User login
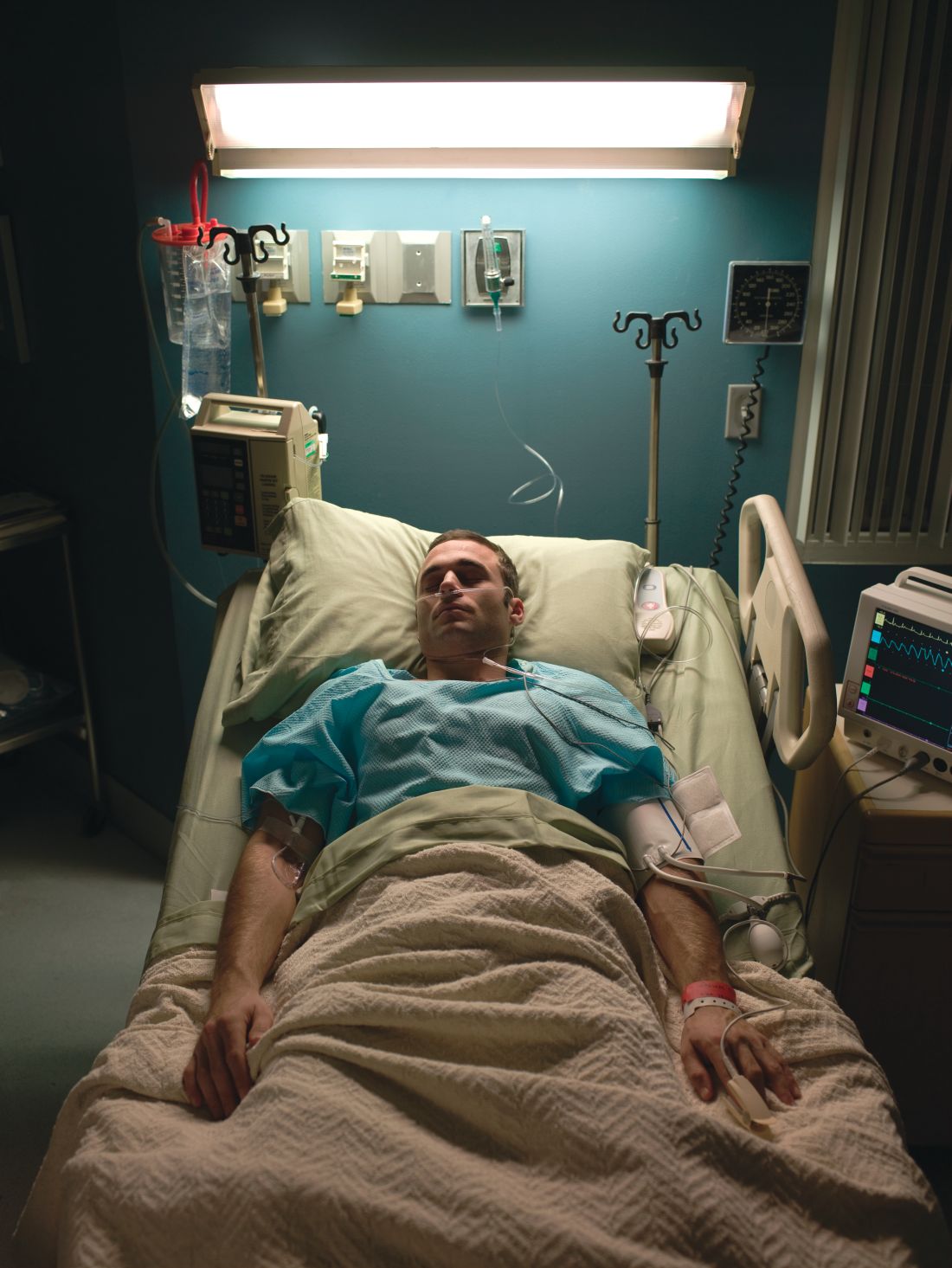
PCPs play role in identifying severe, difficult-to-treat asthma
ORLANDO – Clinicians at the primary care level should learn to distinguish the difference between severe and difficult-to-treat asthma to help facilitate referral to an asthma care specialist, according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Care providers are seeing patients with severe asthma at their primary care practice, whether they realize it or not, and should therefore learn to recognize and diagnose these patients even if they are not directly treating them for asthma, said Michelle R. Dickens, MSN, RN, FNP-C, AE-C, a nurse practitioner at Ferrell-Duncan Clinic department of allergy, asthma, and immunology in Springfield, Mo., said in her presentation.
“There’s still a lot we can do to get the ball rolling,” Ms. Dickens said. “While you may not be prescribing some of these newer biologics and some of the high-level, $30,000-a-year medications for your asthmatic, you still have a role in this in identifying them and helping us to get to where they need to be.”
Ms. Dickens noted that patients may not even disclose their asthma history with their primary care provider if it is not the reason for the office visit. “Because asthma can be such an episodic disease, it’s not right on the top of their radar,” she said. “They have bigger issues they want to talk about with you, and they forget to mention the asthma part.”
Under Global Initiative for Asthma (GINA) criteria, severe asthma is defined as poorly controlled asthma even after patients demonstrate adherence and good technique. However, before diagnosing a patient with severe asthma, clinicians should first rule out whether the patient has asthma that is difficult to treat, said Ms. Dickens. Difficult-to-treat asthma is characterized by inadequate dosing of medication, noncompliance with medication dose (“spreading out” the medication), poor technique when self-administering the medication, and comorbid conditions. It is also possible that difficult-to-treat asthma is not well controlled because the asthma was misdiagnosed and is actually another condition, she added.
If a patient has difficult-to-treat asthma, ensure they are adhering to the therapy, using proper technique, and that the medicine is being administered at the proper dose. Using Expert Panel Report 3 (EPR-3) and Global Initiative for Asthma (GINA) guidelines, clinicians should maximize the treatment for a patient with severe asthma based on the stepwise approach outlined in the guidelines, and referring out to an asthma specialist for add-on therapy or stepping up therapy when indicated.
Researchers are beginning to explore the genotypes, phenotypes, and endotypes of asthma to learn more about severe asthma and potentially identify asthma subtypes, said Ms. Dickens. “We’re not there yet, but we are learning a little bit about why certain patients have certain types of asthma,” she said.
The Severe Asthma Research Program has found three clusters of likely severe asthma candidates: those with classic childhood asthma onset, those who have asthma with chronic obstructive pulmonary disease (COPD), and patients with both obesity and asthma characterized by “high impairment but mildly abnormal lung function.” Clinicians can use spirometry, complete blood count with differential, total immunoglobulin E, body mass index, allergy testing, and exhaled nitric oxide with these data and biomarkers to gain a better idea of a patient’s asthma situation.
Referrals to an asthma specialist should be considered if a patient experiences a life-threatening exacerbation, is not responding to therapy, has an unusual presentation of asthma symptoms, has comorbid conditions, or needs additional testing or additional education. Patients who are at step 2 or higher in EPR-3 guidelines and ready to move to step 3 and are under 4 years of age, and patients who are 5 years or older at step 4 of therapy or higher should be referred to an asthma specialist. “I think the younger the patient and the more severe the [symptoms], the more likely you should be referring to a specialist,” said Ms. Dickens.
Ms. Dickens reports no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – Clinicians at the primary care level should learn to distinguish the difference between severe and difficult-to-treat asthma to help facilitate referral to an asthma care specialist, according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Care providers are seeing patients with severe asthma at their primary care practice, whether they realize it or not, and should therefore learn to recognize and diagnose these patients even if they are not directly treating them for asthma, said Michelle R. Dickens, MSN, RN, FNP-C, AE-C, a nurse practitioner at Ferrell-Duncan Clinic department of allergy, asthma, and immunology in Springfield, Mo., said in her presentation.
“There’s still a lot we can do to get the ball rolling,” Ms. Dickens said. “While you may not be prescribing some of these newer biologics and some of the high-level, $30,000-a-year medications for your asthmatic, you still have a role in this in identifying them and helping us to get to where they need to be.”
Ms. Dickens noted that patients may not even disclose their asthma history with their primary care provider if it is not the reason for the office visit. “Because asthma can be such an episodic disease, it’s not right on the top of their radar,” she said. “They have bigger issues they want to talk about with you, and they forget to mention the asthma part.”
Under Global Initiative for Asthma (GINA) criteria, severe asthma is defined as poorly controlled asthma even after patients demonstrate adherence and good technique. However, before diagnosing a patient with severe asthma, clinicians should first rule out whether the patient has asthma that is difficult to treat, said Ms. Dickens. Difficult-to-treat asthma is characterized by inadequate dosing of medication, noncompliance with medication dose (“spreading out” the medication), poor technique when self-administering the medication, and comorbid conditions. It is also possible that difficult-to-treat asthma is not well controlled because the asthma was misdiagnosed and is actually another condition, she added.
If a patient has difficult-to-treat asthma, ensure they are adhering to the therapy, using proper technique, and that the medicine is being administered at the proper dose. Using Expert Panel Report 3 (EPR-3) and Global Initiative for Asthma (GINA) guidelines, clinicians should maximize the treatment for a patient with severe asthma based on the stepwise approach outlined in the guidelines, and referring out to an asthma specialist for add-on therapy or stepping up therapy when indicated.
Researchers are beginning to explore the genotypes, phenotypes, and endotypes of asthma to learn more about severe asthma and potentially identify asthma subtypes, said Ms. Dickens. “We’re not there yet, but we are learning a little bit about why certain patients have certain types of asthma,” she said.
The Severe Asthma Research Program has found three clusters of likely severe asthma candidates: those with classic childhood asthma onset, those who have asthma with chronic obstructive pulmonary disease (COPD), and patients with both obesity and asthma characterized by “high impairment but mildly abnormal lung function.” Clinicians can use spirometry, complete blood count with differential, total immunoglobulin E, body mass index, allergy testing, and exhaled nitric oxide with these data and biomarkers to gain a better idea of a patient’s asthma situation.
Referrals to an asthma specialist should be considered if a patient experiences a life-threatening exacerbation, is not responding to therapy, has an unusual presentation of asthma symptoms, has comorbid conditions, or needs additional testing or additional education. Patients who are at step 2 or higher in EPR-3 guidelines and ready to move to step 3 and are under 4 years of age, and patients who are 5 years or older at step 4 of therapy or higher should be referred to an asthma specialist. “I think the younger the patient and the more severe the [symptoms], the more likely you should be referring to a specialist,” said Ms. Dickens.
Ms. Dickens reports no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – Clinicians at the primary care level should learn to distinguish the difference between severe and difficult-to-treat asthma to help facilitate referral to an asthma care specialist, according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Care providers are seeing patients with severe asthma at their primary care practice, whether they realize it or not, and should therefore learn to recognize and diagnose these patients even if they are not directly treating them for asthma, said Michelle R. Dickens, MSN, RN, FNP-C, AE-C, a nurse practitioner at Ferrell-Duncan Clinic department of allergy, asthma, and immunology in Springfield, Mo., said in her presentation.
“There’s still a lot we can do to get the ball rolling,” Ms. Dickens said. “While you may not be prescribing some of these newer biologics and some of the high-level, $30,000-a-year medications for your asthmatic, you still have a role in this in identifying them and helping us to get to where they need to be.”
Ms. Dickens noted that patients may not even disclose their asthma history with their primary care provider if it is not the reason for the office visit. “Because asthma can be such an episodic disease, it’s not right on the top of their radar,” she said. “They have bigger issues they want to talk about with you, and they forget to mention the asthma part.”
Under Global Initiative for Asthma (GINA) criteria, severe asthma is defined as poorly controlled asthma even after patients demonstrate adherence and good technique. However, before diagnosing a patient with severe asthma, clinicians should first rule out whether the patient has asthma that is difficult to treat, said Ms. Dickens. Difficult-to-treat asthma is characterized by inadequate dosing of medication, noncompliance with medication dose (“spreading out” the medication), poor technique when self-administering the medication, and comorbid conditions. It is also possible that difficult-to-treat asthma is not well controlled because the asthma was misdiagnosed and is actually another condition, she added.
If a patient has difficult-to-treat asthma, ensure they are adhering to the therapy, using proper technique, and that the medicine is being administered at the proper dose. Using Expert Panel Report 3 (EPR-3) and Global Initiative for Asthma (GINA) guidelines, clinicians should maximize the treatment for a patient with severe asthma based on the stepwise approach outlined in the guidelines, and referring out to an asthma specialist for add-on therapy or stepping up therapy when indicated.
Researchers are beginning to explore the genotypes, phenotypes, and endotypes of asthma to learn more about severe asthma and potentially identify asthma subtypes, said Ms. Dickens. “We’re not there yet, but we are learning a little bit about why certain patients have certain types of asthma,” she said.
The Severe Asthma Research Program has found three clusters of likely severe asthma candidates: those with classic childhood asthma onset, those who have asthma with chronic obstructive pulmonary disease (COPD), and patients with both obesity and asthma characterized by “high impairment but mildly abnormal lung function.” Clinicians can use spirometry, complete blood count with differential, total immunoglobulin E, body mass index, allergy testing, and exhaled nitric oxide with these data and biomarkers to gain a better idea of a patient’s asthma situation.
Referrals to an asthma specialist should be considered if a patient experiences a life-threatening exacerbation, is not responding to therapy, has an unusual presentation of asthma symptoms, has comorbid conditions, or needs additional testing or additional education. Patients who are at step 2 or higher in EPR-3 guidelines and ready to move to step 3 and are under 4 years of age, and patients who are 5 years or older at step 4 of therapy or higher should be referred to an asthma specialist. “I think the younger the patient and the more severe the [symptoms], the more likely you should be referring to a specialist,” said Ms. Dickens.
Ms. Dickens reports no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Keep your staff current – and happy
It goes without saying that as a physician, it’s essential to keep your knowledge and skills current. But too many private practitioners overlook the similar needs of their employees.
Like you, staff members provide better care to patients when they know the latest findings and techniques. They also provide better information: When patients ask questions of your staff, either in the office or over the phone (which happens more often than you probably think), you certainly want their answers to be accurate and up to date.
But there are lots of other good reasons to invest in ongoing staff training. It’s a win-win strategy for you, your staff, and for your practice.
The more your employees know, the more productive they will be. Not only will they complete everyday duties more efficiently, they will be stimulated to learn new tasks and accept more responsibility.
Staffers who have learned new skills are more willing to take on new challenges. And the better their skills and the greater their confidence, the less supervision they need from you, and the more they become involved in their work.
They will also be happier in their jobs. Investing in your employees’ competence makes them feel valued and appreciated. This leads to reduced turnover – which, alone, often pays for the training.
You probably already do some ongoing education: You do your yearly OSHA training because the law requires it, you run HIPAA updates as necessary, and you have everyone recertified periodically in basic or advanced CPR (I hope). But I’m talking about going beyond the basic stuff, which may satisfy legal requirements, but does not motivate your people to loftier goals.
An obvious example is sending your insurance people annually to coding and insurance processing courses – or at the very least, online refreshers – so they are always current on the latest third-party changes. The use of outdated or obsolete codes can cost you thousands of dollars every month. Other opportunities include keyboarding and computer courses for staff who work with your computers, and Excel and QuickBooks updates for your bookkeepers.
Continuing education does not have to be costly, and in some cases it can be free. For example, pharmaceutical representatives will be happy to run an in-service for your staff on a new medication or procedure or instrument, or refresh their memories on an established one. Be sure to make clear to the rep that the presentation must be as objective and impartial as possible, given the obvious potential conflict of interest involved.
Your office manager should join the Association of Dermatology Administrators and Managers. It holds annual meetings at the same time and in the same city as the American Academy of Dermatology winter meetings, with a good selection of refresher courses and lots of opportunities for networking with other managers, both personally or virtually.
Many other venues are available for employee education, in the cloud and in conventional classrooms. Courses are offered in many relevant subjects; a quick Google search turns up an eclectic mix, including medical terminology, record keeping and accounting, laboratory skills, diagnostic tests and procedures, pharmacology and medication administration, patient relations, medical law and ethics, and many others.
By far the most common question I receive on this issue is, “What if I pay for all that training, and then the employees leave?”
My reply: “What if you don’t, and they stay?”
Well-trained employees are vastly preferable to untrained ones. I suppose there is some risk of an occasional staffer accepting training and then moving on; but in 38 years, it has never happened in my office. In my experience, well-trained employees will stay. Education fosters loyalty. Employees who know you care enough about them to advance their skills will sense that they have a stake in the practice, and thus will be less likely to want to leave. Furthermore, continuing education will always be cheaper than training new employees from scratch.
In any case, everyone will benefit from a well-trained staff – you, your employees, your practice, and most importantly your patients.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It goes without saying that as a physician, it’s essential to keep your knowledge and skills current. But too many private practitioners overlook the similar needs of their employees.
Like you, staff members provide better care to patients when they know the latest findings and techniques. They also provide better information: When patients ask questions of your staff, either in the office or over the phone (which happens more often than you probably think), you certainly want their answers to be accurate and up to date.
But there are lots of other good reasons to invest in ongoing staff training. It’s a win-win strategy for you, your staff, and for your practice.
The more your employees know, the more productive they will be. Not only will they complete everyday duties more efficiently, they will be stimulated to learn new tasks and accept more responsibility.
Staffers who have learned new skills are more willing to take on new challenges. And the better their skills and the greater their confidence, the less supervision they need from you, and the more they become involved in their work.
They will also be happier in their jobs. Investing in your employees’ competence makes them feel valued and appreciated. This leads to reduced turnover – which, alone, often pays for the training.
You probably already do some ongoing education: You do your yearly OSHA training because the law requires it, you run HIPAA updates as necessary, and you have everyone recertified periodically in basic or advanced CPR (I hope). But I’m talking about going beyond the basic stuff, which may satisfy legal requirements, but does not motivate your people to loftier goals.
An obvious example is sending your insurance people annually to coding and insurance processing courses – or at the very least, online refreshers – so they are always current on the latest third-party changes. The use of outdated or obsolete codes can cost you thousands of dollars every month. Other opportunities include keyboarding and computer courses for staff who work with your computers, and Excel and QuickBooks updates for your bookkeepers.
Continuing education does not have to be costly, and in some cases it can be free. For example, pharmaceutical representatives will be happy to run an in-service for your staff on a new medication or procedure or instrument, or refresh their memories on an established one. Be sure to make clear to the rep that the presentation must be as objective and impartial as possible, given the obvious potential conflict of interest involved.
Your office manager should join the Association of Dermatology Administrators and Managers. It holds annual meetings at the same time and in the same city as the American Academy of Dermatology winter meetings, with a good selection of refresher courses and lots of opportunities for networking with other managers, both personally or virtually.
Many other venues are available for employee education, in the cloud and in conventional classrooms. Courses are offered in many relevant subjects; a quick Google search turns up an eclectic mix, including medical terminology, record keeping and accounting, laboratory skills, diagnostic tests and procedures, pharmacology and medication administration, patient relations, medical law and ethics, and many others.
By far the most common question I receive on this issue is, “What if I pay for all that training, and then the employees leave?”
My reply: “What if you don’t, and they stay?”
Well-trained employees are vastly preferable to untrained ones. I suppose there is some risk of an occasional staffer accepting training and then moving on; but in 38 years, it has never happened in my office. In my experience, well-trained employees will stay. Education fosters loyalty. Employees who know you care enough about them to advance their skills will sense that they have a stake in the practice, and thus will be less likely to want to leave. Furthermore, continuing education will always be cheaper than training new employees from scratch.
In any case, everyone will benefit from a well-trained staff – you, your employees, your practice, and most importantly your patients.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It goes without saying that as a physician, it’s essential to keep your knowledge and skills current. But too many private practitioners overlook the similar needs of their employees.
Like you, staff members provide better care to patients when they know the latest findings and techniques. They also provide better information: When patients ask questions of your staff, either in the office or over the phone (which happens more often than you probably think), you certainly want their answers to be accurate and up to date.
But there are lots of other good reasons to invest in ongoing staff training. It’s a win-win strategy for you, your staff, and for your practice.
The more your employees know, the more productive they will be. Not only will they complete everyday duties more efficiently, they will be stimulated to learn new tasks and accept more responsibility.
Staffers who have learned new skills are more willing to take on new challenges. And the better their skills and the greater their confidence, the less supervision they need from you, and the more they become involved in their work.
They will also be happier in their jobs. Investing in your employees’ competence makes them feel valued and appreciated. This leads to reduced turnover – which, alone, often pays for the training.
You probably already do some ongoing education: You do your yearly OSHA training because the law requires it, you run HIPAA updates as necessary, and you have everyone recertified periodically in basic or advanced CPR (I hope). But I’m talking about going beyond the basic stuff, which may satisfy legal requirements, but does not motivate your people to loftier goals.
An obvious example is sending your insurance people annually to coding and insurance processing courses – or at the very least, online refreshers – so they are always current on the latest third-party changes. The use of outdated or obsolete codes can cost you thousands of dollars every month. Other opportunities include keyboarding and computer courses for staff who work with your computers, and Excel and QuickBooks updates for your bookkeepers.
Continuing education does not have to be costly, and in some cases it can be free. For example, pharmaceutical representatives will be happy to run an in-service for your staff on a new medication or procedure or instrument, or refresh their memories on an established one. Be sure to make clear to the rep that the presentation must be as objective and impartial as possible, given the obvious potential conflict of interest involved.
Your office manager should join the Association of Dermatology Administrators and Managers. It holds annual meetings at the same time and in the same city as the American Academy of Dermatology winter meetings, with a good selection of refresher courses and lots of opportunities for networking with other managers, both personally or virtually.
Many other venues are available for employee education, in the cloud and in conventional classrooms. Courses are offered in many relevant subjects; a quick Google search turns up an eclectic mix, including medical terminology, record keeping and accounting, laboratory skills, diagnostic tests and procedures, pharmacology and medication administration, patient relations, medical law and ethics, and many others.
By far the most common question I receive on this issue is, “What if I pay for all that training, and then the employees leave?”
My reply: “What if you don’t, and they stay?”
Well-trained employees are vastly preferable to untrained ones. I suppose there is some risk of an occasional staffer accepting training and then moving on; but in 38 years, it has never happened in my office. In my experience, well-trained employees will stay. Education fosters loyalty. Employees who know you care enough about them to advance their skills will sense that they have a stake in the practice, and thus will be less likely to want to leave. Furthermore, continuing education will always be cheaper than training new employees from scratch.
In any case, everyone will benefit from a well-trained staff – you, your employees, your practice, and most importantly your patients.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Study: Cardiac biomarkers predicted CV events in CAP
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
FROM CHEST
Lasmiditan is associated with driving impairment
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
REPORTING FROM AHS 2019
Medicare’s CAR T-cell coverage decision draws praise, but cost issues linger
Physicians are praising the decision by officials at the Centers for Medicare & Medicaid Services to provide coverage of chimeric antigen receptor T-cell therapy, though they say the planned payment structure will still leave hospitals in the red when treatment is administered.
On Aug. 7, 2019, the agency issued a national coverage determination that outlines Medicare coverage of chimeric antigen receptor (CAR) T-cell therapies when they are provided in health care facilities enrolled in the Food and Drug Administration’s Risk Evaluation and Mitigation Strategies program. Medicare will cover treatments for both FDA-approved indications and off-label uses that are recommended in CMS-approved compendia.
“What you’ve seen in both the [Medicare Inpatient Prospective Payment System] rule, as well as this coverage determination, is recognition by CMS that, with CAR T cells, we are dealing with something different and something extraordinary,” Joseph Alvarnas, MD, vice president of government affairs at City of Hope, Duarte, Calif., said in an interview. “For a lot of patients who suffer with non-Hodgkin’s lymphoma, the consequences of being refractory to standard therapies mean that many patients have few great prospects for moving forward with curative treatments. CAR T cells represent a really innovative set of treatments for patients.”
A proposed national coverage determination, issued in February 2019, would have put in place a coverage with evidence development (CED) requirement for CAR T-cell therapy, covering treatment nationwide if it was offered through CMS-approved registries or clinical studies in which patients were monitored for 2 or more years following treatment.
Physicians applauded the decision not to restrict access by imposing the CED requirement.
“I think what CMS has put out is a good thing,” said Navneet Majhail, MD, director of the Cleveland Clinic’s blood and marrow transplant program, and president of the American Society for Transplantation and Cellular Therapy.
“Both at the Cleveland Clinic level and the society level, we have been asking CMS for something similar and we are really glad and excited that CMS did do this. The concern was that CMS might do this in the context of some other regulatory requirements like CED that they sometimes do. I am glad that CMS decided not to put that mechanism into place for the CAR T-cell therapies,” Dr. Majhail said.
Dr. Alvarnas, who also serves as chair of the American Society of Hematology Committee on Practice, agreed. “I see good. I don’t see bad. I have read through this and it strikes me as being written with fairly great clarity.”
Dr. Alvarnas added that he had been worried about potential restrictions, such as CED. “Once you put something under that whole rubric of coverage with evidence development, then what you do is you create a bottleneck around access to therapy because you have to have an accruing clinical trial for patients to, in fact, be able to participate in that form of therapy.”
By not imposing a CED requirement, it opens the door to better understanding the role CAR T cells play in treatment, Dr. Alvarnas noted.
“Over time, the number of patients for whom these therapeutics work, based upon real medical evidence, will escalate and grow at a pace that can far exceed the restrictions placed under a CED model,” he said, adding that the national coverage determination “gives us the license to deliver therapeutics to the right patients based upon medical evidence as it evolves, provided that these things get listed as part of the compendia. I think that is a fantastic recognition that new roles for drugs, agents, therapeutics ... are going to evolve at a pace far faster than what CMS can write rules about.”
While Medicare’s coverage determination garnered positive reviews, the agency’s Inpatient Prospective Payment System final rule – which outlines reimbursement for CAR T-cell therapy and other new technologies – got a more tepid response.
In the final rule, CMS raised the payment it makes to hospitals for administering CAR T-cell therapies through its new technology add-on payment. Payments will rise from 50% of the technology to 65%, an increase from $186,500 to $242,450 for CAR T-cell therapies, beginning on Oct. 1, 2019.
But even the bump up to 65% may not be enough.
“I see the move to 65% as a new technology add-on payment as an incremental step in the correct direction, but what we’ve done to some extent is that we’ve delayed getting to some sort of more wholly conceived system,” Dr. Alvarnas said, noting that a new system will be needed as the new technology add-on payment goes away in 2021.
Abhinav Deol, MD, of the Karmanos Cancer Institute in Detroit said it’s a challenge to cover costs for the treatment. “If you just look at the simple math, it is still going to be an economic challenge. The cells that are approved for lymphoma patients that will probably fall into the Medicare category, the list price of those cells is $373,000. Even with the 65% coverage, it’s about $235,000-$240,000 in reimbursement,” he said. “For a facility to be able to provide the care for patients, you have that delta that is still not covered. It is still going to be an economic challenge for many of the facilities to provide this care.”
Thomas LeBlanc, MD, an associate professor of medicine at Duke University, Durham, N.C., said that, while the coverage determination is a positive step, it’s not clear that it will provide meaningful access to CAR T-cell therapy because of the cost.
“These products are incredibly expensive, and the total cost of providing them is woefully underestimated in only focusing on the sticker price of the product,” he said. “Doing so ignores the significant hospital care, sometimes even critical care, as well as specialized knowledge and high touch supportive care, all of which is required to safely get patients through this revolutionary yet often risky treatment. So when CMS offers to pay just 65% of the sticker price, I suspect that many institutions will still lose six figures for each patient treated.”
Dr. LeBlanc predicted that many centers will decline to provide CAR T-cell therapy despite the increase in the new technology add-on payment, though he added that “I’d love to be wrong about this.”
Dr. Majhail agreed, noting that, even with the bump in the add-on payment, hospitals “won’t be whole in terms of providing care for these patients.”
“The reimbursement piece continues to be a challenge,” he said. “It is better than what it was, but there is still more work to be done. That is something we will have to keep working with the agency on.”
Physicians are praising the decision by officials at the Centers for Medicare & Medicaid Services to provide coverage of chimeric antigen receptor T-cell therapy, though they say the planned payment structure will still leave hospitals in the red when treatment is administered.
On Aug. 7, 2019, the agency issued a national coverage determination that outlines Medicare coverage of chimeric antigen receptor (CAR) T-cell therapies when they are provided in health care facilities enrolled in the Food and Drug Administration’s Risk Evaluation and Mitigation Strategies program. Medicare will cover treatments for both FDA-approved indications and off-label uses that are recommended in CMS-approved compendia.
“What you’ve seen in both the [Medicare Inpatient Prospective Payment System] rule, as well as this coverage determination, is recognition by CMS that, with CAR T cells, we are dealing with something different and something extraordinary,” Joseph Alvarnas, MD, vice president of government affairs at City of Hope, Duarte, Calif., said in an interview. “For a lot of patients who suffer with non-Hodgkin’s lymphoma, the consequences of being refractory to standard therapies mean that many patients have few great prospects for moving forward with curative treatments. CAR T cells represent a really innovative set of treatments for patients.”
A proposed national coverage determination, issued in February 2019, would have put in place a coverage with evidence development (CED) requirement for CAR T-cell therapy, covering treatment nationwide if it was offered through CMS-approved registries or clinical studies in which patients were monitored for 2 or more years following treatment.
Physicians applauded the decision not to restrict access by imposing the CED requirement.
“I think what CMS has put out is a good thing,” said Navneet Majhail, MD, director of the Cleveland Clinic’s blood and marrow transplant program, and president of the American Society for Transplantation and Cellular Therapy.
“Both at the Cleveland Clinic level and the society level, we have been asking CMS for something similar and we are really glad and excited that CMS did do this. The concern was that CMS might do this in the context of some other regulatory requirements like CED that they sometimes do. I am glad that CMS decided not to put that mechanism into place for the CAR T-cell therapies,” Dr. Majhail said.
Dr. Alvarnas, who also serves as chair of the American Society of Hematology Committee on Practice, agreed. “I see good. I don’t see bad. I have read through this and it strikes me as being written with fairly great clarity.”
Dr. Alvarnas added that he had been worried about potential restrictions, such as CED. “Once you put something under that whole rubric of coverage with evidence development, then what you do is you create a bottleneck around access to therapy because you have to have an accruing clinical trial for patients to, in fact, be able to participate in that form of therapy.”
By not imposing a CED requirement, it opens the door to better understanding the role CAR T cells play in treatment, Dr. Alvarnas noted.
“Over time, the number of patients for whom these therapeutics work, based upon real medical evidence, will escalate and grow at a pace that can far exceed the restrictions placed under a CED model,” he said, adding that the national coverage determination “gives us the license to deliver therapeutics to the right patients based upon medical evidence as it evolves, provided that these things get listed as part of the compendia. I think that is a fantastic recognition that new roles for drugs, agents, therapeutics ... are going to evolve at a pace far faster than what CMS can write rules about.”
While Medicare’s coverage determination garnered positive reviews, the agency’s Inpatient Prospective Payment System final rule – which outlines reimbursement for CAR T-cell therapy and other new technologies – got a more tepid response.
In the final rule, CMS raised the payment it makes to hospitals for administering CAR T-cell therapies through its new technology add-on payment. Payments will rise from 50% of the technology to 65%, an increase from $186,500 to $242,450 for CAR T-cell therapies, beginning on Oct. 1, 2019.
But even the bump up to 65% may not be enough.
“I see the move to 65% as a new technology add-on payment as an incremental step in the correct direction, but what we’ve done to some extent is that we’ve delayed getting to some sort of more wholly conceived system,” Dr. Alvarnas said, noting that a new system will be needed as the new technology add-on payment goes away in 2021.
Abhinav Deol, MD, of the Karmanos Cancer Institute in Detroit said it’s a challenge to cover costs for the treatment. “If you just look at the simple math, it is still going to be an economic challenge. The cells that are approved for lymphoma patients that will probably fall into the Medicare category, the list price of those cells is $373,000. Even with the 65% coverage, it’s about $235,000-$240,000 in reimbursement,” he said. “For a facility to be able to provide the care for patients, you have that delta that is still not covered. It is still going to be an economic challenge for many of the facilities to provide this care.”
Thomas LeBlanc, MD, an associate professor of medicine at Duke University, Durham, N.C., said that, while the coverage determination is a positive step, it’s not clear that it will provide meaningful access to CAR T-cell therapy because of the cost.
“These products are incredibly expensive, and the total cost of providing them is woefully underestimated in only focusing on the sticker price of the product,” he said. “Doing so ignores the significant hospital care, sometimes even critical care, as well as specialized knowledge and high touch supportive care, all of which is required to safely get patients through this revolutionary yet often risky treatment. So when CMS offers to pay just 65% of the sticker price, I suspect that many institutions will still lose six figures for each patient treated.”
Dr. LeBlanc predicted that many centers will decline to provide CAR T-cell therapy despite the increase in the new technology add-on payment, though he added that “I’d love to be wrong about this.”
Dr. Majhail agreed, noting that, even with the bump in the add-on payment, hospitals “won’t be whole in terms of providing care for these patients.”
“The reimbursement piece continues to be a challenge,” he said. “It is better than what it was, but there is still more work to be done. That is something we will have to keep working with the agency on.”
Physicians are praising the decision by officials at the Centers for Medicare & Medicaid Services to provide coverage of chimeric antigen receptor T-cell therapy, though they say the planned payment structure will still leave hospitals in the red when treatment is administered.
On Aug. 7, 2019, the agency issued a national coverage determination that outlines Medicare coverage of chimeric antigen receptor (CAR) T-cell therapies when they are provided in health care facilities enrolled in the Food and Drug Administration’s Risk Evaluation and Mitigation Strategies program. Medicare will cover treatments for both FDA-approved indications and off-label uses that are recommended in CMS-approved compendia.
“What you’ve seen in both the [Medicare Inpatient Prospective Payment System] rule, as well as this coverage determination, is recognition by CMS that, with CAR T cells, we are dealing with something different and something extraordinary,” Joseph Alvarnas, MD, vice president of government affairs at City of Hope, Duarte, Calif., said in an interview. “For a lot of patients who suffer with non-Hodgkin’s lymphoma, the consequences of being refractory to standard therapies mean that many patients have few great prospects for moving forward with curative treatments. CAR T cells represent a really innovative set of treatments for patients.”
A proposed national coverage determination, issued in February 2019, would have put in place a coverage with evidence development (CED) requirement for CAR T-cell therapy, covering treatment nationwide if it was offered through CMS-approved registries or clinical studies in which patients were monitored for 2 or more years following treatment.
Physicians applauded the decision not to restrict access by imposing the CED requirement.
“I think what CMS has put out is a good thing,” said Navneet Majhail, MD, director of the Cleveland Clinic’s blood and marrow transplant program, and president of the American Society for Transplantation and Cellular Therapy.
“Both at the Cleveland Clinic level and the society level, we have been asking CMS for something similar and we are really glad and excited that CMS did do this. The concern was that CMS might do this in the context of some other regulatory requirements like CED that they sometimes do. I am glad that CMS decided not to put that mechanism into place for the CAR T-cell therapies,” Dr. Majhail said.
Dr. Alvarnas, who also serves as chair of the American Society of Hematology Committee on Practice, agreed. “I see good. I don’t see bad. I have read through this and it strikes me as being written with fairly great clarity.”
Dr. Alvarnas added that he had been worried about potential restrictions, such as CED. “Once you put something under that whole rubric of coverage with evidence development, then what you do is you create a bottleneck around access to therapy because you have to have an accruing clinical trial for patients to, in fact, be able to participate in that form of therapy.”
By not imposing a CED requirement, it opens the door to better understanding the role CAR T cells play in treatment, Dr. Alvarnas noted.
“Over time, the number of patients for whom these therapeutics work, based upon real medical evidence, will escalate and grow at a pace that can far exceed the restrictions placed under a CED model,” he said, adding that the national coverage determination “gives us the license to deliver therapeutics to the right patients based upon medical evidence as it evolves, provided that these things get listed as part of the compendia. I think that is a fantastic recognition that new roles for drugs, agents, therapeutics ... are going to evolve at a pace far faster than what CMS can write rules about.”
While Medicare’s coverage determination garnered positive reviews, the agency’s Inpatient Prospective Payment System final rule – which outlines reimbursement for CAR T-cell therapy and other new technologies – got a more tepid response.
In the final rule, CMS raised the payment it makes to hospitals for administering CAR T-cell therapies through its new technology add-on payment. Payments will rise from 50% of the technology to 65%, an increase from $186,500 to $242,450 for CAR T-cell therapies, beginning on Oct. 1, 2019.
But even the bump up to 65% may not be enough.
“I see the move to 65% as a new technology add-on payment as an incremental step in the correct direction, but what we’ve done to some extent is that we’ve delayed getting to some sort of more wholly conceived system,” Dr. Alvarnas said, noting that a new system will be needed as the new technology add-on payment goes away in 2021.
Abhinav Deol, MD, of the Karmanos Cancer Institute in Detroit said it’s a challenge to cover costs for the treatment. “If you just look at the simple math, it is still going to be an economic challenge. The cells that are approved for lymphoma patients that will probably fall into the Medicare category, the list price of those cells is $373,000. Even with the 65% coverage, it’s about $235,000-$240,000 in reimbursement,” he said. “For a facility to be able to provide the care for patients, you have that delta that is still not covered. It is still going to be an economic challenge for many of the facilities to provide this care.”
Thomas LeBlanc, MD, an associate professor of medicine at Duke University, Durham, N.C., said that, while the coverage determination is a positive step, it’s not clear that it will provide meaningful access to CAR T-cell therapy because of the cost.
“These products are incredibly expensive, and the total cost of providing them is woefully underestimated in only focusing on the sticker price of the product,” he said. “Doing so ignores the significant hospital care, sometimes even critical care, as well as specialized knowledge and high touch supportive care, all of which is required to safely get patients through this revolutionary yet often risky treatment. So when CMS offers to pay just 65% of the sticker price, I suspect that many institutions will still lose six figures for each patient treated.”
Dr. LeBlanc predicted that many centers will decline to provide CAR T-cell therapy despite the increase in the new technology add-on payment, though he added that “I’d love to be wrong about this.”
Dr. Majhail agreed, noting that, even with the bump in the add-on payment, hospitals “won’t be whole in terms of providing care for these patients.”
“The reimbursement piece continues to be a challenge,” he said. “It is better than what it was, but there is still more work to be done. That is something we will have to keep working with the agency on.”
Treatment of episodic cluster headache deviates from recommendations
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
REPORTING FROM AHS 2019
What is the future of celiac disease management?
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
EXPERT ANALYSIS FROM THE 2019 FRESTON CONFERENCE
Are rigid HPV vaccination schedules really necessary?
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
REPORTING FROM ESPID 2019
Exposure to outdoor air pollutants linked to increased emphysema
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
FROM JAMA
The Impact of Diet on Psoriasis
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
Practice Points
- No single food, supplement, or diet has been shown to have a notable positive impact on all variations of psoriasis. However, foods with systemic anti-inflammatory effects may be worth testing and adding to the patient’s diet.
- A considerable proportion of patients with psoriasis also have elevated levels of antigliadin antibody. If patients have celiac disease or high levels of antigliadin antibody, switching them to a gluten-free diet could have a positive impact on their psoriasis.
- Elevated body mass index, weight gain, smoking, and obesity are all associated with a higher risk for psoriasis appearance and severity.