User login
So Much More than Bald and Bloated
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
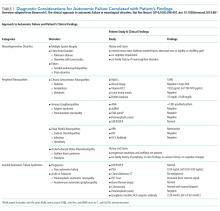
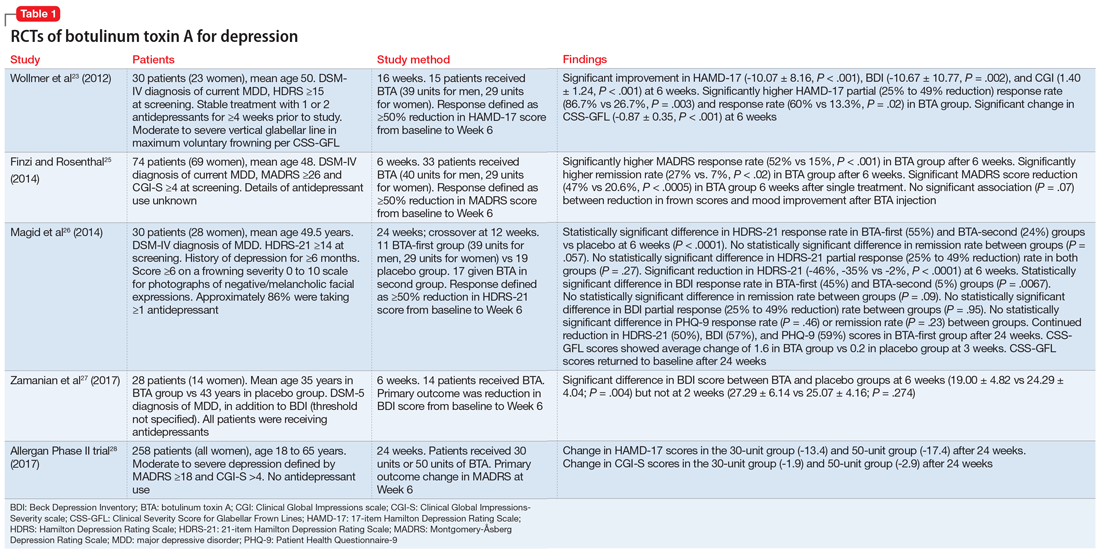
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
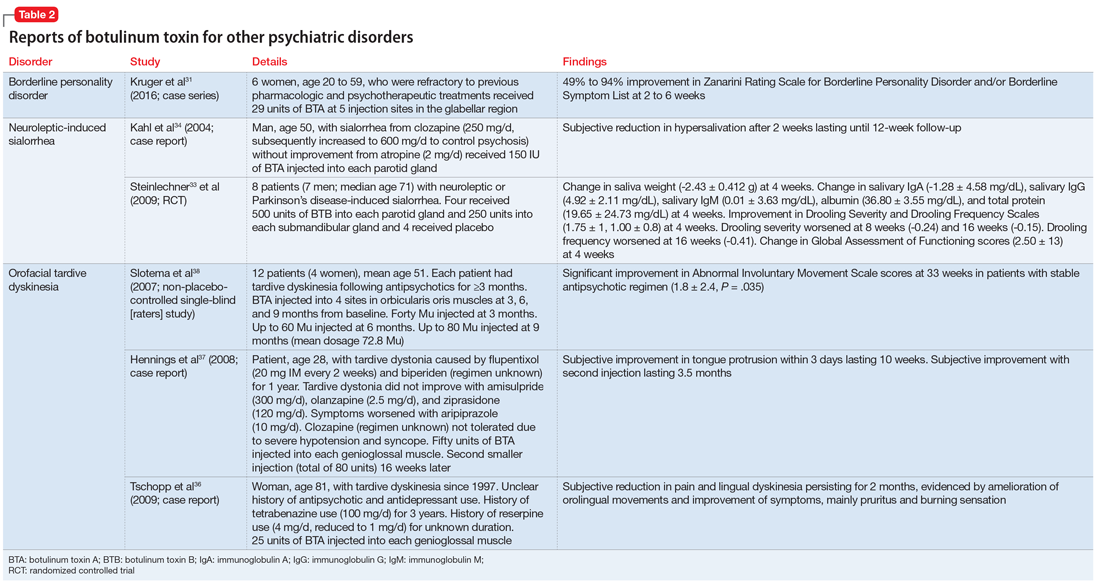
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
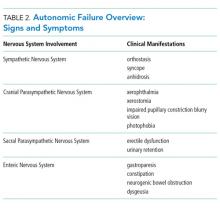
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
© 2018 Society of Hospital Medicine
A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM)
Palliative care is an essential component of inpatient medicine. At its core, it is an interdisciplinary philosophy of care aiming to achieve the best quality of life for patients and families in the physical, psychosocial, and spiritual domains. With the aging population and growing complexity of hospitalized patients, inpatient palliative care needs are only projected to rise. However, a mismatch exists between the number of palliative care–trained physicians and the demand for such physicians. Currently, only 6,600 US physicians are board certified in palliative care – just 37% of the projected need.1 These workforce shortages have serious implications. In fact, it is estimated that nearly 40% of all hospitalized patients who need palliative care go without it.2
Existing efforts to improve access to palliative care have largely focused on bolstering the palliative care workforce. One tactic particularly relevant to hospitalists centers on frontline physicians providing “primary” palliative care: basic symptom management, patient-centered communication, and goals of care assessment, regardless of the disease state.3 Such physicians constitute the base of today’s palliative care workforce model – a three-tiered pyramid built on clinician availability and skills. In this model, the second tier (“secondary” palliative care) includes physicians supported by a palliative care consultant or referral. The third level (“tertiary” palliative care) encompasses care provided directly by specialized palliative care teams, usually within academic medical centers (Figure).4
The practice of primary palliative care is central to the practice of hospital medicine.5,6 After all, hospitalists generate nearly half of all inpatient palliative care consultations7 and routinely interface with social workers, pharmacists, nurses, chaplains, and other consultants in their daily activities. Consequently, they are also well versed in serious illness communication and prognostication.8 In many ways, they are ideal purveyors of palliative care in the hospital.
Why then does the challenge to meet the demands of patients with palliative care needs persist? The truth may lie in at least three central shortcomings within the tiered palliative care workforce model. First, physicians comprising the base (where hospitalists typically fall) possess variable skills and knowledge in caring for seriously ill patients. While training opportunities exist for interested individuals,7 education alone can rarely achieve a systematic change. Second, some physicians may have the requisite skills but lack
The Palliative Care Redistribution Integrated Service Model (PRISM)
To better address the current palliative care access problem, we propose a new model: “The Palliative care Redistribution Integrated Service Model (PRISM; Figure 1).” Using the industrial engineering principle of “task shifting,” this approach leverages disciplinary diversity and shifts specific activities from more specialized to less specialized members.9 In this way, PRISM integrates hospital-based interdisciplinary teams across all tiers of palliative care delivery.
PRISM sheds a tier-based approach in favor of flexible, skill-based verticals that span all physician and nonphysician providers. By dividing the original pyramid into three domains – physical, psychosocial, and spiritual – providers with various spheres of expertise may serve patients on multiple tiers. For example, a bedside nurse may perform basic psychosocial assessment consistent with his or her training, while physicians may focus on code status or prescribe antiemetics or low-dose opiate monotherapy – skills they have refined during medical school. Analogously, secondary palliative care may be delivered by any provider with more advanced skills in communication or symptom management. In this way, we expand the pool of clinicians available to provide palliative care to include nurses, hospitalists, oncologists, intensivists, social workers, and chaplains and also recognize the diversity of skill sets within and between disciplines. Thus, a hospitalist may clarify the goals of care but may ask a social worker trained in psychosocial assessment for assistance with difficult family dynamics or a chaplain for spiritual needs. Interdisciplinary teamwork and cross-disciplinary communication – hallmarks of palliative care – are encouraged and valued. Furthermore, if providers feel uncomfortable providing a certain type of care, they can ask for assistance from more experienced providers within their discipline or outside of it. In rare cases, the most complex patients may be referred to specialist palliative care teams.
Inherent within PRISM is a recognition that all providers must have a basic palliative care skillset obtained through educational initiatives.7 Yet focusing solely on training the workforce as a strategy has and will continue to miss the mark. Rather, structural changes to the means of providing care are also needed. Within hospitals, these changes often rely heavily on hospitalists due to their central position in care delivery. In this way, hospitalists are well primed to be the agents of change in this model.
The Role of Technology
Since many hospitalized patients have unrecognized and underserved palliative care needs, a formal approach to assessment is needed. Lin et al. proposed criteria for a “sentinel hospitalization,” marking a major illness or transition in high-risk patients necessitating palliative interventions.10 Similar screening criteria have been validated among hospitalized oncology patients11 and in critical care.12 While checklists have been shown to help identify hospitalized patients with palliative care needs,13 their implementation has been slow, presumably because they are burdensome for busy providers to complete.
Technological automation may be a solution to the checklist conundrum. For example, if palliative care screening criteria could be automatically extracted from electronic health records, scoring systems could trigger hospitalists to consider the goals of care discussions or engage an interdisciplinary care team to fulfill a variety of needs. Frameworks for such scoring systems already exist and are familiar to most hospitalists. For example, admission order sets routinely calculate the Padua or Caprini score to facilitate decision-making for prophylaxis of deep vein thrombosis. An admission order set that screens and prompts decision-making around palliative care needs is thus feasible. One example is a hard stop for entering code status in the admission order set; in turn, this hard stop could also trigger providers to complete a “check-box” palliative care screening checklist. Automatic extraction of certain data from the record – such as age, prior code status, recent hospitalizations, or mobility scores – could auto-populate to facilitate decision-making. In turn, measuring the influence of such tools on access to palliative care, workflow, and capacity will be important, as most tools may not have quality or value intended.14
Streamlining Workflow
It is common for hospitalists to oversee care for 15-20 patients at a time. Thus, they may not have the time to meaningfully engage patients to assess palliative care needs. Creating designated hospitalist palliative care teams with enhanced interdisciplinary support for patients identified using sentinel hospitalization or checklist-based tools may help to solve this dilemma. These teams may also employ lower “caps,” freeing up time for critical discussions and planning around end of life. At the University of Michigan, we are planning just such an approach, a strategy which has the additional benefit of bypassing the binary “care versus no care” dilemma faced by patients choosing palliation. Rather, patients may continue to receive treatments congruent with the goals of care in such teams.
Making Palliative Care a Standard of Care
A call for health systems to develop and implement palliative care quality metrics has emerged. Given their role in quality improvement and health system reform, hospitalists are well positioned to shepherd this imperative. Creating incentives to screen inpatients for palliative care needs and develop new homes in which to care for these patients are but a few ways to help set the tone. Additionally, developing and sharing quality metrics and benchmarks currently captured in repositories such as the Palliative Care Quality Network, Global Palliative Care Quality Alliance, and Center to Advance Palliative Care can help to assess and continually improve care delivery. Creating and sharing dashboards from these metrics with all providers, regardless of discipline or training, will ensure accountability to deliver quality palliative care.
CONCLUSION
Many hospitalized patients do not receive appropriate attention to their palliative care needs. A new interdisciplinary workforce model that task shifts to physician and nonphysician providers and pairs system-level innovations and quality may solve this problem. Input and endorsement from a wide variety of disciplines (particularly our nonphysician colleagues) are needed to make PRISM operational. The proof of concept will lie in testing feasibility among key stakeholders and rigorously studying the proposed interventions. Through innovation in technology, workflow, and quality improvement, hospitalists are well poised to lead this change. After all, our patients deserve nothing less.
Disclosures
The authors have nothing to disclose.Funding: Dr. Abedini’s work is supported by the University of Michigan National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, as well as the Un
1. Lupu D. American Academy of Hospice and Palliative Medicine Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911. doi: 10.1016/j.jpainsymman.2010.07.004. PubMed
2. Chuang E, Hope AA, Allyn K, Szalkiewicz E, Gary B, Gong MN. Gaps in provision of primary and specialty palliative care in the acute care setting by race and ethnicity. J Pain Symptom Manage. 2017;54(5):645-653. doi: 10.1016/j.jpainsymman.2017.05.001 PubMed
3. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi: 10.1056/NEJMp1215620 PubMed
4. von Gunten CF. Secondary and tertiary palliative care in US hospitals. JAMA. 2002;287(7):875-881. doi: 10.1001/jama.287.7.875 PubMed
5. Pantilat SZ. Hope to reality: the future of hospitalists and palliative care. J Hosp Med. 2015;10(10):701-702. doi: 10.1002/jhm.2401 PubMed
6. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1016/j.cger.2004.07.006 PubMed
7. Fail RE, Meier DE. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. doi: 10.12788/jhm.2896. [Epub ahead of print] PubMed
8. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and barriers to serious illness communication: A national survey of hospitalists. J Palliat Med. 2017;20(9):1013-1019. doi: 10.1089/jpm.2016.0515 PubMed
9. Carayon P, Gurses AP. Nursing workload and patient safety–a human factors engineering perspective. In: Hughes RG, ed.Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160 PubMed
11. Glare PA, Chow K. Validation of a simple screening tool for identifying unmet palliative care needs in patients with cancer. J Oncol Pract. 2015;11(1):e81-e86. doi: 10.1200/JOP.2014.001487. PubMed
12. Zalenski RJ, Jones SS, Courage C, et al. Impact of a palliative care screening and consultation in the ICU: A multihospital quality improvement project. J Pain Symptom Manage. 2017;53(1):5-12.e3. doi: 10.1016/j.jpainsymman.2016.08.003. PubMed
13. Weissman DE, Meier DE. Identifying patients in need of palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi: PubMed
14. MacLean CH, Kerr EA, Qaseem A. Time out-charting a path for improving performance measurement. N Engl J Med. 2018. Epub ahead of print. doi: 10.1056/NEJMp1802595 PubMed
Palliative care is an essential component of inpatient medicine. At its core, it is an interdisciplinary philosophy of care aiming to achieve the best quality of life for patients and families in the physical, psychosocial, and spiritual domains. With the aging population and growing complexity of hospitalized patients, inpatient palliative care needs are only projected to rise. However, a mismatch exists between the number of palliative care–trained physicians and the demand for such physicians. Currently, only 6,600 US physicians are board certified in palliative care – just 37% of the projected need.1 These workforce shortages have serious implications. In fact, it is estimated that nearly 40% of all hospitalized patients who need palliative care go without it.2
Existing efforts to improve access to palliative care have largely focused on bolstering the palliative care workforce. One tactic particularly relevant to hospitalists centers on frontline physicians providing “primary” palliative care: basic symptom management, patient-centered communication, and goals of care assessment, regardless of the disease state.3 Such physicians constitute the base of today’s palliative care workforce model – a three-tiered pyramid built on clinician availability and skills. In this model, the second tier (“secondary” palliative care) includes physicians supported by a palliative care consultant or referral. The third level (“tertiary” palliative care) encompasses care provided directly by specialized palliative care teams, usually within academic medical centers (Figure).4
The practice of primary palliative care is central to the practice of hospital medicine.5,6 After all, hospitalists generate nearly half of all inpatient palliative care consultations7 and routinely interface with social workers, pharmacists, nurses, chaplains, and other consultants in their daily activities. Consequently, they are also well versed in serious illness communication and prognostication.8 In many ways, they are ideal purveyors of palliative care in the hospital.
Why then does the challenge to meet the demands of patients with palliative care needs persist? The truth may lie in at least three central shortcomings within the tiered palliative care workforce model. First, physicians comprising the base (where hospitalists typically fall) possess variable skills and knowledge in caring for seriously ill patients. While training opportunities exist for interested individuals,7 education alone can rarely achieve a systematic change. Second, some physicians may have the requisite skills but lack
The Palliative Care Redistribution Integrated Service Model (PRISM)
To better address the current palliative care access problem, we propose a new model: “The Palliative care Redistribution Integrated Service Model (PRISM; Figure 1).” Using the industrial engineering principle of “task shifting,” this approach leverages disciplinary diversity and shifts specific activities from more specialized to less specialized members.9 In this way, PRISM integrates hospital-based interdisciplinary teams across all tiers of palliative care delivery.
PRISM sheds a tier-based approach in favor of flexible, skill-based verticals that span all physician and nonphysician providers. By dividing the original pyramid into three domains – physical, psychosocial, and spiritual – providers with various spheres of expertise may serve patients on multiple tiers. For example, a bedside nurse may perform basic psychosocial assessment consistent with his or her training, while physicians may focus on code status or prescribe antiemetics or low-dose opiate monotherapy – skills they have refined during medical school. Analogously, secondary palliative care may be delivered by any provider with more advanced skills in communication or symptom management. In this way, we expand the pool of clinicians available to provide palliative care to include nurses, hospitalists, oncologists, intensivists, social workers, and chaplains and also recognize the diversity of skill sets within and between disciplines. Thus, a hospitalist may clarify the goals of care but may ask a social worker trained in psychosocial assessment for assistance with difficult family dynamics or a chaplain for spiritual needs. Interdisciplinary teamwork and cross-disciplinary communication – hallmarks of palliative care – are encouraged and valued. Furthermore, if providers feel uncomfortable providing a certain type of care, they can ask for assistance from more experienced providers within their discipline or outside of it. In rare cases, the most complex patients may be referred to specialist palliative care teams.
Inherent within PRISM is a recognition that all providers must have a basic palliative care skillset obtained through educational initiatives.7 Yet focusing solely on training the workforce as a strategy has and will continue to miss the mark. Rather, structural changes to the means of providing care are also needed. Within hospitals, these changes often rely heavily on hospitalists due to their central position in care delivery. In this way, hospitalists are well primed to be the agents of change in this model.
The Role of Technology
Since many hospitalized patients have unrecognized and underserved palliative care needs, a formal approach to assessment is needed. Lin et al. proposed criteria for a “sentinel hospitalization,” marking a major illness or transition in high-risk patients necessitating palliative interventions.10 Similar screening criteria have been validated among hospitalized oncology patients11 and in critical care.12 While checklists have been shown to help identify hospitalized patients with palliative care needs,13 their implementation has been slow, presumably because they are burdensome for busy providers to complete.
Technological automation may be a solution to the checklist conundrum. For example, if palliative care screening criteria could be automatically extracted from electronic health records, scoring systems could trigger hospitalists to consider the goals of care discussions or engage an interdisciplinary care team to fulfill a variety of needs. Frameworks for such scoring systems already exist and are familiar to most hospitalists. For example, admission order sets routinely calculate the Padua or Caprini score to facilitate decision-making for prophylaxis of deep vein thrombosis. An admission order set that screens and prompts decision-making around palliative care needs is thus feasible. One example is a hard stop for entering code status in the admission order set; in turn, this hard stop could also trigger providers to complete a “check-box” palliative care screening checklist. Automatic extraction of certain data from the record – such as age, prior code status, recent hospitalizations, or mobility scores – could auto-populate to facilitate decision-making. In turn, measuring the influence of such tools on access to palliative care, workflow, and capacity will be important, as most tools may not have quality or value intended.14
Streamlining Workflow
It is common for hospitalists to oversee care for 15-20 patients at a time. Thus, they may not have the time to meaningfully engage patients to assess palliative care needs. Creating designated hospitalist palliative care teams with enhanced interdisciplinary support for patients identified using sentinel hospitalization or checklist-based tools may help to solve this dilemma. These teams may also employ lower “caps,” freeing up time for critical discussions and planning around end of life. At the University of Michigan, we are planning just such an approach, a strategy which has the additional benefit of bypassing the binary “care versus no care” dilemma faced by patients choosing palliation. Rather, patients may continue to receive treatments congruent with the goals of care in such teams.
Making Palliative Care a Standard of Care
A call for health systems to develop and implement palliative care quality metrics has emerged. Given their role in quality improvement and health system reform, hospitalists are well positioned to shepherd this imperative. Creating incentives to screen inpatients for palliative care needs and develop new homes in which to care for these patients are but a few ways to help set the tone. Additionally, developing and sharing quality metrics and benchmarks currently captured in repositories such as the Palliative Care Quality Network, Global Palliative Care Quality Alliance, and Center to Advance Palliative Care can help to assess and continually improve care delivery. Creating and sharing dashboards from these metrics with all providers, regardless of discipline or training, will ensure accountability to deliver quality palliative care.
CONCLUSION
Many hospitalized patients do not receive appropriate attention to their palliative care needs. A new interdisciplinary workforce model that task shifts to physician and nonphysician providers and pairs system-level innovations and quality may solve this problem. Input and endorsement from a wide variety of disciplines (particularly our nonphysician colleagues) are needed to make PRISM operational. The proof of concept will lie in testing feasibility among key stakeholders and rigorously studying the proposed interventions. Through innovation in technology, workflow, and quality improvement, hospitalists are well poised to lead this change. After all, our patients deserve nothing less.
Disclosures
The authors have nothing to disclose.Funding: Dr. Abedini’s work is supported by the University of Michigan National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, as well as the Un
Palliative care is an essential component of inpatient medicine. At its core, it is an interdisciplinary philosophy of care aiming to achieve the best quality of life for patients and families in the physical, psychosocial, and spiritual domains. With the aging population and growing complexity of hospitalized patients, inpatient palliative care needs are only projected to rise. However, a mismatch exists between the number of palliative care–trained physicians and the demand for such physicians. Currently, only 6,600 US physicians are board certified in palliative care – just 37% of the projected need.1 These workforce shortages have serious implications. In fact, it is estimated that nearly 40% of all hospitalized patients who need palliative care go without it.2
Existing efforts to improve access to palliative care have largely focused on bolstering the palliative care workforce. One tactic particularly relevant to hospitalists centers on frontline physicians providing “primary” palliative care: basic symptom management, patient-centered communication, and goals of care assessment, regardless of the disease state.3 Such physicians constitute the base of today’s palliative care workforce model – a three-tiered pyramid built on clinician availability and skills. In this model, the second tier (“secondary” palliative care) includes physicians supported by a palliative care consultant or referral. The third level (“tertiary” palliative care) encompasses care provided directly by specialized palliative care teams, usually within academic medical centers (Figure).4
The practice of primary palliative care is central to the practice of hospital medicine.5,6 After all, hospitalists generate nearly half of all inpatient palliative care consultations7 and routinely interface with social workers, pharmacists, nurses, chaplains, and other consultants in their daily activities. Consequently, they are also well versed in serious illness communication and prognostication.8 In many ways, they are ideal purveyors of palliative care in the hospital.
Why then does the challenge to meet the demands of patients with palliative care needs persist? The truth may lie in at least three central shortcomings within the tiered palliative care workforce model. First, physicians comprising the base (where hospitalists typically fall) possess variable skills and knowledge in caring for seriously ill patients. While training opportunities exist for interested individuals,7 education alone can rarely achieve a systematic change. Second, some physicians may have the requisite skills but lack
The Palliative Care Redistribution Integrated Service Model (PRISM)
To better address the current palliative care access problem, we propose a new model: “The Palliative care Redistribution Integrated Service Model (PRISM; Figure 1).” Using the industrial engineering principle of “task shifting,” this approach leverages disciplinary diversity and shifts specific activities from more specialized to less specialized members.9 In this way, PRISM integrates hospital-based interdisciplinary teams across all tiers of palliative care delivery.
PRISM sheds a tier-based approach in favor of flexible, skill-based verticals that span all physician and nonphysician providers. By dividing the original pyramid into three domains – physical, psychosocial, and spiritual – providers with various spheres of expertise may serve patients on multiple tiers. For example, a bedside nurse may perform basic psychosocial assessment consistent with his or her training, while physicians may focus on code status or prescribe antiemetics or low-dose opiate monotherapy – skills they have refined during medical school. Analogously, secondary palliative care may be delivered by any provider with more advanced skills in communication or symptom management. In this way, we expand the pool of clinicians available to provide palliative care to include nurses, hospitalists, oncologists, intensivists, social workers, and chaplains and also recognize the diversity of skill sets within and between disciplines. Thus, a hospitalist may clarify the goals of care but may ask a social worker trained in psychosocial assessment for assistance with difficult family dynamics or a chaplain for spiritual needs. Interdisciplinary teamwork and cross-disciplinary communication – hallmarks of palliative care – are encouraged and valued. Furthermore, if providers feel uncomfortable providing a certain type of care, they can ask for assistance from more experienced providers within their discipline or outside of it. In rare cases, the most complex patients may be referred to specialist palliative care teams.
Inherent within PRISM is a recognition that all providers must have a basic palliative care skillset obtained through educational initiatives.7 Yet focusing solely on training the workforce as a strategy has and will continue to miss the mark. Rather, structural changes to the means of providing care are also needed. Within hospitals, these changes often rely heavily on hospitalists due to their central position in care delivery. In this way, hospitalists are well primed to be the agents of change in this model.
The Role of Technology
Since many hospitalized patients have unrecognized and underserved palliative care needs, a formal approach to assessment is needed. Lin et al. proposed criteria for a “sentinel hospitalization,” marking a major illness or transition in high-risk patients necessitating palliative interventions.10 Similar screening criteria have been validated among hospitalized oncology patients11 and in critical care.12 While checklists have been shown to help identify hospitalized patients with palliative care needs,13 their implementation has been slow, presumably because they are burdensome for busy providers to complete.
Technological automation may be a solution to the checklist conundrum. For example, if palliative care screening criteria could be automatically extracted from electronic health records, scoring systems could trigger hospitalists to consider the goals of care discussions or engage an interdisciplinary care team to fulfill a variety of needs. Frameworks for such scoring systems already exist and are familiar to most hospitalists. For example, admission order sets routinely calculate the Padua or Caprini score to facilitate decision-making for prophylaxis of deep vein thrombosis. An admission order set that screens and prompts decision-making around palliative care needs is thus feasible. One example is a hard stop for entering code status in the admission order set; in turn, this hard stop could also trigger providers to complete a “check-box” palliative care screening checklist. Automatic extraction of certain data from the record – such as age, prior code status, recent hospitalizations, or mobility scores – could auto-populate to facilitate decision-making. In turn, measuring the influence of such tools on access to palliative care, workflow, and capacity will be important, as most tools may not have quality or value intended.14
Streamlining Workflow
It is common for hospitalists to oversee care for 15-20 patients at a time. Thus, they may not have the time to meaningfully engage patients to assess palliative care needs. Creating designated hospitalist palliative care teams with enhanced interdisciplinary support for patients identified using sentinel hospitalization or checklist-based tools may help to solve this dilemma. These teams may also employ lower “caps,” freeing up time for critical discussions and planning around end of life. At the University of Michigan, we are planning just such an approach, a strategy which has the additional benefit of bypassing the binary “care versus no care” dilemma faced by patients choosing palliation. Rather, patients may continue to receive treatments congruent with the goals of care in such teams.
Making Palliative Care a Standard of Care
A call for health systems to develop and implement palliative care quality metrics has emerged. Given their role in quality improvement and health system reform, hospitalists are well positioned to shepherd this imperative. Creating incentives to screen inpatients for palliative care needs and develop new homes in which to care for these patients are but a few ways to help set the tone. Additionally, developing and sharing quality metrics and benchmarks currently captured in repositories such as the Palliative Care Quality Network, Global Palliative Care Quality Alliance, and Center to Advance Palliative Care can help to assess and continually improve care delivery. Creating and sharing dashboards from these metrics with all providers, regardless of discipline or training, will ensure accountability to deliver quality palliative care.
CONCLUSION
Many hospitalized patients do not receive appropriate attention to their palliative care needs. A new interdisciplinary workforce model that task shifts to physician and nonphysician providers and pairs system-level innovations and quality may solve this problem. Input and endorsement from a wide variety of disciplines (particularly our nonphysician colleagues) are needed to make PRISM operational. The proof of concept will lie in testing feasibility among key stakeholders and rigorously studying the proposed interventions. Through innovation in technology, workflow, and quality improvement, hospitalists are well poised to lead this change. After all, our patients deserve nothing less.
Disclosures
The authors have nothing to disclose.Funding: Dr. Abedini’s work is supported by the University of Michigan National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, as well as the Un
1. Lupu D. American Academy of Hospice and Palliative Medicine Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911. doi: 10.1016/j.jpainsymman.2010.07.004. PubMed
2. Chuang E, Hope AA, Allyn K, Szalkiewicz E, Gary B, Gong MN. Gaps in provision of primary and specialty palliative care in the acute care setting by race and ethnicity. J Pain Symptom Manage. 2017;54(5):645-653. doi: 10.1016/j.jpainsymman.2017.05.001 PubMed
3. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi: 10.1056/NEJMp1215620 PubMed
4. von Gunten CF. Secondary and tertiary palliative care in US hospitals. JAMA. 2002;287(7):875-881. doi: 10.1001/jama.287.7.875 PubMed
5. Pantilat SZ. Hope to reality: the future of hospitalists and palliative care. J Hosp Med. 2015;10(10):701-702. doi: 10.1002/jhm.2401 PubMed
6. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1016/j.cger.2004.07.006 PubMed
7. Fail RE, Meier DE. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. doi: 10.12788/jhm.2896. [Epub ahead of print] PubMed
8. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and barriers to serious illness communication: A national survey of hospitalists. J Palliat Med. 2017;20(9):1013-1019. doi: 10.1089/jpm.2016.0515 PubMed
9. Carayon P, Gurses AP. Nursing workload and patient safety–a human factors engineering perspective. In: Hughes RG, ed.Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160 PubMed
11. Glare PA, Chow K. Validation of a simple screening tool for identifying unmet palliative care needs in patients with cancer. J Oncol Pract. 2015;11(1):e81-e86. doi: 10.1200/JOP.2014.001487. PubMed
12. Zalenski RJ, Jones SS, Courage C, et al. Impact of a palliative care screening and consultation in the ICU: A multihospital quality improvement project. J Pain Symptom Manage. 2017;53(1):5-12.e3. doi: 10.1016/j.jpainsymman.2016.08.003. PubMed
13. Weissman DE, Meier DE. Identifying patients in need of palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi: PubMed
14. MacLean CH, Kerr EA, Qaseem A. Time out-charting a path for improving performance measurement. N Engl J Med. 2018. Epub ahead of print. doi: 10.1056/NEJMp1802595 PubMed
1. Lupu D. American Academy of Hospice and Palliative Medicine Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911. doi: 10.1016/j.jpainsymman.2010.07.004. PubMed
2. Chuang E, Hope AA, Allyn K, Szalkiewicz E, Gary B, Gong MN. Gaps in provision of primary and specialty palliative care in the acute care setting by race and ethnicity. J Pain Symptom Manage. 2017;54(5):645-653. doi: 10.1016/j.jpainsymman.2017.05.001 PubMed
3. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi: 10.1056/NEJMp1215620 PubMed
4. von Gunten CF. Secondary and tertiary palliative care in US hospitals. JAMA. 2002;287(7):875-881. doi: 10.1001/jama.287.7.875 PubMed
5. Pantilat SZ. Hope to reality: the future of hospitalists and palliative care. J Hosp Med. 2015;10(10):701-702. doi: 10.1002/jhm.2401 PubMed
6. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1016/j.cger.2004.07.006 PubMed
7. Fail RE, Meier DE. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. doi: 10.12788/jhm.2896. [Epub ahead of print] PubMed
8. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and barriers to serious illness communication: A national survey of hospitalists. J Palliat Med. 2017;20(9):1013-1019. doi: 10.1089/jpm.2016.0515 PubMed
9. Carayon P, Gurses AP. Nursing workload and patient safety–a human factors engineering perspective. In: Hughes RG, ed.Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160 PubMed
11. Glare PA, Chow K. Validation of a simple screening tool for identifying unmet palliative care needs in patients with cancer. J Oncol Pract. 2015;11(1):e81-e86. doi: 10.1200/JOP.2014.001487. PubMed
12. Zalenski RJ, Jones SS, Courage C, et al. Impact of a palliative care screening and consultation in the ICU: A multihospital quality improvement project. J Pain Symptom Manage. 2017;53(1):5-12.e3. doi: 10.1016/j.jpainsymman.2016.08.003. PubMed
13. Weissman DE, Meier DE. Identifying patients in need of palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi: PubMed
14. MacLean CH, Kerr EA, Qaseem A. Time out-charting a path for improving performance measurement. N Engl J Med. 2018. Epub ahead of print. doi: 10.1056/NEJMp1802595 PubMed
© 2018 Society of Hospital Medicine
Barriers to Earlier Hospital Discharge: What Matters Most?
“Every system is perfectly designed to get the results it gets.”
—W. Edwards Deming inspired quote1
The timing of patient discharge represents a Gordian knot in hospital operations. Moving the time of discharge to earlier in the day is a complex challenge that defies replicable solutions and is often a barrier to optimal throughput and patient experience. In this issue of the Journal of Hospital Medicine, Zoucha et al. identify that discharge orders are frequently delayed due to physicians caring for other patients, heterogeneity in physician rounding styles, and other intrinsic factors such as census size, rounding style, and teaching versus nonteaching services.2 Some of these factors and their negative impact are consistent with the effect of higher hospitalist workload (census) when increasing length of stay that was identified by Elliott et al.3 Others, such as rounding style and balancing teaching and education, are a part of many hospitalist service operations. Other intrinsic factors identified by the authors include awaiting consultant recommendations, care completion by social workers, procedures, labs, radiology, therapy services, and home oxygen.
The authors, however, recognize hospitalist behaviors and hospital operations as intrinsic factors. This is significant because intrinsic factors are theoretically under the control of the hospital’s physicians, administration, and support services. They lend themselves to continuous improvement, re-engineering, and change management. They are a direct result of the people, processes, structure, and supporting information technology (IT).
The findings of this study contrast with the perceived dominance of extrinsic factors such as awaiting a ride, insurance authorization issues, or placement as the cause for discharge delays. Anecdotally, physicians and nurses in organizations often identify such extrinsic factors as causes of discharge delays before they call out intrinsic factors.
Frequently, the first reaction to managing complex intrinsic constraints is to add resources and complexity. Continuous improvement often reveals the culprit is poor design and waste found throughout the system. Zoucha et al. refer to LEAN successes by others4 as an example of how to approach these complex intrinsic issues. Increasing early discharge with improvement in length of stay and reducing or maintaining the readmission rate has been achieved using the Institute for Healthcare Improvement Model for Improvement,5 the Red/Yellow/Green Discharge Tool within the electronic medical record,6 and a comprehensive management plan.7 These examples were often accomplished through improving the deployment of existing resources and reducing wasted activity. New predictive tools using supervised machine learning can help identify appropriate patients for discharge earlier in the day.8 This approach is built on the concepts of “efficiency and communication as components of quality healthcare delivery.”6
Perhaps a practical reductionist approach is to start with the end in mind, and ask the question “what matters most?” Three key times occur in each discharge and the authors capture two of these: the discharge order time and discharge time. Not captured is the time the patient and family are told they are being discharged. It is against this backdrop that we can look at four perspectives: caregiver, organization, community, and the patient and family. “What matters most?” depends on the perspective of each one of the parties involved.
From the perspective of the caregivers (physicians and residents), the conclusions support prioritizing rounding on patients ready to discharge, lowering team census, and restructuring teaching rounds to drive earlier discharges. But only 7% of encounters prioritized patients ready for discharge first. Seventy-six percent prioritized sickest patients first (33%), room-by-room (27%), and newest patients (16%).2 The authors emphasize that such an approach needs to be balanced against the needs of the entire team census to ensure optimal care for all patients. Team and individual hospitalist census and processes must be optimized to improve the efficiency and effectiveness of the work. For teaching services, the goal is to accomplish effective teaching while maintaining or improving throughput. When
Healthcare is delivered by teams. As we look at supporting and structuring our hospitalist teams’ inpatient rounding we need to include the contributions of advanced practice professionals, pharmacists, nurses, care managers, social workers, and others. Achieving a team focus on a goal can be supported by number-by-time (n-by-T) target initiatives, which have been used successfully.10,11 Team-based solutions must be developed to address these complex issues and in recognition of the need to distribute this responsibility across the system, not just depending on physician changes to ensure optimal outcomes.
The perspectives of organization and community have the common goals of delivering healthcare value (outcomes, quality, safety, and sustainability) and ensuring access. To achieve these, it is important to separate the discharge curve (by shifting these patients’ time of discharge to the left) from the arrival curve, which is more fixed. The organization and community benefit from reduced cost of care, improved value delivery, and better access to services. For hospitals and health systems facing high occupancy, this becomes important for access and serving the community, especially during the peak hours for admissions and discharges.
Against this backdrop is the most important perspective, which is that of the patients and families. What matters most to them? When does their clock start? For patients and families, we believe that their expectations begin when the physician or APP says, “you are doing well and we can get you home today.” In the current study, the median time to discharge from the discharge order for four of the five hospitals was about three hours.2 It is reasonable to assume the time interval is on the order of four to six hours or more for many patients. Is this acceptable? We have little data to answer this question directly, and while the Hospital Consumers Assessment of Healthcare Providers and Systems (HCAHPS) survey asks select questions regarding the effectiveness of discharge information, it is silent on matters of discharge timeliness and expectations. While on the administrative side we often use readmission rates as a proxy for a safe and “effective” discharge, in reality, we lack meaningful patient-reported outcome measures to assess our effectiveness, which is a necessity for performance improvement.
The opportunities for improvement suggested by this study include restructuring rounding to prioritize discharges, managing census per provider, and rethinking resident education to accommodate both education and service. The authors’ approach includes identifying ways to improve the efficiency of the work through other team members (such as pharmacy techs for medication reconciliation) and balancing ancillary services support for all inpatient care and the outpatients they serve. Alternatively, tying incentives to the goal could be a convenient leadership response. The 2016 Society of Hospital Medicine State of Hospital Medicine Report notes that more than half (54%) of nonacademic hospitalist groups that treat adults have an incentive tied to early morning discharge orders or times. We believe that by keeping the patients and families at the center of this discussion, we are more likely to accomplish the goal of improved safety, efficiency, effectiveness, and patient experience.
The literature supports discharge delays as an international challenge with research on the topic in healthcare systems across the world.12 This may be related to an aging population, improvements, and access to advanced healthcare, and the challenges of occupancy and capacity mismatches in many healthcare systems worldwide. The authors have identified important intrinsic factors for these throughput and discharge delays. The results beg the question, are we willing to do the redesign and behavior change in our delivery of healthcare and healthcare education to achieve a more optimized system of care delivery?
A now-retired Cleveland Clinic performance improvement engineer frequently referenced W. Edwards Deming on “what makes the biggest difference in improving internal service quality?” He distilled this to two axioms based on Deming’s work: reducing cycle time and reducing defects. Both must be accomplished from the customer’s (patient’s) perspective without tradeoffs between the two. Cycle time is the time to accomplish a completed process or action, such as patient discharge or LOS. Defects are all the waste or “impossible” challenges that contribute to the feeling of resignation that lead to people dismissing the possibility of improvement, stating “it is what it is.” The challenge in the service of our patients and families, organizations, and communities is to move this dialog forward to “it is what we make it.”13
When we tell the patient and family they are being discharged it should happen safely, efficiently, predictably, and with empathy. From the perspective of clinicians, it should be as easy as possible to consistently do the right thing and do the work to which they have dedicated themselves. For communities and organizations struggling with access, improving throughput is vital.
Disclosures
Neither author has any conflicts to disclose. There are no external funding sources for this manuscript.
1. Institute for Healthcare Improvement. Available at: http://www.ihi.org/communities/blogs/origin-of-every-system-is-perfectly-designed-quote. Accessed August 2, 2018.
2. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
3. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786. doi: 10.1001/jamainternmed.2014.300. PubMed
4. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
5. Patel H, Morduchowicz S, Mourad M. Using a Systematic Framework of Interventions to Improve Early Discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. doi: 10.1016/j.jcjq.2016.12.003. PubMed
6. Mathews KS, Corso P, Bacon S, Jenq GY. Using the Red/Yellow/Green Discharge Tool to Improve the Timeliness of Hospital Discharges. Jt Comm J Qual Patient Saf. 2014;40(6). doi:10.1016/s1553-7250(14)40033-3. PubMed
7. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
8. Barnes S, Hamrock E, Toerper M, Siddiqui S, Levin S. Real-time prediction of inpatient length of stay for discharge prioritization. J Am Med Inform Assoc. 2015;23(e1). doi: 10.1093/jamia/ocv106. PubMed
9. Wachter RM. Hospitalist Workload. JAMA Intern Med. 2014;174(5):794. doi:1 0.1001/jamainternmed.2014.18. PubMed
10. Parikh PJ, Ballester N, Ramsey K, Kong N, Pook N. The n-by-T Target Discharge Strategy for Inpatient Units. Med Decis Making. 2017;37(5):534-543. doi:10.1177/0272989x17691735. PubMed
11. Kane M, Weinacker A, Arthofer R, et al. A Multidisciplinary Initiative to Increase Inpatient Discharges Before Noon. J Nurs Adm. 2016; 46(12):630-635.doi: 10.1097/NNA.0000000000000418 PubMed
12. Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: A mixed-studies systematic review. Health Expect. 2017;21(1):41-56. doi: 10.1111/hex.12619. PubMed
13. Emmelhainz L. Achieving Excellence: Some Last Thoughts. Lecture presented: Health System Leadership at Cleveland Clinic Akron General; May 16, 2018; Akron, OH. PubMed
“Every system is perfectly designed to get the results it gets.”
—W. Edwards Deming inspired quote1
The timing of patient discharge represents a Gordian knot in hospital operations. Moving the time of discharge to earlier in the day is a complex challenge that defies replicable solutions and is often a barrier to optimal throughput and patient experience. In this issue of the Journal of Hospital Medicine, Zoucha et al. identify that discharge orders are frequently delayed due to physicians caring for other patients, heterogeneity in physician rounding styles, and other intrinsic factors such as census size, rounding style, and teaching versus nonteaching services.2 Some of these factors and their negative impact are consistent with the effect of higher hospitalist workload (census) when increasing length of stay that was identified by Elliott et al.3 Others, such as rounding style and balancing teaching and education, are a part of many hospitalist service operations. Other intrinsic factors identified by the authors include awaiting consultant recommendations, care completion by social workers, procedures, labs, radiology, therapy services, and home oxygen.
The authors, however, recognize hospitalist behaviors and hospital operations as intrinsic factors. This is significant because intrinsic factors are theoretically under the control of the hospital’s physicians, administration, and support services. They lend themselves to continuous improvement, re-engineering, and change management. They are a direct result of the people, processes, structure, and supporting information technology (IT).
The findings of this study contrast with the perceived dominance of extrinsic factors such as awaiting a ride, insurance authorization issues, or placement as the cause for discharge delays. Anecdotally, physicians and nurses in organizations often identify such extrinsic factors as causes of discharge delays before they call out intrinsic factors.
Frequently, the first reaction to managing complex intrinsic constraints is to add resources and complexity. Continuous improvement often reveals the culprit is poor design and waste found throughout the system. Zoucha et al. refer to LEAN successes by others4 as an example of how to approach these complex intrinsic issues. Increasing early discharge with improvement in length of stay and reducing or maintaining the readmission rate has been achieved using the Institute for Healthcare Improvement Model for Improvement,5 the Red/Yellow/Green Discharge Tool within the electronic medical record,6 and a comprehensive management plan.7 These examples were often accomplished through improving the deployment of existing resources and reducing wasted activity. New predictive tools using supervised machine learning can help identify appropriate patients for discharge earlier in the day.8 This approach is built on the concepts of “efficiency and communication as components of quality healthcare delivery.”6
Perhaps a practical reductionist approach is to start with the end in mind, and ask the question “what matters most?” Three key times occur in each discharge and the authors capture two of these: the discharge order time and discharge time. Not captured is the time the patient and family are told they are being discharged. It is against this backdrop that we can look at four perspectives: caregiver, organization, community, and the patient and family. “What matters most?” depends on the perspective of each one of the parties involved.
From the perspective of the caregivers (physicians and residents), the conclusions support prioritizing rounding on patients ready to discharge, lowering team census, and restructuring teaching rounds to drive earlier discharges. But only 7% of encounters prioritized patients ready for discharge first. Seventy-six percent prioritized sickest patients first (33%), room-by-room (27%), and newest patients (16%).2 The authors emphasize that such an approach needs to be balanced against the needs of the entire team census to ensure optimal care for all patients. Team and individual hospitalist census and processes must be optimized to improve the efficiency and effectiveness of the work. For teaching services, the goal is to accomplish effective teaching while maintaining or improving throughput. When
Healthcare is delivered by teams. As we look at supporting and structuring our hospitalist teams’ inpatient rounding we need to include the contributions of advanced practice professionals, pharmacists, nurses, care managers, social workers, and others. Achieving a team focus on a goal can be supported by number-by-time (n-by-T) target initiatives, which have been used successfully.10,11 Team-based solutions must be developed to address these complex issues and in recognition of the need to distribute this responsibility across the system, not just depending on physician changes to ensure optimal outcomes.
The perspectives of organization and community have the common goals of delivering healthcare value (outcomes, quality, safety, and sustainability) and ensuring access. To achieve these, it is important to separate the discharge curve (by shifting these patients’ time of discharge to the left) from the arrival curve, which is more fixed. The organization and community benefit from reduced cost of care, improved value delivery, and better access to services. For hospitals and health systems facing high occupancy, this becomes important for access and serving the community, especially during the peak hours for admissions and discharges.
Against this backdrop is the most important perspective, which is that of the patients and families. What matters most to them? When does their clock start? For patients and families, we believe that their expectations begin when the physician or APP says, “you are doing well and we can get you home today.” In the current study, the median time to discharge from the discharge order for four of the five hospitals was about three hours.2 It is reasonable to assume the time interval is on the order of four to six hours or more for many patients. Is this acceptable? We have little data to answer this question directly, and while the Hospital Consumers Assessment of Healthcare Providers and Systems (HCAHPS) survey asks select questions regarding the effectiveness of discharge information, it is silent on matters of discharge timeliness and expectations. While on the administrative side we often use readmission rates as a proxy for a safe and “effective” discharge, in reality, we lack meaningful patient-reported outcome measures to assess our effectiveness, which is a necessity for performance improvement.
The opportunities for improvement suggested by this study include restructuring rounding to prioritize discharges, managing census per provider, and rethinking resident education to accommodate both education and service. The authors’ approach includes identifying ways to improve the efficiency of the work through other team members (such as pharmacy techs for medication reconciliation) and balancing ancillary services support for all inpatient care and the outpatients they serve. Alternatively, tying incentives to the goal could be a convenient leadership response. The 2016 Society of Hospital Medicine State of Hospital Medicine Report notes that more than half (54%) of nonacademic hospitalist groups that treat adults have an incentive tied to early morning discharge orders or times. We believe that by keeping the patients and families at the center of this discussion, we are more likely to accomplish the goal of improved safety, efficiency, effectiveness, and patient experience.
The literature supports discharge delays as an international challenge with research on the topic in healthcare systems across the world.12 This may be related to an aging population, improvements, and access to advanced healthcare, and the challenges of occupancy and capacity mismatches in many healthcare systems worldwide. The authors have identified important intrinsic factors for these throughput and discharge delays. The results beg the question, are we willing to do the redesign and behavior change in our delivery of healthcare and healthcare education to achieve a more optimized system of care delivery?
A now-retired Cleveland Clinic performance improvement engineer frequently referenced W. Edwards Deming on “what makes the biggest difference in improving internal service quality?” He distilled this to two axioms based on Deming’s work: reducing cycle time and reducing defects. Both must be accomplished from the customer’s (patient’s) perspective without tradeoffs between the two. Cycle time is the time to accomplish a completed process or action, such as patient discharge or LOS. Defects are all the waste or “impossible” challenges that contribute to the feeling of resignation that lead to people dismissing the possibility of improvement, stating “it is what it is.” The challenge in the service of our patients and families, organizations, and communities is to move this dialog forward to “it is what we make it.”13
When we tell the patient and family they are being discharged it should happen safely, efficiently, predictably, and with empathy. From the perspective of clinicians, it should be as easy as possible to consistently do the right thing and do the work to which they have dedicated themselves. For communities and organizations struggling with access, improving throughput is vital.
Disclosures
Neither author has any conflicts to disclose. There are no external funding sources for this manuscript.
“Every system is perfectly designed to get the results it gets.”
—W. Edwards Deming inspired quote1
The timing of patient discharge represents a Gordian knot in hospital operations. Moving the time of discharge to earlier in the day is a complex challenge that defies replicable solutions and is often a barrier to optimal throughput and patient experience. In this issue of the Journal of Hospital Medicine, Zoucha et al. identify that discharge orders are frequently delayed due to physicians caring for other patients, heterogeneity in physician rounding styles, and other intrinsic factors such as census size, rounding style, and teaching versus nonteaching services.2 Some of these factors and their negative impact are consistent with the effect of higher hospitalist workload (census) when increasing length of stay that was identified by Elliott et al.3 Others, such as rounding style and balancing teaching and education, are a part of many hospitalist service operations. Other intrinsic factors identified by the authors include awaiting consultant recommendations, care completion by social workers, procedures, labs, radiology, therapy services, and home oxygen.
The authors, however, recognize hospitalist behaviors and hospital operations as intrinsic factors. This is significant because intrinsic factors are theoretically under the control of the hospital’s physicians, administration, and support services. They lend themselves to continuous improvement, re-engineering, and change management. They are a direct result of the people, processes, structure, and supporting information technology (IT).
The findings of this study contrast with the perceived dominance of extrinsic factors such as awaiting a ride, insurance authorization issues, or placement as the cause for discharge delays. Anecdotally, physicians and nurses in organizations often identify such extrinsic factors as causes of discharge delays before they call out intrinsic factors.
Frequently, the first reaction to managing complex intrinsic constraints is to add resources and complexity. Continuous improvement often reveals the culprit is poor design and waste found throughout the system. Zoucha et al. refer to LEAN successes by others4 as an example of how to approach these complex intrinsic issues. Increasing early discharge with improvement in length of stay and reducing or maintaining the readmission rate has been achieved using the Institute for Healthcare Improvement Model for Improvement,5 the Red/Yellow/Green Discharge Tool within the electronic medical record,6 and a comprehensive management plan.7 These examples were often accomplished through improving the deployment of existing resources and reducing wasted activity. New predictive tools using supervised machine learning can help identify appropriate patients for discharge earlier in the day.8 This approach is built on the concepts of “efficiency and communication as components of quality healthcare delivery.”6
Perhaps a practical reductionist approach is to start with the end in mind, and ask the question “what matters most?” Three key times occur in each discharge and the authors capture two of these: the discharge order time and discharge time. Not captured is the time the patient and family are told they are being discharged. It is against this backdrop that we can look at four perspectives: caregiver, organization, community, and the patient and family. “What matters most?” depends on the perspective of each one of the parties involved.
From the perspective of the caregivers (physicians and residents), the conclusions support prioritizing rounding on patients ready to discharge, lowering team census, and restructuring teaching rounds to drive earlier discharges. But only 7% of encounters prioritized patients ready for discharge first. Seventy-six percent prioritized sickest patients first (33%), room-by-room (27%), and newest patients (16%).2 The authors emphasize that such an approach needs to be balanced against the needs of the entire team census to ensure optimal care for all patients. Team and individual hospitalist census and processes must be optimized to improve the efficiency and effectiveness of the work. For teaching services, the goal is to accomplish effective teaching while maintaining or improving throughput. When
Healthcare is delivered by teams. As we look at supporting and structuring our hospitalist teams’ inpatient rounding we need to include the contributions of advanced practice professionals, pharmacists, nurses, care managers, social workers, and others. Achieving a team focus on a goal can be supported by number-by-time (n-by-T) target initiatives, which have been used successfully.10,11 Team-based solutions must be developed to address these complex issues and in recognition of the need to distribute this responsibility across the system, not just depending on physician changes to ensure optimal outcomes.
The perspectives of organization and community have the common goals of delivering healthcare value (outcomes, quality, safety, and sustainability) and ensuring access. To achieve these, it is important to separate the discharge curve (by shifting these patients’ time of discharge to the left) from the arrival curve, which is more fixed. The organization and community benefit from reduced cost of care, improved value delivery, and better access to services. For hospitals and health systems facing high occupancy, this becomes important for access and serving the community, especially during the peak hours for admissions and discharges.
Against this backdrop is the most important perspective, which is that of the patients and families. What matters most to them? When does their clock start? For patients and families, we believe that their expectations begin when the physician or APP says, “you are doing well and we can get you home today.” In the current study, the median time to discharge from the discharge order for four of the five hospitals was about three hours.2 It is reasonable to assume the time interval is on the order of four to six hours or more for many patients. Is this acceptable? We have little data to answer this question directly, and while the Hospital Consumers Assessment of Healthcare Providers and Systems (HCAHPS) survey asks select questions regarding the effectiveness of discharge information, it is silent on matters of discharge timeliness and expectations. While on the administrative side we often use readmission rates as a proxy for a safe and “effective” discharge, in reality, we lack meaningful patient-reported outcome measures to assess our effectiveness, which is a necessity for performance improvement.
The opportunities for improvement suggested by this study include restructuring rounding to prioritize discharges, managing census per provider, and rethinking resident education to accommodate both education and service. The authors’ approach includes identifying ways to improve the efficiency of the work through other team members (such as pharmacy techs for medication reconciliation) and balancing ancillary services support for all inpatient care and the outpatients they serve. Alternatively, tying incentives to the goal could be a convenient leadership response. The 2016 Society of Hospital Medicine State of Hospital Medicine Report notes that more than half (54%) of nonacademic hospitalist groups that treat adults have an incentive tied to early morning discharge orders or times. We believe that by keeping the patients and families at the center of this discussion, we are more likely to accomplish the goal of improved safety, efficiency, effectiveness, and patient experience.
The literature supports discharge delays as an international challenge with research on the topic in healthcare systems across the world.12 This may be related to an aging population, improvements, and access to advanced healthcare, and the challenges of occupancy and capacity mismatches in many healthcare systems worldwide. The authors have identified important intrinsic factors for these throughput and discharge delays. The results beg the question, are we willing to do the redesign and behavior change in our delivery of healthcare and healthcare education to achieve a more optimized system of care delivery?
A now-retired Cleveland Clinic performance improvement engineer frequently referenced W. Edwards Deming on “what makes the biggest difference in improving internal service quality?” He distilled this to two axioms based on Deming’s work: reducing cycle time and reducing defects. Both must be accomplished from the customer’s (patient’s) perspective without tradeoffs between the two. Cycle time is the time to accomplish a completed process or action, such as patient discharge or LOS. Defects are all the waste or “impossible” challenges that contribute to the feeling of resignation that lead to people dismissing the possibility of improvement, stating “it is what it is.” The challenge in the service of our patients and families, organizations, and communities is to move this dialog forward to “it is what we make it.”13
When we tell the patient and family they are being discharged it should happen safely, efficiently, predictably, and with empathy. From the perspective of clinicians, it should be as easy as possible to consistently do the right thing and do the work to which they have dedicated themselves. For communities and organizations struggling with access, improving throughput is vital.
Disclosures
Neither author has any conflicts to disclose. There are no external funding sources for this manuscript.
1. Institute for Healthcare Improvement. Available at: http://www.ihi.org/communities/blogs/origin-of-every-system-is-perfectly-designed-quote. Accessed August 2, 2018.
2. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
3. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786. doi: 10.1001/jamainternmed.2014.300. PubMed
4. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
5. Patel H, Morduchowicz S, Mourad M. Using a Systematic Framework of Interventions to Improve Early Discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. doi: 10.1016/j.jcjq.2016.12.003. PubMed
6. Mathews KS, Corso P, Bacon S, Jenq GY. Using the Red/Yellow/Green Discharge Tool to Improve the Timeliness of Hospital Discharges. Jt Comm J Qual Patient Saf. 2014;40(6). doi:10.1016/s1553-7250(14)40033-3. PubMed
7. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
8. Barnes S, Hamrock E, Toerper M, Siddiqui S, Levin S. Real-time prediction of inpatient length of stay for discharge prioritization. J Am Med Inform Assoc. 2015;23(e1). doi: 10.1093/jamia/ocv106. PubMed
9. Wachter RM. Hospitalist Workload. JAMA Intern Med. 2014;174(5):794. doi:1 0.1001/jamainternmed.2014.18. PubMed
10. Parikh PJ, Ballester N, Ramsey K, Kong N, Pook N. The n-by-T Target Discharge Strategy for Inpatient Units. Med Decis Making. 2017;37(5):534-543. doi:10.1177/0272989x17691735. PubMed
11. Kane M, Weinacker A, Arthofer R, et al. A Multidisciplinary Initiative to Increase Inpatient Discharges Before Noon. J Nurs Adm. 2016; 46(12):630-635.doi: 10.1097/NNA.0000000000000418 PubMed
12. Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: A mixed-studies systematic review. Health Expect. 2017;21(1):41-56. doi: 10.1111/hex.12619. PubMed
13. Emmelhainz L. Achieving Excellence: Some Last Thoughts. Lecture presented: Health System Leadership at Cleveland Clinic Akron General; May 16, 2018; Akron, OH. PubMed
1. Institute for Healthcare Improvement. Available at: http://www.ihi.org/communities/blogs/origin-of-every-system-is-perfectly-designed-quote. Accessed August 2, 2018.
2. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
3. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786. doi: 10.1001/jamainternmed.2014.300. PubMed
4. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
5. Patel H, Morduchowicz S, Mourad M. Using a Systematic Framework of Interventions to Improve Early Discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. doi: 10.1016/j.jcjq.2016.12.003. PubMed
6. Mathews KS, Corso P, Bacon S, Jenq GY. Using the Red/Yellow/Green Discharge Tool to Improve the Timeliness of Hospital Discharges. Jt Comm J Qual Patient Saf. 2014;40(6). doi:10.1016/s1553-7250(14)40033-3. PubMed
7. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
8. Barnes S, Hamrock E, Toerper M, Siddiqui S, Levin S. Real-time prediction of inpatient length of stay for discharge prioritization. J Am Med Inform Assoc. 2015;23(e1). doi: 10.1093/jamia/ocv106. PubMed
9. Wachter RM. Hospitalist Workload. JAMA Intern Med. 2014;174(5):794. doi:1 0.1001/jamainternmed.2014.18. PubMed
10. Parikh PJ, Ballester N, Ramsey K, Kong N, Pook N. The n-by-T Target Discharge Strategy for Inpatient Units. Med Decis Making. 2017;37(5):534-543. doi:10.1177/0272989x17691735. PubMed
11. Kane M, Weinacker A, Arthofer R, et al. A Multidisciplinary Initiative to Increase Inpatient Discharges Before Noon. J Nurs Adm. 2016; 46(12):630-635.doi: 10.1097/NNA.0000000000000418 PubMed
12. Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: A mixed-studies systematic review. Health Expect. 2017;21(1):41-56. doi: 10.1111/hex.12619. PubMed
13. Emmelhainz L. Achieving Excellence: Some Last Thoughts. Lecture presented: Health System Leadership at Cleveland Clinic Akron General; May 16, 2018; Akron, OH. PubMed
© 2018 Society of Hospital Medicine
Is Hospital Discharge the Rube Goldberg Machine of Academic Internal Medicine?
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
© 2018 Society of Hospital Medicine
We May Not “Have It All,” But We Can Make It Better through Structural Changes
In this issue of the Journal of Hospital Medicine, the paper by Gottenborg et al. captures the experiences of female academic hospitalists navigating one of the most significant transitions they will face—becoming new mothers.1 This article gives an accessible voice to impersonal statistics about the barriers women physicians encounter within and across specialties in academia. The challenges and anecdotes shared by the study participants were eminently relatable and captured the all-too-familiar circumstances most of us with children have faced in our careers as physician mothers.
STUDY COMMENTARY AND DISCUSSION
This study uses qualitative research methods to illustrate the hurdles faced by mothers in hospital medicine beyond what is demonstrated by quantitative measures and provides the helpful step of proposing some solutions to the obstacles they have faced. While the sample size was very small, the women interviewed were diverse in their years in practice, geographic distribution, and percent clinical effort, providing evidence that the themes discussed prevail across demographic categories.
The snowball sampling via the Society of Hospital Medicine committees may not have yielded a representative sample of female hospitalists. It seems possible that women who are involved in this type of leadership may be better supported and/or have different work schedules than their peers who are not in leadership positions. We also wish there had been more emphasis on the systemic and structural factors that can improve the quality of life of physician mothers. These policies include paternity leave and other creative ways of acknowledging the useful skills and experience that motherhood brings to bear on clinical practice, such as increased empathy and compassion, as mentioned by one of the study participants.
Even with the aforementioned limitations, this study is important because it combines authentic quotes from practicing academic hospitalists with concrete and tangible suggestions for structural changes. The most striking element is that the majority of the study participants experienced uncertainty and a lack of transparency around parental leave policies. As nearly half of hospitalists are women and 80% are under age 40,2 it seems unimaginable that there would not be explicit policies in place for what is a common and largely anticipated life event. Medicine has made great strides toward gender equality, but we are unlikely to ever reach the goal of true parity without openly addressing the disproportionate effect of childbearing and child rearing on women physicians. Standardized, readily available, and equitable parental leave policies (for both birth parents and nonbirth parents) are the first and most critical step.
The absence of standard leave policies naturally puts physician mothers in the position of having to negotiate or “haggle” with various supervisors, the majority of whom are male division chiefs and department chairs,3 which places all parties in an uncomfortable position, further reinforcing inequities and sowing discord and resentment. Having formal policies around leave protects not only those who utilize parental leave but also the other members of a hospital medicine practice who take on the workload of the person on leave.
Uncertainty around how to address the increased clinical load and for how long, also creates anxiety among other group members and may lead to feelings of bitterness toward clinicians on leave, further contributing to the negative impact of new parenthood on female hospitalists. We can think of no other medical circumstance in which there is as much advance notice of the need for significant time away from work. Yet pregnancy, which is subject to complications and emergencies just like other medical conditions, is treated with so little concern that one may be asked to arrange for their own coverage during such an emergency, as one study subject reported.
We also empathize with the study participants’ reports of feeling that supervisors often mentally discounted their ability to participate in projects on return to work. These pernicious assumptions can compound a cycle of lost productivity, disengagement, and attrition from the workforce.
Female hospitalists returning from leave face additional challenges that place an undue burden on their professional activities, most notably related to breastfeeding. This is particularly relevant in the context of the intensity inherent in practicing hospital medicine, which includes long days of being the primary provider for acutely ill inpatients, as well as long stretches of many consecutive days when it may not be possible to return home before children’s bedtime. Even at our own institution, which has been recognized as a “Healthy Mothers Workplace,” breastfeeding accommodations are not set up to allow for ongoing clinical activities while taking time to express breastmilk, and the clinical schedule does not build in adjustments for this time-consuming and psychologically taxing commitment. Breastfeeding for at least one year is the medical recommendation of the American Academy of Pediatrics in line with a substantial body of evidence.4 One quote from the article poignantly notes, “Pumping every 3-4 hours: stopping what you’re doing, finding an empty room to pump, finding a place to store your milk, then going back to work, three times per shift, for the next 9 months of your life, was hell.” If we cannot enable our own medical providers to follow evidence-based recommendations, how can we possibly expect this of our patients?
CONCLUSIONS
The notion of women “having it all” is an impossible ideal—both work and life outside of work will inevitably require tradeoffs. However, there is an abundance of evidence and recommendations for concrete steps that can be taken to improve the experience of female physicians who have children. These include formal policies for childbearing and child rearing leave (the American Academy of Pediatrics recommends at least six to nine months5), convenient space and protected time for pumping milk during the first year, on-site childcare services and back-up child care, and flexible work schedules.6 It is time to stop treating childbirth among female physicians like an unexpected inconvenience and acknowledge the undeniable demographics of physicians in hospital medicine and the duty of healthcare systems and hospital medicine leaders to effectively plan for the needs of half of their workforce.
Disclosures
Neither of the authors have any financial conflicts of interest to disclose.
1. Gottenborg E, Maw A, Ngov LK, Burden M, Ponomaryova A, Jones CD. You can’t have it all: The experience of academic hospitalists during pregnancy, parental leave, and return to work. J Hosp Med. 2018;13(12):836-839. doi: 10.12788/jhm.3076. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
3. Association of American Medical Colleges. The state of women in academic medicine: The pipeline and pathways to leadership, 2015-2016. https://www.aamc.org/members/gwims/statistics/. Accessed October 1, 2018.
4. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
5. National Public Radio. A Pediatrician’s View of Paid Parental Leave. https://www.npr.org/sections/health-shots/2016/10/10/497052014/a-pediatricians-view-of-paid-parental-leave. Accessed September 26, 2018.
6. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership? (2018, June 19). Harvard Business Review. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership. Accessed October 1, 2018.
In this issue of the Journal of Hospital Medicine, the paper by Gottenborg et al. captures the experiences of female academic hospitalists navigating one of the most significant transitions they will face—becoming new mothers.1 This article gives an accessible voice to impersonal statistics about the barriers women physicians encounter within and across specialties in academia. The challenges and anecdotes shared by the study participants were eminently relatable and captured the all-too-familiar circumstances most of us with children have faced in our careers as physician mothers.
STUDY COMMENTARY AND DISCUSSION
This study uses qualitative research methods to illustrate the hurdles faced by mothers in hospital medicine beyond what is demonstrated by quantitative measures and provides the helpful step of proposing some solutions to the obstacles they have faced. While the sample size was very small, the women interviewed were diverse in their years in practice, geographic distribution, and percent clinical effort, providing evidence that the themes discussed prevail across demographic categories.
The snowball sampling via the Society of Hospital Medicine committees may not have yielded a representative sample of female hospitalists. It seems possible that women who are involved in this type of leadership may be better supported and/or have different work schedules than their peers who are not in leadership positions. We also wish there had been more emphasis on the systemic and structural factors that can improve the quality of life of physician mothers. These policies include paternity leave and other creative ways of acknowledging the useful skills and experience that motherhood brings to bear on clinical practice, such as increased empathy and compassion, as mentioned by one of the study participants.
Even with the aforementioned limitations, this study is important because it combines authentic quotes from practicing academic hospitalists with concrete and tangible suggestions for structural changes. The most striking element is that the majority of the study participants experienced uncertainty and a lack of transparency around parental leave policies. As nearly half of hospitalists are women and 80% are under age 40,2 it seems unimaginable that there would not be explicit policies in place for what is a common and largely anticipated life event. Medicine has made great strides toward gender equality, but we are unlikely to ever reach the goal of true parity without openly addressing the disproportionate effect of childbearing and child rearing on women physicians. Standardized, readily available, and equitable parental leave policies (for both birth parents and nonbirth parents) are the first and most critical step.
The absence of standard leave policies naturally puts physician mothers in the position of having to negotiate or “haggle” with various supervisors, the majority of whom are male division chiefs and department chairs,3 which places all parties in an uncomfortable position, further reinforcing inequities and sowing discord and resentment. Having formal policies around leave protects not only those who utilize parental leave but also the other members of a hospital medicine practice who take on the workload of the person on leave.
Uncertainty around how to address the increased clinical load and for how long, also creates anxiety among other group members and may lead to feelings of bitterness toward clinicians on leave, further contributing to the negative impact of new parenthood on female hospitalists. We can think of no other medical circumstance in which there is as much advance notice of the need for significant time away from work. Yet pregnancy, which is subject to complications and emergencies just like other medical conditions, is treated with so little concern that one may be asked to arrange for their own coverage during such an emergency, as one study subject reported.
We also empathize with the study participants’ reports of feeling that supervisors often mentally discounted their ability to participate in projects on return to work. These pernicious assumptions can compound a cycle of lost productivity, disengagement, and attrition from the workforce.
Female hospitalists returning from leave face additional challenges that place an undue burden on their professional activities, most notably related to breastfeeding. This is particularly relevant in the context of the intensity inherent in practicing hospital medicine, which includes long days of being the primary provider for acutely ill inpatients, as well as long stretches of many consecutive days when it may not be possible to return home before children’s bedtime. Even at our own institution, which has been recognized as a “Healthy Mothers Workplace,” breastfeeding accommodations are not set up to allow for ongoing clinical activities while taking time to express breastmilk, and the clinical schedule does not build in adjustments for this time-consuming and psychologically taxing commitment. Breastfeeding for at least one year is the medical recommendation of the American Academy of Pediatrics in line with a substantial body of evidence.4 One quote from the article poignantly notes, “Pumping every 3-4 hours: stopping what you’re doing, finding an empty room to pump, finding a place to store your milk, then going back to work, three times per shift, for the next 9 months of your life, was hell.” If we cannot enable our own medical providers to follow evidence-based recommendations, how can we possibly expect this of our patients?
CONCLUSIONS
The notion of women “having it all” is an impossible ideal—both work and life outside of work will inevitably require tradeoffs. However, there is an abundance of evidence and recommendations for concrete steps that can be taken to improve the experience of female physicians who have children. These include formal policies for childbearing and child rearing leave (the American Academy of Pediatrics recommends at least six to nine months5), convenient space and protected time for pumping milk during the first year, on-site childcare services and back-up child care, and flexible work schedules.6 It is time to stop treating childbirth among female physicians like an unexpected inconvenience and acknowledge the undeniable demographics of physicians in hospital medicine and the duty of healthcare systems and hospital medicine leaders to effectively plan for the needs of half of their workforce.
Disclosures
Neither of the authors have any financial conflicts of interest to disclose.
In this issue of the Journal of Hospital Medicine, the paper by Gottenborg et al. captures the experiences of female academic hospitalists navigating one of the most significant transitions they will face—becoming new mothers.1 This article gives an accessible voice to impersonal statistics about the barriers women physicians encounter within and across specialties in academia. The challenges and anecdotes shared by the study participants were eminently relatable and captured the all-too-familiar circumstances most of us with children have faced in our careers as physician mothers.
STUDY COMMENTARY AND DISCUSSION
This study uses qualitative research methods to illustrate the hurdles faced by mothers in hospital medicine beyond what is demonstrated by quantitative measures and provides the helpful step of proposing some solutions to the obstacles they have faced. While the sample size was very small, the women interviewed were diverse in their years in practice, geographic distribution, and percent clinical effort, providing evidence that the themes discussed prevail across demographic categories.
The snowball sampling via the Society of Hospital Medicine committees may not have yielded a representative sample of female hospitalists. It seems possible that women who are involved in this type of leadership may be better supported and/or have different work schedules than their peers who are not in leadership positions. We also wish there had been more emphasis on the systemic and structural factors that can improve the quality of life of physician mothers. These policies include paternity leave and other creative ways of acknowledging the useful skills and experience that motherhood brings to bear on clinical practice, such as increased empathy and compassion, as mentioned by one of the study participants.
Even with the aforementioned limitations, this study is important because it combines authentic quotes from practicing academic hospitalists with concrete and tangible suggestions for structural changes. The most striking element is that the majority of the study participants experienced uncertainty and a lack of transparency around parental leave policies. As nearly half of hospitalists are women and 80% are under age 40,2 it seems unimaginable that there would not be explicit policies in place for what is a common and largely anticipated life event. Medicine has made great strides toward gender equality, but we are unlikely to ever reach the goal of true parity without openly addressing the disproportionate effect of childbearing and child rearing on women physicians. Standardized, readily available, and equitable parental leave policies (for both birth parents and nonbirth parents) are the first and most critical step.
The absence of standard leave policies naturally puts physician mothers in the position of having to negotiate or “haggle” with various supervisors, the majority of whom are male division chiefs and department chairs,3 which places all parties in an uncomfortable position, further reinforcing inequities and sowing discord and resentment. Having formal policies around leave protects not only those who utilize parental leave but also the other members of a hospital medicine practice who take on the workload of the person on leave.
Uncertainty around how to address the increased clinical load and for how long, also creates anxiety among other group members and may lead to feelings of bitterness toward clinicians on leave, further contributing to the negative impact of new parenthood on female hospitalists. We can think of no other medical circumstance in which there is as much advance notice of the need for significant time away from work. Yet pregnancy, which is subject to complications and emergencies just like other medical conditions, is treated with so little concern that one may be asked to arrange for their own coverage during such an emergency, as one study subject reported.
We also empathize with the study participants’ reports of feeling that supervisors often mentally discounted their ability to participate in projects on return to work. These pernicious assumptions can compound a cycle of lost productivity, disengagement, and attrition from the workforce.
Female hospitalists returning from leave face additional challenges that place an undue burden on their professional activities, most notably related to breastfeeding. This is particularly relevant in the context of the intensity inherent in practicing hospital medicine, which includes long days of being the primary provider for acutely ill inpatients, as well as long stretches of many consecutive days when it may not be possible to return home before children’s bedtime. Even at our own institution, which has been recognized as a “Healthy Mothers Workplace,” breastfeeding accommodations are not set up to allow for ongoing clinical activities while taking time to express breastmilk, and the clinical schedule does not build in adjustments for this time-consuming and psychologically taxing commitment. Breastfeeding for at least one year is the medical recommendation of the American Academy of Pediatrics in line with a substantial body of evidence.4 One quote from the article poignantly notes, “Pumping every 3-4 hours: stopping what you’re doing, finding an empty room to pump, finding a place to store your milk, then going back to work, three times per shift, for the next 9 months of your life, was hell.” If we cannot enable our own medical providers to follow evidence-based recommendations, how can we possibly expect this of our patients?
CONCLUSIONS
The notion of women “having it all” is an impossible ideal—both work and life outside of work will inevitably require tradeoffs. However, there is an abundance of evidence and recommendations for concrete steps that can be taken to improve the experience of female physicians who have children. These include formal policies for childbearing and child rearing leave (the American Academy of Pediatrics recommends at least six to nine months5), convenient space and protected time for pumping milk during the first year, on-site childcare services and back-up child care, and flexible work schedules.6 It is time to stop treating childbirth among female physicians like an unexpected inconvenience and acknowledge the undeniable demographics of physicians in hospital medicine and the duty of healthcare systems and hospital medicine leaders to effectively plan for the needs of half of their workforce.
Disclosures
Neither of the authors have any financial conflicts of interest to disclose.
1. Gottenborg E, Maw A, Ngov LK, Burden M, Ponomaryova A, Jones CD. You can’t have it all: The experience of academic hospitalists during pregnancy, parental leave, and return to work. J Hosp Med. 2018;13(12):836-839. doi: 10.12788/jhm.3076. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
3. Association of American Medical Colleges. The state of women in academic medicine: The pipeline and pathways to leadership, 2015-2016. https://www.aamc.org/members/gwims/statistics/. Accessed October 1, 2018.
4. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
5. National Public Radio. A Pediatrician’s View of Paid Parental Leave. https://www.npr.org/sections/health-shots/2016/10/10/497052014/a-pediatricians-view-of-paid-parental-leave. Accessed September 26, 2018.
6. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership? (2018, June 19). Harvard Business Review. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership. Accessed October 1, 2018.
1. Gottenborg E, Maw A, Ngov LK, Burden M, Ponomaryova A, Jones CD. You can’t have it all: The experience of academic hospitalists during pregnancy, parental leave, and return to work. J Hosp Med. 2018;13(12):836-839. doi: 10.12788/jhm.3076. PubMed
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
3. Association of American Medical Colleges. The state of women in academic medicine: The pipeline and pathways to leadership, 2015-2016. https://www.aamc.org/members/gwims/statistics/. Accessed October 1, 2018.
4. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
5. National Public Radio. A Pediatrician’s View of Paid Parental Leave. https://www.npr.org/sections/health-shots/2016/10/10/497052014/a-pediatricians-view-of-paid-parental-leave. Accessed September 26, 2018.
6. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership? (2018, June 19). Harvard Business Review. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership. Accessed October 1, 2018.
© 2018 Society of Hospital Medicine
On Decreasing Utilization: Models of Care for Frequently Hospitalized Patients and Their Effect on Outcomes
In this month’s edition of the Journal of Hospital Medicine, Goodwin and colleagues report their findings from their systematic review of models of care for frequently hospitalized patients. The authors reviewed the literature for interventions to reduce hospital admissions in frequently hospitalized patients with the goal of assessing the success of the interventions. This report contributes to the literature base of interventions to reduce healthcare utilization, particularly in the area of inpatient hospitalization.1
Goodwin et al. report that only nine studies met their criteria for review after a thorough search of the published literature. Of these nine studies, only four were determined to be high-quality studies. Interestingly, the low-quality studies found positive results in reducing hospital utilization, whereas the high-quality studies found decreases that were mirrored by their control groups. Impressive heterogeneity was found in the range of definitions, interventions, and outcome measures in the studies. These studies highlight the issue of “regression to the mean” for sicker individuals; however, they may not address readmission rates of specific medical systems or procedures that are also cost drivers, even if the patients are not considered critically ill. They also show where research partnerships can assist in increasing the number of members included in the studies for robust analyses.
From the perspective of a health plan, we applaud all efforts to improve patient outcomes and reduce cost. This report states that efforts to reduce chronic hospitalizations have not been unqualified successes. We must reflect upon how reducing utilization and improving outcomes align with our overall goals as a society. Recently, Federal Reserve Chairman Jay Powell summed up our nation’s particular issue, stating, “It is widely understood that the United States is on an unsustainable fiscal path, largely due to the interaction between an aging population and a healthcare system that delivers pretty average healthcare at a cost that is much higher than that of any other advanced economy.”2
A recent Kaiser Family Foundation analysis showed that 1% of patients accounted for 23% of all medical spending in the United States, and 97% of medical spending is attributed to the top 50% of patients.3 Pharmaceutical costs also play a role in this trend. Blue Cross and Blue Shield of Texas (BCBSTX) found that 2.5% of our population accounted for just under 50% of total medical spending. Conversely, when looking at patients with very high costs, only 0.4% had over $100,000 in spending exclusive of pharmacy. When including pharmacy, that number rises to 0.5%. As we consider annual medical and pharmacy trends year over year, we find that pharmacy spending may outpace hospital expenses in the near future.
Our internal data are also consistent with published reports that fewer than half of high-cost patients in one year continue to be high-cost cases the following year. Niall Brennan et al. reported that only 39% of the top 5% of spenders in a given year are also high spenders the following year.4 This finding not only coincides with the author’s statement around regression to the mean for the high admission utilizers, but it may be instructive to those looking to a Pareto method of attacking cost. If more than half of targeted patients will move out of the high cost category on their own, then demonstrating the effectiveness of interventions becomes challenging. Moreover, this regression finding speaks to the need to create effective programs to manage population health on a broad basis, which can address quality to all members and streamline costs for a large group that covers well more than 50% of medical spending.
BCBSTX emphasizes the creation of systems that let providers become responsible and accountable to outcomes and cost. Accountable Care Organizations (ACOs) and Intensive Medical Homes (IMHs) have played important roles in this journey, but physicians need to continue to invent and prioritize interventions that may achieve both goals. In particular, hospitalists have an important role to play. As ACOs flourish, hospitalists will need to join under the value-based umbrella and continue to intervene in patient care, policies, and procedures to reduce avoidable hospitalizations.
The development of value-based arrangements offers the healthcare system a unique opportunity to bring much-needed change. In our medical partnerships, direct communication with providers regarding their member experience and sharing of vital information about their patients’ health status have helped improve patient outcomes and decrease cost. Our IMH partnerships show a savings of up to $45,000 per member per year driven by decreases in admissions and ER visits, and in some cases, expensive medications. The hard work in these successes lies within the subtleties of fostering the relationship between payers and providers. Each pillar within the ecosystem plays a key role offering strengths, but the upside toward change comes in how we support each other’s weaknesses. This support is manifested in two ways: collaboration through communication and transparency through data sharing.
The road to change is one less traveled but not unpaved; advances in technology allow us to take experiences and build from them. At its core, technology has enhanced our collaboration and data capabilities. The ability to stay in touch with providers allows for almost real-time addressing of issues, promoting efficiency. The connection we have with providers has evolved from being solely paper contracts to a multichannel, multifunctional system. The ability to take claims experience, insert clinical acumen, and perform data analysis brings actionable solutions to be executed by our providers.
Those in the healthcare system will need to come together to continue to create interventions that improve quality while decreasing costs. The second part may require even more work than the first. The Health Care Cost Institute recently published data showing that inpatient utilization over a five-year period fell 12.9% in the commercially insured.5 However, over that same period, hospital prices for inpatient care rose 24.3%. The fundamental reason for the excess amount of money spent in US healthcare is that the prices are incredibly high.6 Currently, when diligence is exercised in reducing utilization, hospitals simply raise prices as a response to compensate for the lost income. Likewise, although prescription drug utilization only increased 1.8% during that period, the prices increased by 24.9%.
For the United States healthcare system to improve its quality and reduce its cost, we will need inventive partnerships to continue to create new systems to interact with patients in the most efficient and effective way possible. Readmissions and hospital utilization will be a large part of that improvement. Hospitals and hospitalists should ensure that they continue to focus on making healthcare more affordable by improving efficiency and outcomes and by resisting the tendencies of hospitals and pharmaceutical companies to raise prices in reaction to the improved efficiency.
Disclosures
The authors have nothing to disclose.
1. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for Frequently Hospitalized Patients and their Effect on Outcomes: A Systematic Review. J Hosp Med. 2018; 13(12):853-859. doi: 10.12788/jhm.3089. PubMed
2. Marketplace. Fed Chair Jay Powel. https://www.marketplace.org/2018/07/12/economy/powell-transcript. Accessed August 3, 2018.
3. Health System Tracker. https://www.healthsystemtracker.org/chart-collection/health-expenditures-vary-across-population/#item-start%2012/01/2017. Accessed August 3, 2018.
4. NEJM Catalyst. Consistently High Turnover in the Group of Top Health Care Spenders. https://catalyst.nejm.org/high-turnover-top-health-care-spenders/. Accessed August 3, 2018.
5. Health Care Cost Institute. 2016 Health Care Cost and Utilization Report. http://www.healthcostinstitute.org/report/2016-health-care-cost-utilization-report/. Accessed August 3, 2018.
6. Anderson GF, Reinhardt UE, Hussey PS, Peterosyan V. It’s the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood). 2003;22(3):89-105. doi: 10.1377/hlthaff.22.3.89. PubMed
In this month’s edition of the Journal of Hospital Medicine, Goodwin and colleagues report their findings from their systematic review of models of care for frequently hospitalized patients. The authors reviewed the literature for interventions to reduce hospital admissions in frequently hospitalized patients with the goal of assessing the success of the interventions. This report contributes to the literature base of interventions to reduce healthcare utilization, particularly in the area of inpatient hospitalization.1
Goodwin et al. report that only nine studies met their criteria for review after a thorough search of the published literature. Of these nine studies, only four were determined to be high-quality studies. Interestingly, the low-quality studies found positive results in reducing hospital utilization, whereas the high-quality studies found decreases that were mirrored by their control groups. Impressive heterogeneity was found in the range of definitions, interventions, and outcome measures in the studies. These studies highlight the issue of “regression to the mean” for sicker individuals; however, they may not address readmission rates of specific medical systems or procedures that are also cost drivers, even if the patients are not considered critically ill. They also show where research partnerships can assist in increasing the number of members included in the studies for robust analyses.
From the perspective of a health plan, we applaud all efforts to improve patient outcomes and reduce cost. This report states that efforts to reduce chronic hospitalizations have not been unqualified successes. We must reflect upon how reducing utilization and improving outcomes align with our overall goals as a society. Recently, Federal Reserve Chairman Jay Powell summed up our nation’s particular issue, stating, “It is widely understood that the United States is on an unsustainable fiscal path, largely due to the interaction between an aging population and a healthcare system that delivers pretty average healthcare at a cost that is much higher than that of any other advanced economy.”2
A recent Kaiser Family Foundation analysis showed that 1% of patients accounted for 23% of all medical spending in the United States, and 97% of medical spending is attributed to the top 50% of patients.3 Pharmaceutical costs also play a role in this trend. Blue Cross and Blue Shield of Texas (BCBSTX) found that 2.5% of our population accounted for just under 50% of total medical spending. Conversely, when looking at patients with very high costs, only 0.4% had over $100,000 in spending exclusive of pharmacy. When including pharmacy, that number rises to 0.5%. As we consider annual medical and pharmacy trends year over year, we find that pharmacy spending may outpace hospital expenses in the near future.
Our internal data are also consistent with published reports that fewer than half of high-cost patients in one year continue to be high-cost cases the following year. Niall Brennan et al. reported that only 39% of the top 5% of spenders in a given year are also high spenders the following year.4 This finding not only coincides with the author’s statement around regression to the mean for the high admission utilizers, but it may be instructive to those looking to a Pareto method of attacking cost. If more than half of targeted patients will move out of the high cost category on their own, then demonstrating the effectiveness of interventions becomes challenging. Moreover, this regression finding speaks to the need to create effective programs to manage population health on a broad basis, which can address quality to all members and streamline costs for a large group that covers well more than 50% of medical spending.
BCBSTX emphasizes the creation of systems that let providers become responsible and accountable to outcomes and cost. Accountable Care Organizations (ACOs) and Intensive Medical Homes (IMHs) have played important roles in this journey, but physicians need to continue to invent and prioritize interventions that may achieve both goals. In particular, hospitalists have an important role to play. As ACOs flourish, hospitalists will need to join under the value-based umbrella and continue to intervene in patient care, policies, and procedures to reduce avoidable hospitalizations.
The development of value-based arrangements offers the healthcare system a unique opportunity to bring much-needed change. In our medical partnerships, direct communication with providers regarding their member experience and sharing of vital information about their patients’ health status have helped improve patient outcomes and decrease cost. Our IMH partnerships show a savings of up to $45,000 per member per year driven by decreases in admissions and ER visits, and in some cases, expensive medications. The hard work in these successes lies within the subtleties of fostering the relationship between payers and providers. Each pillar within the ecosystem plays a key role offering strengths, but the upside toward change comes in how we support each other’s weaknesses. This support is manifested in two ways: collaboration through communication and transparency through data sharing.
The road to change is one less traveled but not unpaved; advances in technology allow us to take experiences and build from them. At its core, technology has enhanced our collaboration and data capabilities. The ability to stay in touch with providers allows for almost real-time addressing of issues, promoting efficiency. The connection we have with providers has evolved from being solely paper contracts to a multichannel, multifunctional system. The ability to take claims experience, insert clinical acumen, and perform data analysis brings actionable solutions to be executed by our providers.
Those in the healthcare system will need to come together to continue to create interventions that improve quality while decreasing costs. The second part may require even more work than the first. The Health Care Cost Institute recently published data showing that inpatient utilization over a five-year period fell 12.9% in the commercially insured.5 However, over that same period, hospital prices for inpatient care rose 24.3%. The fundamental reason for the excess amount of money spent in US healthcare is that the prices are incredibly high.6 Currently, when diligence is exercised in reducing utilization, hospitals simply raise prices as a response to compensate for the lost income. Likewise, although prescription drug utilization only increased 1.8% during that period, the prices increased by 24.9%.
For the United States healthcare system to improve its quality and reduce its cost, we will need inventive partnerships to continue to create new systems to interact with patients in the most efficient and effective way possible. Readmissions and hospital utilization will be a large part of that improvement. Hospitals and hospitalists should ensure that they continue to focus on making healthcare more affordable by improving efficiency and outcomes and by resisting the tendencies of hospitals and pharmaceutical companies to raise prices in reaction to the improved efficiency.
Disclosures
The authors have nothing to disclose.
In this month’s edition of the Journal of Hospital Medicine, Goodwin and colleagues report their findings from their systematic review of models of care for frequently hospitalized patients. The authors reviewed the literature for interventions to reduce hospital admissions in frequently hospitalized patients with the goal of assessing the success of the interventions. This report contributes to the literature base of interventions to reduce healthcare utilization, particularly in the area of inpatient hospitalization.1
Goodwin et al. report that only nine studies met their criteria for review after a thorough search of the published literature. Of these nine studies, only four were determined to be high-quality studies. Interestingly, the low-quality studies found positive results in reducing hospital utilization, whereas the high-quality studies found decreases that were mirrored by their control groups. Impressive heterogeneity was found in the range of definitions, interventions, and outcome measures in the studies. These studies highlight the issue of “regression to the mean” for sicker individuals; however, they may not address readmission rates of specific medical systems or procedures that are also cost drivers, even if the patients are not considered critically ill. They also show where research partnerships can assist in increasing the number of members included in the studies for robust analyses.
From the perspective of a health plan, we applaud all efforts to improve patient outcomes and reduce cost. This report states that efforts to reduce chronic hospitalizations have not been unqualified successes. We must reflect upon how reducing utilization and improving outcomes align with our overall goals as a society. Recently, Federal Reserve Chairman Jay Powell summed up our nation’s particular issue, stating, “It is widely understood that the United States is on an unsustainable fiscal path, largely due to the interaction between an aging population and a healthcare system that delivers pretty average healthcare at a cost that is much higher than that of any other advanced economy.”2
A recent Kaiser Family Foundation analysis showed that 1% of patients accounted for 23% of all medical spending in the United States, and 97% of medical spending is attributed to the top 50% of patients.3 Pharmaceutical costs also play a role in this trend. Blue Cross and Blue Shield of Texas (BCBSTX) found that 2.5% of our population accounted for just under 50% of total medical spending. Conversely, when looking at patients with very high costs, only 0.4% had over $100,000 in spending exclusive of pharmacy. When including pharmacy, that number rises to 0.5%. As we consider annual medical and pharmacy trends year over year, we find that pharmacy spending may outpace hospital expenses in the near future.
Our internal data are also consistent with published reports that fewer than half of high-cost patients in one year continue to be high-cost cases the following year. Niall Brennan et al. reported that only 39% of the top 5% of spenders in a given year are also high spenders the following year.4 This finding not only coincides with the author’s statement around regression to the mean for the high admission utilizers, but it may be instructive to those looking to a Pareto method of attacking cost. If more than half of targeted patients will move out of the high cost category on their own, then demonstrating the effectiveness of interventions becomes challenging. Moreover, this regression finding speaks to the need to create effective programs to manage population health on a broad basis, which can address quality to all members and streamline costs for a large group that covers well more than 50% of medical spending.
BCBSTX emphasizes the creation of systems that let providers become responsible and accountable to outcomes and cost. Accountable Care Organizations (ACOs) and Intensive Medical Homes (IMHs) have played important roles in this journey, but physicians need to continue to invent and prioritize interventions that may achieve both goals. In particular, hospitalists have an important role to play. As ACOs flourish, hospitalists will need to join under the value-based umbrella and continue to intervene in patient care, policies, and procedures to reduce avoidable hospitalizations.
The development of value-based arrangements offers the healthcare system a unique opportunity to bring much-needed change. In our medical partnerships, direct communication with providers regarding their member experience and sharing of vital information about their patients’ health status have helped improve patient outcomes and decrease cost. Our IMH partnerships show a savings of up to $45,000 per member per year driven by decreases in admissions and ER visits, and in some cases, expensive medications. The hard work in these successes lies within the subtleties of fostering the relationship between payers and providers. Each pillar within the ecosystem plays a key role offering strengths, but the upside toward change comes in how we support each other’s weaknesses. This support is manifested in two ways: collaboration through communication and transparency through data sharing.
The road to change is one less traveled but not unpaved; advances in technology allow us to take experiences and build from them. At its core, technology has enhanced our collaboration and data capabilities. The ability to stay in touch with providers allows for almost real-time addressing of issues, promoting efficiency. The connection we have with providers has evolved from being solely paper contracts to a multichannel, multifunctional system. The ability to take claims experience, insert clinical acumen, and perform data analysis brings actionable solutions to be executed by our providers.
Those in the healthcare system will need to come together to continue to create interventions that improve quality while decreasing costs. The second part may require even more work than the first. The Health Care Cost Institute recently published data showing that inpatient utilization over a five-year period fell 12.9% in the commercially insured.5 However, over that same period, hospital prices for inpatient care rose 24.3%. The fundamental reason for the excess amount of money spent in US healthcare is that the prices are incredibly high.6 Currently, when diligence is exercised in reducing utilization, hospitals simply raise prices as a response to compensate for the lost income. Likewise, although prescription drug utilization only increased 1.8% during that period, the prices increased by 24.9%.
For the United States healthcare system to improve its quality and reduce its cost, we will need inventive partnerships to continue to create new systems to interact with patients in the most efficient and effective way possible. Readmissions and hospital utilization will be a large part of that improvement. Hospitals and hospitalists should ensure that they continue to focus on making healthcare more affordable by improving efficiency and outcomes and by resisting the tendencies of hospitals and pharmaceutical companies to raise prices in reaction to the improved efficiency.
Disclosures
The authors have nothing to disclose.
1. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for Frequently Hospitalized Patients and their Effect on Outcomes: A Systematic Review. J Hosp Med. 2018; 13(12):853-859. doi: 10.12788/jhm.3089. PubMed
2. Marketplace. Fed Chair Jay Powel. https://www.marketplace.org/2018/07/12/economy/powell-transcript. Accessed August 3, 2018.
3. Health System Tracker. https://www.healthsystemtracker.org/chart-collection/health-expenditures-vary-across-population/#item-start%2012/01/2017. Accessed August 3, 2018.
4. NEJM Catalyst. Consistently High Turnover in the Group of Top Health Care Spenders. https://catalyst.nejm.org/high-turnover-top-health-care-spenders/. Accessed August 3, 2018.
5. Health Care Cost Institute. 2016 Health Care Cost and Utilization Report. http://www.healthcostinstitute.org/report/2016-health-care-cost-utilization-report/. Accessed August 3, 2018.
6. Anderson GF, Reinhardt UE, Hussey PS, Peterosyan V. It’s the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood). 2003;22(3):89-105. doi: 10.1377/hlthaff.22.3.89. PubMed
1. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for Frequently Hospitalized Patients and their Effect on Outcomes: A Systematic Review. J Hosp Med. 2018; 13(12):853-859. doi: 10.12788/jhm.3089. PubMed
2. Marketplace. Fed Chair Jay Powel. https://www.marketplace.org/2018/07/12/economy/powell-transcript. Accessed August 3, 2018.
3. Health System Tracker. https://www.healthsystemtracker.org/chart-collection/health-expenditures-vary-across-population/#item-start%2012/01/2017. Accessed August 3, 2018.
4. NEJM Catalyst. Consistently High Turnover in the Group of Top Health Care Spenders. https://catalyst.nejm.org/high-turnover-top-health-care-spenders/. Accessed August 3, 2018.
5. Health Care Cost Institute. 2016 Health Care Cost and Utilization Report. http://www.healthcostinstitute.org/report/2016-health-care-cost-utilization-report/. Accessed August 3, 2018.
6. Anderson GF, Reinhardt UE, Hussey PS, Peterosyan V. It’s the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood). 2003;22(3):89-105. doi: 10.1377/hlthaff.22.3.89. PubMed
© 2018 Society of Hospital Medicine
Towards Scalable Hospital-Based Palliative Care: Challenges and Opportunities for Hospitalists
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
There is growing evidence that supports the ability of specialty palliative care to achieve the Triple Aim in healthcare: (1) improve patient and family experience of care, (2) improve health outcomes, and (3) reduce healthcare costs.1,2 However, the full realization of this value remains elusive due, in large part, to the increasing demand for specialty palliative care services outpacing the supply of specialists.3 Because expansion of the specialty palliative care workforce will never be sufficient to meet the needs of seriously ill patients, and nonspecialist physicians often fail to recognize palliative care needs in a timely manner,4 innovative and systematic solutions are needed to provide high-quality palliative care in a manner that is sustainable.5
To close the gap between workforce and patient needs, experts have largely advocated for two care delivery models that aim to improve the organization and allocation of limited palliative care resources: (1) a tier-based approach in which primary palliative care (basic skills for all clinicians) and specialty palliative care (advanced skills requiring additional training) have distinct but supportive roles, and (2) a need-based approach where different types of palliative care clinicians are deployed based on specific needs.5,6 In this issue, Abedini and Chopra propose a “Palliative Care Redistribution Integrated System Model” (PRISM) that combines these two approaches, with need-based care delivery that escalates through skill tiers to improve hospital-based palliative care.7
PRISM is attractive because it leverages the skill sets of clinicians across disciplines and is designed for the hospital, where the vast majority of specialty palliative care is provided in the United States. Moreover, it employs hospitalists who routinely care for a high volume of seriously ill patients, and are therefore well positioned to expand the palliative care workforce. The authors suggest several approaches to implement PRISM, such as designating certain hospitalist teams for palliative care, more interdisciplinary support, automated patient risk stratification or mandatory screening checklists, and strategic use of bedside nurses and social workers to facilitate early basic needs assessments. Although sound in principle, there are several foreseeable barriers to each of these approaches and potential unintended consequences of PRISM in the fields of hospital and palliative medicine.
Applying insights from behavioral economics will be essential for the successful implementation and dissemination of PRISM. Changing clinician behavior is not a challenge unique to palliative care interventions, but it may be particularly difficult due to misperceptions that palliative care is synonymous with end-of-life care and that such conversations are always time-intensive. Indeed, Abedini and Chopra acknowledge that all clinicians need to be well versed in basic palliative care skills for PRISM to succeed, yet most educational initiatives have shown modest results at best. The most promising clinician education programs, such as the Serious Illness Care Program and VitalTalk require intensive training simulations and are most effective when implemented on a system level to promote cultural change.8.9 Thus, training hospitalists in preparation for PRISM will require considerable upfront investment by hospitals. While policy efforts to improve palliative care training in medical education are progressing (Palliative Care and Hospice Education and Training Act, H.R.1676), any evidence of impact is nearly a generation away.
The authors also advocate for a technology-driven solution for systematic and early identification of palliative care needs. However, ideal clinical decision support would not rely on checklists to be completed by bedside clinicians or “hard stop” alerts in the electronic health record, as both of these approaches rely heavily upon consistent and accurate data entry by busy clinicians. Rather, innovative predictive analytics with machine learning and natural language processing methods hold great promise to support an electronic precision medicine approach for palliative care delivery. Even after such prediction models are developed, rigorous studies are needed to understand how they can change clinician behavior and impact the quality and cost of care.
Shifting palliative care tasks to nonspecialists has implications beyond quality and access. First, there are likely to be reimbursement implications as nonbillable clinicians such as social workers provide palliative care services that were previously provided by physicians and advance practice providers. As value-based payment models grow, healthcare systems may be wise to invest in innovative palliative care delivery models such as PRISM, but obtaining financial support will require rigorous evidence of value. Second, it will be important to monitor the already high rates of burnout and emotional exhaustion among palliative care clinicians10 when implementing care delivery models that select only the most complex patients for referral to specialty palliative care. Finally, new palliative care delivery models must fit within a larger national strategy to grow palliative care across the care continuum.11 This is of particular importance with hospital-focused solutions such as PRISM due to concerns about the growing split in care coordination between inpatient and outpatient care. Since seriously ill patients spend the majority of time outside the hospital and evidence for the value of palliative care is most robust in home and ambulatory settings,1 an important role for hospitalists could be to systematically identify and refer high-risk patients to community-based palliative care services after discharge from a sentinel hospitalization.
In conclusion, innovative palliative care delivery models such as PRISM are critical to ensuring that seriously ill patients have access to high-quality palliative care; however, more work is still needed to create the training programs, patient identification tools, scalable implementation, and evaluation processes necessary for success.
Disclosures
Dr. Courtright and Dr. O’Connor have nothing to disclose.
Funding
This work was funded in part by a career development award from the National Palliative Care Research Center (KRC). The views expressed herein solely represent those of the authors.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. Jama. 2016;316(20):2104. doi: 10.1001/jama.2016.16840. PubMed
2. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820. doi: 10.1001/jamainternmed.2018.0750. PubMed
3. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2016;19(1):8-15. doi: 10.1089/jpm.2015.0351. PubMed
4. Heyland DK. Failure to Engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778. doi: 10.1001/jamainternmed.2013.180. PubMed
5. Courtright KR, Cassel JB, Halpern SD. A research agenda for high-value palliative care. Ann Intern Med. 2017;168(1):71. doi: 10.7326/m17-2164. PubMed
6. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions. JAMA Intern Med. 2014;174(4):620. doi: 10.1001/jamainternmed.2013.14384. PubMed
7. Abedini NC, Chopra V. A Model to Improve Hospital-Based Palliative Care: The Palliative Care Redistribution Integrated System Model (PRISM). J Hosp Med. 2018;13(12):868-871. doi: 10.12788/jhm.3065 PubMed
8. Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomized controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032. doi: 10.1136/bmjopen-2015-009032. PubMed
9. Clayton JM, Butow PN, Waters A, et al. Evaluation of a novel individualized communication-skills training intervention to improve doctors’ confidence and skills in end-of-life communication. Palliat Med. 2012;27(3):236-243. doi: 10.1177/0269216312449683. PubMed
10. Kamal AH, Bull JH, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manag. 2016;51(4):690-696. doi: 10.1016/j.jpainsymman.2015.10.020. PubMed
11. Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164. PubMed
© 2018 Society of Hospital Medicine
December 2018 Digital Edition
Click here to access the December 2018 Digital Edition.
Table of Contents
- Anesthesia Care Practice Models in the Veterans Health Administration
- Role of Point-of-Care Ultrasonography in the Evaluation and Management of Kidney Disease
- PACT ICU Model: Interprofessional Case Conferences for High-Risk/High-Need Patients
- A Pharmacist-Led Transitional Care Program to Reduce Hospital Readmissions in Older Adults
- The Gift and the Thought Both Count
- Overemphasizing Communities in the National Suicide Strategy Could Undercut VA Successes

Click here to access the December 2018 Digital Edition.
Table of Contents
- Anesthesia Care Practice Models in the Veterans Health Administration
- Role of Point-of-Care Ultrasonography in the Evaluation and Management of Kidney Disease
- PACT ICU Model: Interprofessional Case Conferences for High-Risk/High-Need Patients
- A Pharmacist-Led Transitional Care Program to Reduce Hospital Readmissions in Older Adults
- The Gift and the Thought Both Count
- Overemphasizing Communities in the National Suicide Strategy Could Undercut VA Successes

Click here to access the December 2018 Digital Edition.
Table of Contents
- Anesthesia Care Practice Models in the Veterans Health Administration
- Role of Point-of-Care Ultrasonography in the Evaluation and Management of Kidney Disease
- PACT ICU Model: Interprofessional Case Conferences for High-Risk/High-Need Patients
- A Pharmacist-Led Transitional Care Program to Reduce Hospital Readmissions in Older Adults
- The Gift and the Thought Both Count
- Overemphasizing Communities in the National Suicide Strategy Could Undercut VA Successes

Botulinum toxin: Emerging psychiatric indications
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
Treating negative symptoms of schizophrenia
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.