Therapeutic System] was developed to locally control production of IL-12 in the tumor microenvironment. In this system, transcription of the IL-12 transgene occurs only in the presence of the activator ligand, veledimex,” they noted.
The primary aim of the study was to evaluate safety and determine the optimal dose of veledimex; four dose levels were tested: 10, 20, 30, and 40 mg. Survival outcomes also were reported.
The protocol-defined maximum tolerated dose was not reached; however, the 20-mg dose was chosen, based on observed tolerability. At this dose level, the most common grade 3 or higher adverse events were lymphopenia (20.3%), thrombocytopenia (13.3%), and hyponatremia (13.3%). Specifically for grade 3 or higher neurologic adverse events, headache was most common, occurring in 13.3% of patients. Grade 2 cytokine release syndrome occurred in about one-fourth of patients (26.7%), whereas grade 3 cytokine release syndrome occurred about half as frequently (13.3%). All adverse events, including cytokine release syndrome, were reversible upon discontinuation of veledimex.
After a mean follow-up of 13.1 months, the median overall survival among patients receiving the 20-mg dose was 12.7 months. The investigators pointed out that this compared favorably with historical controls, who had a weighted median overall survival of 8.1 months. Those who received 30- or 40-mg doses had the poorest survival, which the investigators attributed to intolerability and other subgroup factors.
Data analysis also revealed a negative correlation between dexamethasone use and survival. Among patients in the 20-mg veledimex group who received 20 mg or less of dexamethasone during active veledimex dosing, median overall survival was extended to 17.8 months. The investigators speculated that this was because of reduced immune suppression, although dexamethasone could have induced cytochrome P450 3A4, which may have increased elimination of veledimex.
“In summary, this phase 1 trial reports the use of a transcriptional switch to safely control dosing of [IL-12], highlighting that this can be accomplished across the [blood-brain barrier] to remodel the tumor microenvironment with an influx of activated immune cells,” the investigators wrote.
They noted that this strategy could potentially be applied to other types of cancer, particularly those that are immunologically cold. “These data contribute to our understanding of IL-12 as a ‘master regulator’ of the immune system and highlight that even the transient production of this cytokine may function as a match to turn tumors from cold to hot.”
The study was funded by Ziopharm Oncology and the National Institutes of Health. The investigators reported additional relationships with Advantagene, Stemgen, Sigilon Therapeutics, and others.
SOURCE: Chiocca EA et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aaw5680.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: For patients with recurrent, high-grade glioblastoma, localized, drug-inducible gene therapy could unlock the anticancer potential of interleukin-12.
Major finding: After 13.1 months of follow-up, median overall survival was 12.7 months, compared with a weighted median overall survival among historical controls of 8.1 months.
Study details: A phase 1 trial involving 31 patients with recurrent glioblastoma.
Disclosures: The study was funded by Ziopharm Oncology and the National Institutes of Health. The investigators reported additional relationships with Advantagene, Stemgen, Sigilon Therapeutics, and others.
Source: Chiocca EA et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aaw5680.