User login
Evidence-Based Medicine for the Hospitalist
In the last installment of this series, we introduced the concept of critical appraisal of the statistical methods used in a paper. The statistical analysis in a study is often the final barrier between the study’s results and application of those results to patient care, so making sure that the findings have been properly evaluated is of obvious importance.
We have previously discussed P values and confidence intervals—two of the most common statistical outcomes upon which clinical decisions are based. In this segment, we will discuss several specific issues that can help a reader decide how much faith to place in a study’s results.
Test Assumptions
Statistical tests generally require that a variety of assumptions be satisfied for the test procedure to be valid. These assumptions vary from test to test, and unfortunately most computer packages do not ask users whether they want to examine these assumptions more closely. This is one of the dangers of “black box” analysis, when researchers with little statistical training run their data through a statistical package without fully understanding how the output is generated.
Many statistical tests are based on the theory of the bell curve, or normal distribution. These tests require a large enough sample size, usually at least 30 subjects per group and sometimes much greater, for this theory to hold. In addition, the data should not be skewed excessively. For example, consider a study comparing two treatments for mild pain for which scores on a continuous 0-10 visual analog scale are expected to be between 0 and 2. Because of the asymmetry of the data, an underlying bell curve isn’t likely to make much sense. Therefore, a two-sample t-test may not be appropriate for this study even with two large samples.
Another commonly violated assumption is that the two groups being compared may need to be independent. The simplest case occurs when the same subjects are measured before and after a procedure. A two-sample statistical test is not appropriate here because the two groups are actually the same, and therefore clearly not independent. In this case, a paired analysis is required. The issue of independence becomes more complicated when we consider tests of multiple variables that may be related to one another, or studies of effects over time. In these instances, additional expertise in selecting the correct analysis approach is usually needed.
The best way to ensure that these assumptions and the many others required for valid statistical testing are met is to plan your analyses with the help of a trained statistician. If this is not an option, it is incumbent upon the researcher to learn about these assumptions and evaluate their study to make sure the appropriate methods are applied.
Negative Study Results
A more straightforward issue concerns interpretation of negative study results. Most clinicians are familiar with statistical power: A small study may yield a negative finding because this is the correct result or because there is not enough power to discern a difference between the groups being tested. Often, the width of the confidence interval provides insight into this problem. If the confidence interval includes a difference that would be clinically meaningful, a negative study should be viewed skeptically. In such cases, a larger study or a meta-analysis may be needed to better address the question. If, on the other hand, the confidence interval suggests that no clinically relevant result is likely, the negative study finding becomes more compelling.
Multiple Statistical Tests
When we perform a statistical test and set the level of significance at 0.05, we are acknowledging a 5% chance that if the null hypothesis were in fact true we would nonetheless falsely reject it with our test. Turned around, this loosely means a 95% chance of “getting it right,” subject to the limitations of P value interpretation described in the previous segment of this series. This seems reasonable for a single test, but what about the typical research study in which dozens of statistical tests are run? For two independent tests, the chance of “getting it right” in both cases would be 0.95 x 0.95 = 90%. For 20 tests, this probability would be only 36%, meaning a more than 50% chance of drawing at least one false conclusion. The trouble is that there is no way to know which of the 20 tests might have yielded a wrong conclusion!
To address this issue, researchers may set their initial level of significance at a stricter level—perhaps 0.01. There are also mathematical ways to adjust the level of significance to help with multiple comparisons. The key point is that the more tests you run, the more chances you have to draw a false conclusion. Neither you nor your patients can know when this occurs, though. The same arguments apply to subgroup analyses and data-driven, or post hoc, analyses. Such analyses should be regarded as hypothesis-generating rather than hypothesis-testing, and any findings from these analyses should be evaluated more directly by additional research.
Sensitivity Analysis
A rarely considered aspect of study interpretation is whether the results would change if only a few data points changed. Studies with rare events and wide confidence intervals are often sensitive to a change in even one data point. For example, a study published in 2000 by Kernan, et al., presented a statistically significant finding of increased risk of hemorrhagic stroke in women using appetite suppressants containing phenylpropanolamine. This result was based on six cases and one control, with an unadjusted odds ratio of 11.9 (95% CI, 1.4-99.4).
Shifting just one patient who had used phenylpropanolamine from the case group to the control group would change the odds ratio to 5.0, with a nonsignificant CI of 0.9-25.8. Such an analysis should make readers question how quickly they wish to apply the study results to their own patients, especially if the benefits of the drug are significant. A result that is sensitive to small changes in the study population is probably not stable enough to warrant application to the entire patient population.
Back to the Common-Sense Test
An excellent way to judge whether a study’s results should be believed is to step back and consider whether they make sense based on current scientific knowledge. If they do not, either the study represents a breakthrough in our understanding of disease or the study’s results are flawed. Remember, if the prevalence of a disease is very low, even a positive diagnostic test with high sensitivity and specificity is likely to be a false positive. Similarly, a small P value may represent a false result if the hypothesis being tested does not meet standard epidemiologic criteria for causality such as biological plausibility. Statistics are primarily a tool to help us make sense of complex study data. They can often suggest when new theories should be evaluated, but they should not determine by themselves which results we apply to patient care.
Series Conclusion
This series has been intended as a brief introduction to many different facets of evidence-based medicine. The primary message of evidence-based medicine is that critical assessment of every aspect of research is necessary to ensure that we make the best possible decisions for our patients. Understanding the important concepts in study design and analysis may seem daunting, but this effort is made worthwhile every time we positively affect patient care.
Hospitalists are uniquely situated at the interface of internal medicine and essentially every other area of medicine and because of this have a tremendous opportunity to broadly impact patient care. My hope is that evidence-based medicine-savvy hospitalists will capitalize on this for the benefit of our patients, will play a prominent role in educating future clinicians on the importance of evidence-based medicine, and will use it to lead the next wave of patient care advances. TH
Dr. West practices in the Division of General Internal Medicine, Mayo Clinic College of Medicine, Rochester, Minn.
References
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. I: Different types of data need different statistical tests. BMJ. 1997;315:364-366.
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. II: “Significant” relations and their pitfalls. BMJ. 1997;315:422-425.
- Guyatt G and Rennie D, eds. Users’ guides to the medical literature. Chicago: AMA Press; 2002.
- Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
In the last installment of this series, we introduced the concept of critical appraisal of the statistical methods used in a paper. The statistical analysis in a study is often the final barrier between the study’s results and application of those results to patient care, so making sure that the findings have been properly evaluated is of obvious importance.
We have previously discussed P values and confidence intervals—two of the most common statistical outcomes upon which clinical decisions are based. In this segment, we will discuss several specific issues that can help a reader decide how much faith to place in a study’s results.
Test Assumptions
Statistical tests generally require that a variety of assumptions be satisfied for the test procedure to be valid. These assumptions vary from test to test, and unfortunately most computer packages do not ask users whether they want to examine these assumptions more closely. This is one of the dangers of “black box” analysis, when researchers with little statistical training run their data through a statistical package without fully understanding how the output is generated.
Many statistical tests are based on the theory of the bell curve, or normal distribution. These tests require a large enough sample size, usually at least 30 subjects per group and sometimes much greater, for this theory to hold. In addition, the data should not be skewed excessively. For example, consider a study comparing two treatments for mild pain for which scores on a continuous 0-10 visual analog scale are expected to be between 0 and 2. Because of the asymmetry of the data, an underlying bell curve isn’t likely to make much sense. Therefore, a two-sample t-test may not be appropriate for this study even with two large samples.
Another commonly violated assumption is that the two groups being compared may need to be independent. The simplest case occurs when the same subjects are measured before and after a procedure. A two-sample statistical test is not appropriate here because the two groups are actually the same, and therefore clearly not independent. In this case, a paired analysis is required. The issue of independence becomes more complicated when we consider tests of multiple variables that may be related to one another, or studies of effects over time. In these instances, additional expertise in selecting the correct analysis approach is usually needed.
The best way to ensure that these assumptions and the many others required for valid statistical testing are met is to plan your analyses with the help of a trained statistician. If this is not an option, it is incumbent upon the researcher to learn about these assumptions and evaluate their study to make sure the appropriate methods are applied.
Negative Study Results
A more straightforward issue concerns interpretation of negative study results. Most clinicians are familiar with statistical power: A small study may yield a negative finding because this is the correct result or because there is not enough power to discern a difference between the groups being tested. Often, the width of the confidence interval provides insight into this problem. If the confidence interval includes a difference that would be clinically meaningful, a negative study should be viewed skeptically. In such cases, a larger study or a meta-analysis may be needed to better address the question. If, on the other hand, the confidence interval suggests that no clinically relevant result is likely, the negative study finding becomes more compelling.
Multiple Statistical Tests
When we perform a statistical test and set the level of significance at 0.05, we are acknowledging a 5% chance that if the null hypothesis were in fact true we would nonetheless falsely reject it with our test. Turned around, this loosely means a 95% chance of “getting it right,” subject to the limitations of P value interpretation described in the previous segment of this series. This seems reasonable for a single test, but what about the typical research study in which dozens of statistical tests are run? For two independent tests, the chance of “getting it right” in both cases would be 0.95 x 0.95 = 90%. For 20 tests, this probability would be only 36%, meaning a more than 50% chance of drawing at least one false conclusion. The trouble is that there is no way to know which of the 20 tests might have yielded a wrong conclusion!
To address this issue, researchers may set their initial level of significance at a stricter level—perhaps 0.01. There are also mathematical ways to adjust the level of significance to help with multiple comparisons. The key point is that the more tests you run, the more chances you have to draw a false conclusion. Neither you nor your patients can know when this occurs, though. The same arguments apply to subgroup analyses and data-driven, or post hoc, analyses. Such analyses should be regarded as hypothesis-generating rather than hypothesis-testing, and any findings from these analyses should be evaluated more directly by additional research.
Sensitivity Analysis
A rarely considered aspect of study interpretation is whether the results would change if only a few data points changed. Studies with rare events and wide confidence intervals are often sensitive to a change in even one data point. For example, a study published in 2000 by Kernan, et al., presented a statistically significant finding of increased risk of hemorrhagic stroke in women using appetite suppressants containing phenylpropanolamine. This result was based on six cases and one control, with an unadjusted odds ratio of 11.9 (95% CI, 1.4-99.4).
Shifting just one patient who had used phenylpropanolamine from the case group to the control group would change the odds ratio to 5.0, with a nonsignificant CI of 0.9-25.8. Such an analysis should make readers question how quickly they wish to apply the study results to their own patients, especially if the benefits of the drug are significant. A result that is sensitive to small changes in the study population is probably not stable enough to warrant application to the entire patient population.
Back to the Common-Sense Test
An excellent way to judge whether a study’s results should be believed is to step back and consider whether they make sense based on current scientific knowledge. If they do not, either the study represents a breakthrough in our understanding of disease or the study’s results are flawed. Remember, if the prevalence of a disease is very low, even a positive diagnostic test with high sensitivity and specificity is likely to be a false positive. Similarly, a small P value may represent a false result if the hypothesis being tested does not meet standard epidemiologic criteria for causality such as biological plausibility. Statistics are primarily a tool to help us make sense of complex study data. They can often suggest when new theories should be evaluated, but they should not determine by themselves which results we apply to patient care.
Series Conclusion
This series has been intended as a brief introduction to many different facets of evidence-based medicine. The primary message of evidence-based medicine is that critical assessment of every aspect of research is necessary to ensure that we make the best possible decisions for our patients. Understanding the important concepts in study design and analysis may seem daunting, but this effort is made worthwhile every time we positively affect patient care.
Hospitalists are uniquely situated at the interface of internal medicine and essentially every other area of medicine and because of this have a tremendous opportunity to broadly impact patient care. My hope is that evidence-based medicine-savvy hospitalists will capitalize on this for the benefit of our patients, will play a prominent role in educating future clinicians on the importance of evidence-based medicine, and will use it to lead the next wave of patient care advances. TH
Dr. West practices in the Division of General Internal Medicine, Mayo Clinic College of Medicine, Rochester, Minn.
References
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. I: Different types of data need different statistical tests. BMJ. 1997;315:364-366.
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. II: “Significant” relations and their pitfalls. BMJ. 1997;315:422-425.
- Guyatt G and Rennie D, eds. Users’ guides to the medical literature. Chicago: AMA Press; 2002.
- Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
In the last installment of this series, we introduced the concept of critical appraisal of the statistical methods used in a paper. The statistical analysis in a study is often the final barrier between the study’s results and application of those results to patient care, so making sure that the findings have been properly evaluated is of obvious importance.
We have previously discussed P values and confidence intervals—two of the most common statistical outcomes upon which clinical decisions are based. In this segment, we will discuss several specific issues that can help a reader decide how much faith to place in a study’s results.
Test Assumptions
Statistical tests generally require that a variety of assumptions be satisfied for the test procedure to be valid. These assumptions vary from test to test, and unfortunately most computer packages do not ask users whether they want to examine these assumptions more closely. This is one of the dangers of “black box” analysis, when researchers with little statistical training run their data through a statistical package without fully understanding how the output is generated.
Many statistical tests are based on the theory of the bell curve, or normal distribution. These tests require a large enough sample size, usually at least 30 subjects per group and sometimes much greater, for this theory to hold. In addition, the data should not be skewed excessively. For example, consider a study comparing two treatments for mild pain for which scores on a continuous 0-10 visual analog scale are expected to be between 0 and 2. Because of the asymmetry of the data, an underlying bell curve isn’t likely to make much sense. Therefore, a two-sample t-test may not be appropriate for this study even with two large samples.
Another commonly violated assumption is that the two groups being compared may need to be independent. The simplest case occurs when the same subjects are measured before and after a procedure. A two-sample statistical test is not appropriate here because the two groups are actually the same, and therefore clearly not independent. In this case, a paired analysis is required. The issue of independence becomes more complicated when we consider tests of multiple variables that may be related to one another, or studies of effects over time. In these instances, additional expertise in selecting the correct analysis approach is usually needed.
The best way to ensure that these assumptions and the many others required for valid statistical testing are met is to plan your analyses with the help of a trained statistician. If this is not an option, it is incumbent upon the researcher to learn about these assumptions and evaluate their study to make sure the appropriate methods are applied.
Negative Study Results
A more straightforward issue concerns interpretation of negative study results. Most clinicians are familiar with statistical power: A small study may yield a negative finding because this is the correct result or because there is not enough power to discern a difference between the groups being tested. Often, the width of the confidence interval provides insight into this problem. If the confidence interval includes a difference that would be clinically meaningful, a negative study should be viewed skeptically. In such cases, a larger study or a meta-analysis may be needed to better address the question. If, on the other hand, the confidence interval suggests that no clinically relevant result is likely, the negative study finding becomes more compelling.
Multiple Statistical Tests
When we perform a statistical test and set the level of significance at 0.05, we are acknowledging a 5% chance that if the null hypothesis were in fact true we would nonetheless falsely reject it with our test. Turned around, this loosely means a 95% chance of “getting it right,” subject to the limitations of P value interpretation described in the previous segment of this series. This seems reasonable for a single test, but what about the typical research study in which dozens of statistical tests are run? For two independent tests, the chance of “getting it right” in both cases would be 0.95 x 0.95 = 90%. For 20 tests, this probability would be only 36%, meaning a more than 50% chance of drawing at least one false conclusion. The trouble is that there is no way to know which of the 20 tests might have yielded a wrong conclusion!
To address this issue, researchers may set their initial level of significance at a stricter level—perhaps 0.01. There are also mathematical ways to adjust the level of significance to help with multiple comparisons. The key point is that the more tests you run, the more chances you have to draw a false conclusion. Neither you nor your patients can know when this occurs, though. The same arguments apply to subgroup analyses and data-driven, or post hoc, analyses. Such analyses should be regarded as hypothesis-generating rather than hypothesis-testing, and any findings from these analyses should be evaluated more directly by additional research.
Sensitivity Analysis
A rarely considered aspect of study interpretation is whether the results would change if only a few data points changed. Studies with rare events and wide confidence intervals are often sensitive to a change in even one data point. For example, a study published in 2000 by Kernan, et al., presented a statistically significant finding of increased risk of hemorrhagic stroke in women using appetite suppressants containing phenylpropanolamine. This result was based on six cases and one control, with an unadjusted odds ratio of 11.9 (95% CI, 1.4-99.4).
Shifting just one patient who had used phenylpropanolamine from the case group to the control group would change the odds ratio to 5.0, with a nonsignificant CI of 0.9-25.8. Such an analysis should make readers question how quickly they wish to apply the study results to their own patients, especially if the benefits of the drug are significant. A result that is sensitive to small changes in the study population is probably not stable enough to warrant application to the entire patient population.
Back to the Common-Sense Test
An excellent way to judge whether a study’s results should be believed is to step back and consider whether they make sense based on current scientific knowledge. If they do not, either the study represents a breakthrough in our understanding of disease or the study’s results are flawed. Remember, if the prevalence of a disease is very low, even a positive diagnostic test with high sensitivity and specificity is likely to be a false positive. Similarly, a small P value may represent a false result if the hypothesis being tested does not meet standard epidemiologic criteria for causality such as biological plausibility. Statistics are primarily a tool to help us make sense of complex study data. They can often suggest when new theories should be evaluated, but they should not determine by themselves which results we apply to patient care.
Series Conclusion
This series has been intended as a brief introduction to many different facets of evidence-based medicine. The primary message of evidence-based medicine is that critical assessment of every aspect of research is necessary to ensure that we make the best possible decisions for our patients. Understanding the important concepts in study design and analysis may seem daunting, but this effort is made worthwhile every time we positively affect patient care.
Hospitalists are uniquely situated at the interface of internal medicine and essentially every other area of medicine and because of this have a tremendous opportunity to broadly impact patient care. My hope is that evidence-based medicine-savvy hospitalists will capitalize on this for the benefit of our patients, will play a prominent role in educating future clinicians on the importance of evidence-based medicine, and will use it to lead the next wave of patient care advances. TH
Dr. West practices in the Division of General Internal Medicine, Mayo Clinic College of Medicine, Rochester, Minn.
References
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. I: Different types of data need different statistical tests. BMJ. 1997;315:364-366.
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. II: “Significant” relations and their pitfalls. BMJ. 1997;315:422-425.
- Guyatt G and Rennie D, eds. Users’ guides to the medical literature. Chicago: AMA Press; 2002.
- Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
HELPS Really Helps
Spend time with hospitalists and their competitive natures readily emerge. Striving for excellence in clinical care, hospital efficiency, and patient satisfaction, they are their hospitals’ beacons for attracting patients from referring physicians. As hospitalists’ capabilities grow, they hone pathways and procedures, improving their hospitals over time. What would happen if those hospitalists systematically shared their collective knowledge?
That’s what’s happening for hospitalists from nine health systems in southeast Michigan. Transcending their individual pursuits of excellence, they have united as Hospitalists as Emerging Leaders in Patient Safety (HELPS), a unique two-year consortium to improve patient safety regionally. Through large and small group meetings, HELPS is defining and tackling paramount patient safety issues, and collecting and sharing data about what works best.
A $117,000 grant from the Blue Cross/Blue Shield Foundation of Michigan awarded in 2005 to the University of Michigan Health Systems (UMHS) spurred the regional collaboration. Co-principal Investigator Scott Flanders, MD, UMHS’ chief of the hospitalist service and an SHM board member, conceived the project several years ago.
“What galvanized me is when I realized that we are a relatively small number of hospitalists overseeing a large number of patients—between 80,000 to 85,000 admissions annually,” he explains. “Those numbers indicated that we need to share our knowledge, treatment guidelines, and processes if we are to significantly improve patient safety.
—Scott Flanders, MD
“I assumed that many hospitalist groups wanted to improve patient safety,” continues Dr. Flanders. “But we were all working in isolation and were too busy caring for our patients to take the time to collaborate with colleagues in other programs.”
Dr. Flanders and HELPS’ other co-principal investigator, Sanjay Saint, MD, MPH, a hospitalist who heads UMHS’ Patient Safety Enhancement Program, were willing to spearhead a regional patient safety initiative with input from fellow hospitalists and patient safety officers. The Blue Cross/Blue Shield Foundation grant allowed the hospitalists to meet face-to-face periodically, target patient safety concerns, share hospitalist medicine group initiatives, collect data, and disseminate findings and best practices among the participants.
Dr. Saint and his colleagues provided one template for change. By using reminders and automatic order sets to prompt doctors to remove urinary catheters in a timely manner and by using anti-bacterial catheters, the team has shown that it can reduce bloodstream and urinary tract infections among its patients.
Drs. Flanders and Saint outlined a broad range of targets for the consortium hospitalists, including eliminating medication errors, creating a culture of safety, increasing the use of prophylactic medications for surgical patients, improving intensive care practices including pneumonia prevention, and examining end-of-life care practices such as pain management and the use of advance directives. Focusing on the elderly, who often fare poorly during hospitalizations, HELPS is looking for ways to prevent falls and delirium for that patient population. Through regular meetings, the hospitalists are developing techniques and benchmarks for performing quality improvement research and compiling lessons learned.
Nora Maloy, who works for Blue Cross/Blue Shield Foundation and who is Michigan’s senior program officer, positions the hospitalist collaboration as part of her foundation’s broader initiative to improve patient safety in response to the Institute of Medicine’s 1999 “Crossing the Quality Chasm” report that 98,000 unnecessary deaths occur annually in U.S. hospitals.
“We are very excited about the HELPS initiative,” says Maloy. “We hope to see outcomes data and best practices emerge from the nine different systems in the project, and to support a hospitalist consortium that can serve as a national model.”
At early meetings the hospitalists developed this process for their work together:
- Identify a common problem to study;
- Present data on the individual hospitalist or hospitalist group’s experience with the problem and a patient safety initiative to correct it;
- Create a steering committee and a team to research and present data on the initiative;
- Capture and organize data;
- Have an on-site visit from a principal investigator who participates in rounds and discusses data collection capabilities;
- Present to the group key steps in performing the patient safety initiative;
- Implement the initiative in as many of the nine hospitals that want to participate;
- Collect data from the larger group and report to the consortium; and
- Disseminate results through other regional and national meetings, and peer-reviewed journals.
HELPS’ funding frees participating hospitalists to attend quarterly meetings. Reflecting on their busy professional lives, Dr. Flanders says that groups are participating on different levels.
“We know that some hospitalist groups are stable, and they will propose initiatives, collect data, etc.,” he explains. “Other groups that may have recruiting and turnover issues and are just surviving won’t be able to do so, but their attendance at the meetings is very important. There are also small ad hoc meetings for those working on specific patient safety projects.
Took the Challenge
Bobby Lee, MD, director of inpatient medical education at the 600-bed Oakwood Hospital and Medical Center in Dearborn, Mich., eagerly joined the consortium when he realized that a large number of patients were being managed by a small number of hospitalist physicians.
“Scott [Flanders] and Sanjay [Saint] were very inclusive of hospitalists from different programs,” says Dr. Lee. “They articulated what’s important to us as hospitalists—that we bring something special to a hospital, to make it a safer place than when we got there.”
Sharing an Idea
Dr. Lee’s initiative, “Preventing Failure to Resuscitate,” addresses the issue that—on average—between 66% and 70% of patients outside the ICU on whom a code blue is called have alterations in their vital signs six to eight hours before the code. Dr. Lee’s solution was a rapid response team (RRT), developed after process analysis and data collection. And he has shared the initiative with HELPS.
“We did a literature review and then collected historical data on code blues at Oakwood,” explains Dr. Lee. “I took the data to our director of accreditation, an RN, and we felt that we could do better.”
After conducting several small pilot projects on different units to determine optimal staffing, equipment, and medications necessary for a quick response to a code, Dr. Lee presented his findings to Oakwood’s senior management, who committed the necessary resources. That includes a CCU nurse, respiratory therapist, either a hospitalist or intensivist, and a medical service resident—four teams in all for 24/7 coverage.
“There were a surprising number of models and variables we had to look at, such as streamlining lab results, getting test results to the bedside faster, and getting emergency boxes with the right pharmaceuticals on each unit,” adds Dr. Lee.
One interesting twist was Oakwood’s inclusion of hospitalists from private hospital medicine groups on the RRT. “Involving both community-based and academic medicine hospitalists has fostered a culture of inclusiveness, and that works,” says Dr. Lee. His final word: “We can’t leave our patients on the edge of the quality chasm. For not a lot of money, an RRT can help us help someone survive a code blue, and beat the odds that only 17% of code blues live to be discharged from the hospital.”
As the HELPS team continues on its two-year journey to better patient safety, the hospitalists will share what works, what doesn’t work, and what obstacles need to be removed. Overall, though, the HELPS’ vision that a small number of hospitalists joining together can have a huge effect on the care of upward of 80,000 patients has already succeeded. TH
Marlene Piturro is a frequent contributor to The Hospitalist.
Spend time with hospitalists and their competitive natures readily emerge. Striving for excellence in clinical care, hospital efficiency, and patient satisfaction, they are their hospitals’ beacons for attracting patients from referring physicians. As hospitalists’ capabilities grow, they hone pathways and procedures, improving their hospitals over time. What would happen if those hospitalists systematically shared their collective knowledge?
That’s what’s happening for hospitalists from nine health systems in southeast Michigan. Transcending their individual pursuits of excellence, they have united as Hospitalists as Emerging Leaders in Patient Safety (HELPS), a unique two-year consortium to improve patient safety regionally. Through large and small group meetings, HELPS is defining and tackling paramount patient safety issues, and collecting and sharing data about what works best.
A $117,000 grant from the Blue Cross/Blue Shield Foundation of Michigan awarded in 2005 to the University of Michigan Health Systems (UMHS) spurred the regional collaboration. Co-principal Investigator Scott Flanders, MD, UMHS’ chief of the hospitalist service and an SHM board member, conceived the project several years ago.
“What galvanized me is when I realized that we are a relatively small number of hospitalists overseeing a large number of patients—between 80,000 to 85,000 admissions annually,” he explains. “Those numbers indicated that we need to share our knowledge, treatment guidelines, and processes if we are to significantly improve patient safety.
—Scott Flanders, MD
“I assumed that many hospitalist groups wanted to improve patient safety,” continues Dr. Flanders. “But we were all working in isolation and were too busy caring for our patients to take the time to collaborate with colleagues in other programs.”
Dr. Flanders and HELPS’ other co-principal investigator, Sanjay Saint, MD, MPH, a hospitalist who heads UMHS’ Patient Safety Enhancement Program, were willing to spearhead a regional patient safety initiative with input from fellow hospitalists and patient safety officers. The Blue Cross/Blue Shield Foundation grant allowed the hospitalists to meet face-to-face periodically, target patient safety concerns, share hospitalist medicine group initiatives, collect data, and disseminate findings and best practices among the participants.
Dr. Saint and his colleagues provided one template for change. By using reminders and automatic order sets to prompt doctors to remove urinary catheters in a timely manner and by using anti-bacterial catheters, the team has shown that it can reduce bloodstream and urinary tract infections among its patients.
Drs. Flanders and Saint outlined a broad range of targets for the consortium hospitalists, including eliminating medication errors, creating a culture of safety, increasing the use of prophylactic medications for surgical patients, improving intensive care practices including pneumonia prevention, and examining end-of-life care practices such as pain management and the use of advance directives. Focusing on the elderly, who often fare poorly during hospitalizations, HELPS is looking for ways to prevent falls and delirium for that patient population. Through regular meetings, the hospitalists are developing techniques and benchmarks for performing quality improvement research and compiling lessons learned.
Nora Maloy, who works for Blue Cross/Blue Shield Foundation and who is Michigan’s senior program officer, positions the hospitalist collaboration as part of her foundation’s broader initiative to improve patient safety in response to the Institute of Medicine’s 1999 “Crossing the Quality Chasm” report that 98,000 unnecessary deaths occur annually in U.S. hospitals.
“We are very excited about the HELPS initiative,” says Maloy. “We hope to see outcomes data and best practices emerge from the nine different systems in the project, and to support a hospitalist consortium that can serve as a national model.”
At early meetings the hospitalists developed this process for their work together:
- Identify a common problem to study;
- Present data on the individual hospitalist or hospitalist group’s experience with the problem and a patient safety initiative to correct it;
- Create a steering committee and a team to research and present data on the initiative;
- Capture and organize data;
- Have an on-site visit from a principal investigator who participates in rounds and discusses data collection capabilities;
- Present to the group key steps in performing the patient safety initiative;
- Implement the initiative in as many of the nine hospitals that want to participate;
- Collect data from the larger group and report to the consortium; and
- Disseminate results through other regional and national meetings, and peer-reviewed journals.
HELPS’ funding frees participating hospitalists to attend quarterly meetings. Reflecting on their busy professional lives, Dr. Flanders says that groups are participating on different levels.
“We know that some hospitalist groups are stable, and they will propose initiatives, collect data, etc.,” he explains. “Other groups that may have recruiting and turnover issues and are just surviving won’t be able to do so, but their attendance at the meetings is very important. There are also small ad hoc meetings for those working on specific patient safety projects.
Took the Challenge
Bobby Lee, MD, director of inpatient medical education at the 600-bed Oakwood Hospital and Medical Center in Dearborn, Mich., eagerly joined the consortium when he realized that a large number of patients were being managed by a small number of hospitalist physicians.
“Scott [Flanders] and Sanjay [Saint] were very inclusive of hospitalists from different programs,” says Dr. Lee. “They articulated what’s important to us as hospitalists—that we bring something special to a hospital, to make it a safer place than when we got there.”
Sharing an Idea
Dr. Lee’s initiative, “Preventing Failure to Resuscitate,” addresses the issue that—on average—between 66% and 70% of patients outside the ICU on whom a code blue is called have alterations in their vital signs six to eight hours before the code. Dr. Lee’s solution was a rapid response team (RRT), developed after process analysis and data collection. And he has shared the initiative with HELPS.
“We did a literature review and then collected historical data on code blues at Oakwood,” explains Dr. Lee. “I took the data to our director of accreditation, an RN, and we felt that we could do better.”
After conducting several small pilot projects on different units to determine optimal staffing, equipment, and medications necessary for a quick response to a code, Dr. Lee presented his findings to Oakwood’s senior management, who committed the necessary resources. That includes a CCU nurse, respiratory therapist, either a hospitalist or intensivist, and a medical service resident—four teams in all for 24/7 coverage.
“There were a surprising number of models and variables we had to look at, such as streamlining lab results, getting test results to the bedside faster, and getting emergency boxes with the right pharmaceuticals on each unit,” adds Dr. Lee.
One interesting twist was Oakwood’s inclusion of hospitalists from private hospital medicine groups on the RRT. “Involving both community-based and academic medicine hospitalists has fostered a culture of inclusiveness, and that works,” says Dr. Lee. His final word: “We can’t leave our patients on the edge of the quality chasm. For not a lot of money, an RRT can help us help someone survive a code blue, and beat the odds that only 17% of code blues live to be discharged from the hospital.”
As the HELPS team continues on its two-year journey to better patient safety, the hospitalists will share what works, what doesn’t work, and what obstacles need to be removed. Overall, though, the HELPS’ vision that a small number of hospitalists joining together can have a huge effect on the care of upward of 80,000 patients has already succeeded. TH
Marlene Piturro is a frequent contributor to The Hospitalist.
Spend time with hospitalists and their competitive natures readily emerge. Striving for excellence in clinical care, hospital efficiency, and patient satisfaction, they are their hospitals’ beacons for attracting patients from referring physicians. As hospitalists’ capabilities grow, they hone pathways and procedures, improving their hospitals over time. What would happen if those hospitalists systematically shared their collective knowledge?
That’s what’s happening for hospitalists from nine health systems in southeast Michigan. Transcending their individual pursuits of excellence, they have united as Hospitalists as Emerging Leaders in Patient Safety (HELPS), a unique two-year consortium to improve patient safety regionally. Through large and small group meetings, HELPS is defining and tackling paramount patient safety issues, and collecting and sharing data about what works best.
A $117,000 grant from the Blue Cross/Blue Shield Foundation of Michigan awarded in 2005 to the University of Michigan Health Systems (UMHS) spurred the regional collaboration. Co-principal Investigator Scott Flanders, MD, UMHS’ chief of the hospitalist service and an SHM board member, conceived the project several years ago.
“What galvanized me is when I realized that we are a relatively small number of hospitalists overseeing a large number of patients—between 80,000 to 85,000 admissions annually,” he explains. “Those numbers indicated that we need to share our knowledge, treatment guidelines, and processes if we are to significantly improve patient safety.
—Scott Flanders, MD
“I assumed that many hospitalist groups wanted to improve patient safety,” continues Dr. Flanders. “But we were all working in isolation and were too busy caring for our patients to take the time to collaborate with colleagues in other programs.”
Dr. Flanders and HELPS’ other co-principal investigator, Sanjay Saint, MD, MPH, a hospitalist who heads UMHS’ Patient Safety Enhancement Program, were willing to spearhead a regional patient safety initiative with input from fellow hospitalists and patient safety officers. The Blue Cross/Blue Shield Foundation grant allowed the hospitalists to meet face-to-face periodically, target patient safety concerns, share hospitalist medicine group initiatives, collect data, and disseminate findings and best practices among the participants.
Dr. Saint and his colleagues provided one template for change. By using reminders and automatic order sets to prompt doctors to remove urinary catheters in a timely manner and by using anti-bacterial catheters, the team has shown that it can reduce bloodstream and urinary tract infections among its patients.
Drs. Flanders and Saint outlined a broad range of targets for the consortium hospitalists, including eliminating medication errors, creating a culture of safety, increasing the use of prophylactic medications for surgical patients, improving intensive care practices including pneumonia prevention, and examining end-of-life care practices such as pain management and the use of advance directives. Focusing on the elderly, who often fare poorly during hospitalizations, HELPS is looking for ways to prevent falls and delirium for that patient population. Through regular meetings, the hospitalists are developing techniques and benchmarks for performing quality improvement research and compiling lessons learned.
Nora Maloy, who works for Blue Cross/Blue Shield Foundation and who is Michigan’s senior program officer, positions the hospitalist collaboration as part of her foundation’s broader initiative to improve patient safety in response to the Institute of Medicine’s 1999 “Crossing the Quality Chasm” report that 98,000 unnecessary deaths occur annually in U.S. hospitals.
“We are very excited about the HELPS initiative,” says Maloy. “We hope to see outcomes data and best practices emerge from the nine different systems in the project, and to support a hospitalist consortium that can serve as a national model.”
At early meetings the hospitalists developed this process for their work together:
- Identify a common problem to study;
- Present data on the individual hospitalist or hospitalist group’s experience with the problem and a patient safety initiative to correct it;
- Create a steering committee and a team to research and present data on the initiative;
- Capture and organize data;
- Have an on-site visit from a principal investigator who participates in rounds and discusses data collection capabilities;
- Present to the group key steps in performing the patient safety initiative;
- Implement the initiative in as many of the nine hospitals that want to participate;
- Collect data from the larger group and report to the consortium; and
- Disseminate results through other regional and national meetings, and peer-reviewed journals.
HELPS’ funding frees participating hospitalists to attend quarterly meetings. Reflecting on their busy professional lives, Dr. Flanders says that groups are participating on different levels.
“We know that some hospitalist groups are stable, and they will propose initiatives, collect data, etc.,” he explains. “Other groups that may have recruiting and turnover issues and are just surviving won’t be able to do so, but their attendance at the meetings is very important. There are also small ad hoc meetings for those working on specific patient safety projects.
Took the Challenge
Bobby Lee, MD, director of inpatient medical education at the 600-bed Oakwood Hospital and Medical Center in Dearborn, Mich., eagerly joined the consortium when he realized that a large number of patients were being managed by a small number of hospitalist physicians.
“Scott [Flanders] and Sanjay [Saint] were very inclusive of hospitalists from different programs,” says Dr. Lee. “They articulated what’s important to us as hospitalists—that we bring something special to a hospital, to make it a safer place than when we got there.”
Sharing an Idea
Dr. Lee’s initiative, “Preventing Failure to Resuscitate,” addresses the issue that—on average—between 66% and 70% of patients outside the ICU on whom a code blue is called have alterations in their vital signs six to eight hours before the code. Dr. Lee’s solution was a rapid response team (RRT), developed after process analysis and data collection. And he has shared the initiative with HELPS.
“We did a literature review and then collected historical data on code blues at Oakwood,” explains Dr. Lee. “I took the data to our director of accreditation, an RN, and we felt that we could do better.”
After conducting several small pilot projects on different units to determine optimal staffing, equipment, and medications necessary for a quick response to a code, Dr. Lee presented his findings to Oakwood’s senior management, who committed the necessary resources. That includes a CCU nurse, respiratory therapist, either a hospitalist or intensivist, and a medical service resident—four teams in all for 24/7 coverage.
“There were a surprising number of models and variables we had to look at, such as streamlining lab results, getting test results to the bedside faster, and getting emergency boxes with the right pharmaceuticals on each unit,” adds Dr. Lee.
One interesting twist was Oakwood’s inclusion of hospitalists from private hospital medicine groups on the RRT. “Involving both community-based and academic medicine hospitalists has fostered a culture of inclusiveness, and that works,” says Dr. Lee. His final word: “We can’t leave our patients on the edge of the quality chasm. For not a lot of money, an RRT can help us help someone survive a code blue, and beat the odds that only 17% of code blues live to be discharged from the hospital.”
As the HELPS team continues on its two-year journey to better patient safety, the hospitalists will share what works, what doesn’t work, and what obstacles need to be removed. Overall, though, the HELPS’ vision that a small number of hospitalists joining together can have a huge effect on the care of upward of 80,000 patients has already succeeded. TH
Marlene Piturro is a frequent contributor to The Hospitalist.
Retention Recommendations
Retaining good hospitalists is one of the major factors in building a successful hospital medicine group—but it’s also one of the biggest challenges faced in the industry today. Why is hospitalist retention a problem, and what can be done to ensure your hospitalists stay for the long haul?
“As in any profession, there are some [hospitalists] who constantly look for bigger and better opportunities in the employment world,” admits Kenneth G. Simone, DO, founder and president of Hospitalist and Practice Solutions, Veazie, Maine, and a consultant to hospital medicine groups. “Sometimes this involves individuals looking for a better practice fit or for a better financial compensation package. In addition, hospitalist medicine can lead to burnout if the practice is not created or operated in a prudent manner, thus leading to turnover.”
Retention Is Crucial
Why is it so important to retain your hospitalists? For one thing, keeping physicians in your program for the long term can directly decrease costs, time, and effort related to recruitment and training.
More importantly, by decreasing staff turnover, retention can stabilize your hospitalist program. “A stable staff is influential in maintaining the providers’ focus on the practice mission, goals and objectives, and values,” points out Dr. Simone. “It de-emphasizes personal agendas, which may develop if an individual was looking to move on to another practice or up the ladder at the expense of the practice and fellow providers, and allows the practice team to gel over time. This is in some ways similar to a professional sports team, where chemistry and trust in one’s teammates are created over time.”
You can get your own hospitalist team to gel by retaining physicians for years if you initiate a program with that very goal.
How-Tos of Retention Programs
“There are a lot of [hospitalist] programs that haven’t developed retention programs at the outset,” says Dr. Simone. “They may be behind the eight ball, but they can and should create one. Successful programs identify their practice mission, values, and objectives, and clearly and concisely spell them out. This policy can then be utilized with the existing staff to align the team’s values and can also be used in recruiting future candidates.”
Once you have a solid written mission and vision statement, check to see if your hospitalists share the same values. Have your clinical director or an administrator sit down with providers one-on-one and find out their vision, values, and objectives. If what you hear differs from the core values of the practice, then you must develop a plan on which you can all agree.
“You may also have to consider altering some of the program’s vision and objectives, if appropriate,” says Dr. Simone.
Scheduling: A Core Value
Your group’s values can be reflected in the schedule you set for staff. “The practice structure and schedule plays a very important role in provider retention,” says Dr. Simone. “In general, various schedule types work for different individuals, and—in all probability—the provider will seek out the practices that offer a particular schedule to their liking.”
A hospital group that values time over money may offer larger chunks of time off. “We’ve found that our recruitment and retention improved when we went to a schedule of seven days on, seven off,” says Dr. Simone. “A lot of individuals are attracted to this because it gives them a week at a time to spend with their families or to pursue other interests, such as travel or educational pursuits.”
Whatever schedule you choose, there are some basic tenets Dr. Simone recommends, including:
- Create a schedule that is consistent and stable rather than constantly changing;
- Make sure the schedule is perceived as fair for all providers;
- Ensure that all providers get appropriate time off;
- Give providers enough flexibility to participate in other projects such as teaching, subspecialty clinics, administrative duties, or special projects; and
- Adhere to a schedule that promotes/accommodates a safe patient to provider ratio.
Salary and Bonuses
Money does matter in retention. As long as the pay you offer is perceived as fair, you have a good start. “In the end, you can only afford what your finances dictate,” says Dr. Simone. “Smaller hospitals may fall short on salary, so it’s important for them to recognize their strengths and sell them in order to compete.”
Your hospitalists may be attracted to participate in an incentive program that rewards them for hard work and productivity. “Incentives help change behavior, and people are stimulated when they have direct control over their own pay,” says Dr. Simone. “There are hospitalist programs with incentive plans, but many programs aren’t sure how to incentivize. You don’t want to reward doctors solely on the amount of work they do.”
For instance, a hospital-based program that rewards hospitalists on the basis of how many patients they admit is basically encouraging them to hospitalize every patient they see.
“I recommend finding a way to reward quality work and dedication, while not neglecting productivity,” says Dr. Simone. “In my opinion, the focus needs to be on increasing the program’s ability to standardize care following evidence-based protocols, encouraging participation in the value-added services that hospitalists are so good at, like participating in a rapid response team or a code blue team, acting as hospital leaders, educating hospital staff and residents, etc.”
Feeling Connected
Unlike many other physicians, hospitalists don’t get many opportunities to connect with patients or a community.
“In my opinion, a hospitalist’s professional job satisfaction and retention is influenced by the perception of feeling connected to the practice and providers, patients, colleagues, and the hospital,” says Dr. Simone. “There are various characteristics of an employment arrangement that may help an individual feel connected. When an employee feels connected, he/she will typically dedicate themselves to the mission of the company and perform at or above expectations.”
Create or revisit your group’s retention program today. By ensuring that the values and objectives of your practice are clearly stated, and catering to those values and objectives in scheduling and other management practices, you can begin to build your retention. TH
Jane Jerrard writes the “Career Development” column every month for The Hospitalist.
Retaining good hospitalists is one of the major factors in building a successful hospital medicine group—but it’s also one of the biggest challenges faced in the industry today. Why is hospitalist retention a problem, and what can be done to ensure your hospitalists stay for the long haul?
“As in any profession, there are some [hospitalists] who constantly look for bigger and better opportunities in the employment world,” admits Kenneth G. Simone, DO, founder and president of Hospitalist and Practice Solutions, Veazie, Maine, and a consultant to hospital medicine groups. “Sometimes this involves individuals looking for a better practice fit or for a better financial compensation package. In addition, hospitalist medicine can lead to burnout if the practice is not created or operated in a prudent manner, thus leading to turnover.”
Retention Is Crucial
Why is it so important to retain your hospitalists? For one thing, keeping physicians in your program for the long term can directly decrease costs, time, and effort related to recruitment and training.
More importantly, by decreasing staff turnover, retention can stabilize your hospitalist program. “A stable staff is influential in maintaining the providers’ focus on the practice mission, goals and objectives, and values,” points out Dr. Simone. “It de-emphasizes personal agendas, which may develop if an individual was looking to move on to another practice or up the ladder at the expense of the practice and fellow providers, and allows the practice team to gel over time. This is in some ways similar to a professional sports team, where chemistry and trust in one’s teammates are created over time.”
You can get your own hospitalist team to gel by retaining physicians for years if you initiate a program with that very goal.
How-Tos of Retention Programs
“There are a lot of [hospitalist] programs that haven’t developed retention programs at the outset,” says Dr. Simone. “They may be behind the eight ball, but they can and should create one. Successful programs identify their practice mission, values, and objectives, and clearly and concisely spell them out. This policy can then be utilized with the existing staff to align the team’s values and can also be used in recruiting future candidates.”
Once you have a solid written mission and vision statement, check to see if your hospitalists share the same values. Have your clinical director or an administrator sit down with providers one-on-one and find out their vision, values, and objectives. If what you hear differs from the core values of the practice, then you must develop a plan on which you can all agree.
“You may also have to consider altering some of the program’s vision and objectives, if appropriate,” says Dr. Simone.
Scheduling: A Core Value
Your group’s values can be reflected in the schedule you set for staff. “The practice structure and schedule plays a very important role in provider retention,” says Dr. Simone. “In general, various schedule types work for different individuals, and—in all probability—the provider will seek out the practices that offer a particular schedule to their liking.”
A hospital group that values time over money may offer larger chunks of time off. “We’ve found that our recruitment and retention improved when we went to a schedule of seven days on, seven off,” says Dr. Simone. “A lot of individuals are attracted to this because it gives them a week at a time to spend with their families or to pursue other interests, such as travel or educational pursuits.”
Whatever schedule you choose, there are some basic tenets Dr. Simone recommends, including:
- Create a schedule that is consistent and stable rather than constantly changing;
- Make sure the schedule is perceived as fair for all providers;
- Ensure that all providers get appropriate time off;
- Give providers enough flexibility to participate in other projects such as teaching, subspecialty clinics, administrative duties, or special projects; and
- Adhere to a schedule that promotes/accommodates a safe patient to provider ratio.
Salary and Bonuses
Money does matter in retention. As long as the pay you offer is perceived as fair, you have a good start. “In the end, you can only afford what your finances dictate,” says Dr. Simone. “Smaller hospitals may fall short on salary, so it’s important for them to recognize their strengths and sell them in order to compete.”
Your hospitalists may be attracted to participate in an incentive program that rewards them for hard work and productivity. “Incentives help change behavior, and people are stimulated when they have direct control over their own pay,” says Dr. Simone. “There are hospitalist programs with incentive plans, but many programs aren’t sure how to incentivize. You don’t want to reward doctors solely on the amount of work they do.”
For instance, a hospital-based program that rewards hospitalists on the basis of how many patients they admit is basically encouraging them to hospitalize every patient they see.
“I recommend finding a way to reward quality work and dedication, while not neglecting productivity,” says Dr. Simone. “In my opinion, the focus needs to be on increasing the program’s ability to standardize care following evidence-based protocols, encouraging participation in the value-added services that hospitalists are so good at, like participating in a rapid response team or a code blue team, acting as hospital leaders, educating hospital staff and residents, etc.”
Feeling Connected
Unlike many other physicians, hospitalists don’t get many opportunities to connect with patients or a community.
“In my opinion, a hospitalist’s professional job satisfaction and retention is influenced by the perception of feeling connected to the practice and providers, patients, colleagues, and the hospital,” says Dr. Simone. “There are various characteristics of an employment arrangement that may help an individual feel connected. When an employee feels connected, he/she will typically dedicate themselves to the mission of the company and perform at or above expectations.”
Create or revisit your group’s retention program today. By ensuring that the values and objectives of your practice are clearly stated, and catering to those values and objectives in scheduling and other management practices, you can begin to build your retention. TH
Jane Jerrard writes the “Career Development” column every month for The Hospitalist.
Retaining good hospitalists is one of the major factors in building a successful hospital medicine group—but it’s also one of the biggest challenges faced in the industry today. Why is hospitalist retention a problem, and what can be done to ensure your hospitalists stay for the long haul?
“As in any profession, there are some [hospitalists] who constantly look for bigger and better opportunities in the employment world,” admits Kenneth G. Simone, DO, founder and president of Hospitalist and Practice Solutions, Veazie, Maine, and a consultant to hospital medicine groups. “Sometimes this involves individuals looking for a better practice fit or for a better financial compensation package. In addition, hospitalist medicine can lead to burnout if the practice is not created or operated in a prudent manner, thus leading to turnover.”
Retention Is Crucial
Why is it so important to retain your hospitalists? For one thing, keeping physicians in your program for the long term can directly decrease costs, time, and effort related to recruitment and training.
More importantly, by decreasing staff turnover, retention can stabilize your hospitalist program. “A stable staff is influential in maintaining the providers’ focus on the practice mission, goals and objectives, and values,” points out Dr. Simone. “It de-emphasizes personal agendas, which may develop if an individual was looking to move on to another practice or up the ladder at the expense of the practice and fellow providers, and allows the practice team to gel over time. This is in some ways similar to a professional sports team, where chemistry and trust in one’s teammates are created over time.”
You can get your own hospitalist team to gel by retaining physicians for years if you initiate a program with that very goal.
How-Tos of Retention Programs
“There are a lot of [hospitalist] programs that haven’t developed retention programs at the outset,” says Dr. Simone. “They may be behind the eight ball, but they can and should create one. Successful programs identify their practice mission, values, and objectives, and clearly and concisely spell them out. This policy can then be utilized with the existing staff to align the team’s values and can also be used in recruiting future candidates.”
Once you have a solid written mission and vision statement, check to see if your hospitalists share the same values. Have your clinical director or an administrator sit down with providers one-on-one and find out their vision, values, and objectives. If what you hear differs from the core values of the practice, then you must develop a plan on which you can all agree.
“You may also have to consider altering some of the program’s vision and objectives, if appropriate,” says Dr. Simone.
Scheduling: A Core Value
Your group’s values can be reflected in the schedule you set for staff. “The practice structure and schedule plays a very important role in provider retention,” says Dr. Simone. “In general, various schedule types work for different individuals, and—in all probability—the provider will seek out the practices that offer a particular schedule to their liking.”
A hospital group that values time over money may offer larger chunks of time off. “We’ve found that our recruitment and retention improved when we went to a schedule of seven days on, seven off,” says Dr. Simone. “A lot of individuals are attracted to this because it gives them a week at a time to spend with their families or to pursue other interests, such as travel or educational pursuits.”
Whatever schedule you choose, there are some basic tenets Dr. Simone recommends, including:
- Create a schedule that is consistent and stable rather than constantly changing;
- Make sure the schedule is perceived as fair for all providers;
- Ensure that all providers get appropriate time off;
- Give providers enough flexibility to participate in other projects such as teaching, subspecialty clinics, administrative duties, or special projects; and
- Adhere to a schedule that promotes/accommodates a safe patient to provider ratio.
Salary and Bonuses
Money does matter in retention. As long as the pay you offer is perceived as fair, you have a good start. “In the end, you can only afford what your finances dictate,” says Dr. Simone. “Smaller hospitals may fall short on salary, so it’s important for them to recognize their strengths and sell them in order to compete.”
Your hospitalists may be attracted to participate in an incentive program that rewards them for hard work and productivity. “Incentives help change behavior, and people are stimulated when they have direct control over their own pay,” says Dr. Simone. “There are hospitalist programs with incentive plans, but many programs aren’t sure how to incentivize. You don’t want to reward doctors solely on the amount of work they do.”
For instance, a hospital-based program that rewards hospitalists on the basis of how many patients they admit is basically encouraging them to hospitalize every patient they see.
“I recommend finding a way to reward quality work and dedication, while not neglecting productivity,” says Dr. Simone. “In my opinion, the focus needs to be on increasing the program’s ability to standardize care following evidence-based protocols, encouraging participation in the value-added services that hospitalists are so good at, like participating in a rapid response team or a code blue team, acting as hospital leaders, educating hospital staff and residents, etc.”
Feeling Connected
Unlike many other physicians, hospitalists don’t get many opportunities to connect with patients or a community.
“In my opinion, a hospitalist’s professional job satisfaction and retention is influenced by the perception of feeling connected to the practice and providers, patients, colleagues, and the hospital,” says Dr. Simone. “There are various characteristics of an employment arrangement that may help an individual feel connected. When an employee feels connected, he/she will typically dedicate themselves to the mission of the company and perform at or above expectations.”
Create or revisit your group’s retention program today. By ensuring that the values and objectives of your practice are clearly stated, and catering to those values and objectives in scheduling and other management practices, you can begin to build your retention. TH
Jane Jerrard writes the “Career Development” column every month for The Hospitalist.
Exenatide and pramlintide: New glucose-lowering agents for treating diabetes mellitus
Heel pain: Diagnosis and treatment, step by step
Routine Rapid HIV Testing / Greenwald
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.
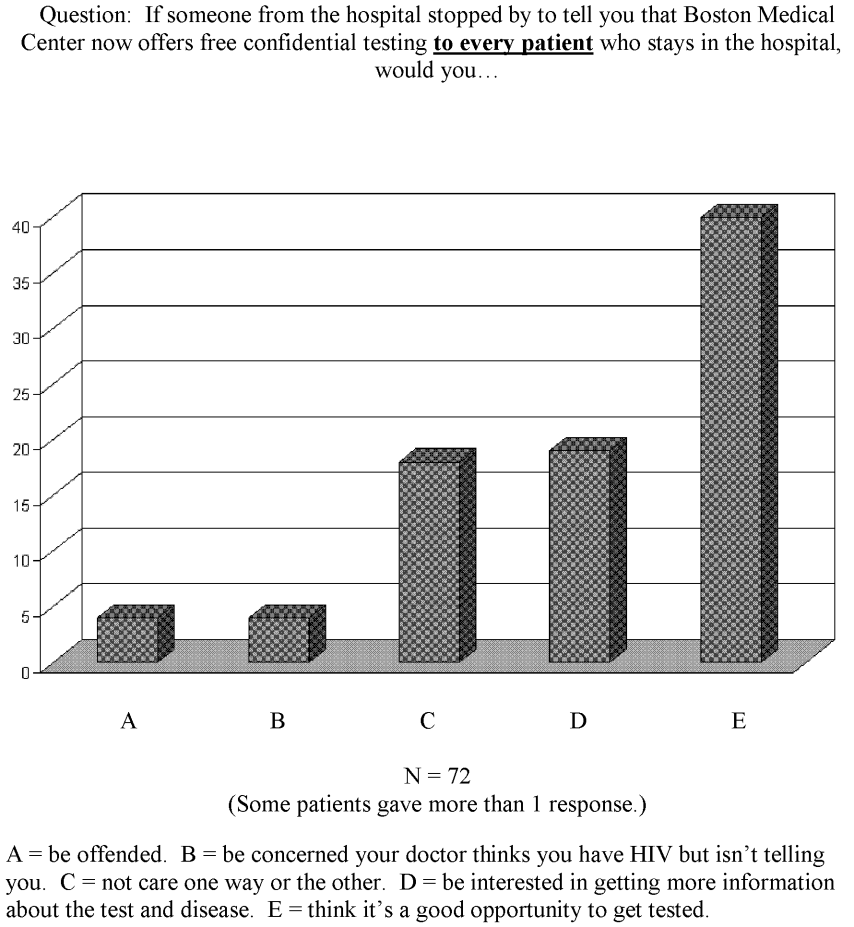
From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
The Venous Thromboembolism Quality Improvement Resource Room
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).

Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.
Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).


Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.

Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).


Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.

Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
Copyright © 2006 Society of Hospital Medicine
Clinical Conundrum
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).
The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.
Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.
The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
Pandemic Influenza and the Hospitalist
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
Handoffs
She is not, however, without a certain, well, grim sense of humor.
The voice throws you at first. It's deceptively mild and calm, more Gwyneth Paltrow than James Earl Jones. To the best of my knowledge, however, Gwyneth Paltrow has never shown up in a patient's room wearing a hooded black robe and sporting a scythe.
Hmm, bad case of sepsis, huh? she asked.
I was still cleaning up from the central line, so I tried to get rid of her gracefully. Now's not really a good time. That line always seems to work with pesky administrators.
Death, however, would not be dissuaded. HIV positive for nearly 10 years now, flirting with CD4 counts in double digits, rising viral load the past 3 years. Picked a bad time to have her gallbladder go bad on her, didn't she?
Or a bad time to pick a surgeon who was less than fastidious about his sterile fields, I muttered.
Death, however, chose to ignore me. Ventilator, pressors, antibiotics. She reached for the girl's right hand, attached as it was to an arterial line blood pressure monitor, and somethingdamn, if it didn't look like a scarabfell out of the robe and then scrambled across my patient's chest, right next to my newly placed and oh‐so‐deftly sutured plastic catheter, ducking away from the light.
Hey, that was my sterile field, I complained. I, unlike the aforementioned surgeon, had been fastidious about it. I hoped the scarab was sterile, at least.
Splinter hemorrhages, Death noted with obvious glee, taking particular interest in the little streaks of blood under my patient's fingernails. Septic emboli. Death seems to have Gwnyeth Paltrow's laugh along with her voice. It's a light and airy thing, almost like a favorite wind chime. That laugh could almost make you think Death had a heart. Endocarditis, she said fondly. One of my favorites.
Almost make you think she had a heart.
Go away. I tried to look more busy than worried, but I didn't think she was buying it.
It's been a good month for me here in the ICU. Heck, you yourself have 4 kills. Why don't you throw in this one and call yourself an ace?
Very funny. Now get out. I would imagine it's best not to lose your temper with Death. Or with any of the other three Horsemen of the Apocalypse, for that matter. Didn't anyone ever tell you it's poor form to make fun of your host? You're in Donna Smith's house now, buddy. Don't be dissin' my nurse manager.
Come on, she pushed. She's pathetic, she's tired, she lives alone in a tiny room in a forgotten crack house that she pretends is her apartment, and her parents haven't taken her calls for years.
Donna? I asked. That was some serious disrespect.
Don't go all medical student on me. I was talking about your patient, she explained evenly. She'd be better off with me anyway. You know that.
She can be persuasive when she might be right. Death and I have been having similar conversations all the way back to my surgical rotation as a third‐year medical student. Although she has never been above making the easy score, she typically only makes serious plays for patients who, in all honesty, just might be better off heading with her beyond the vale. That's not my decision to make, I told Death, tweaking the pressors. I have this thing about systolic blood pressure less than room temperature. By being here, she's placed her life in my hands, I said, staring down into what almost looked like a lifeless face. Until and unless I have a compelling reason to do otherwise, I will do whatever is necessary to ensure she comes out of this thing as well as she can. I turned to look Death in those beady little eyes. Even go 15 rounds with you, if that's what it takes.
There was a moment of silence, during which I began to think that perhaps I had made my point. Rock, paper, scissors? Death asked with a hopeful lilt.
I sighed. Damn, but she was persistent. I don't gamble with death. That's something my mommy taught me, way back when I used to think I'd be able to fly if I jumped from that really tall tree on the hill.
Oh, come on. Your patient in the next bed did. Overdosed on Ativan with a fifth of Johnny Walker Black as a chaser? Now there's a lifestyle choice I can find myself endorsing.
I was out of witty comebacks. Everyone deserves another chance, I told her, my eyes fixed now on the hollowed, closed eyes of my patient in the ICU bed. Evenor maybe even especiallymy patient in the next bed. I don't know what it was about his life that had driven him to the lifestyle choices he had made. There's a part of every physician that thinks that he or she can make that difference in a patient's life. All too often, though, we see enough repeat business to learn, the hard way, that we rarely make the kind of differences we'd like to. With a little luck, and no small amount of medical diligence, both my patients would survive this hospitalization.
But would they survive their next?
Would there be a next?
Would this be the time they'd turn the corner?
I had to hope so, because I still consider myself far too young to be any more cynical than I already am.
What about your partner? Death asked suddenly.
No, I said with something I hoped sounded like authority. You can't have her either.
No, I meanhow about I work a deal with her? She's young, she's impressionable, she's idealisticwe could make it not your fault.
She's got enough to do; please just leave her alone.
The hood shook slowly back and forth, so I assumed she was shaking her head. Or her skull. Whichever. Look, you wouldn't even have to do anything, that sweet, seductive voice told me. Just head back to your call room for a few minutes. Turn off your pager.
I don't know why we're even having this conversation, I groaned. We both know that you're just a hallucination caused by way too little sleep and way too much caffeine. Last call night I caught myself discussing the relative merits of high‐frequency jet ventilation with Galen.
Galen, Death reflected, suddenly nostalgic. Highly overrated, a positive trait in a doctor if you don't mind my saying so. Has an ego that would make an orthopedist seem humble.
You're lying, I decided.
Yes, I am. And you're stalling. Her voice suddenly became a whole lot more James Earl Jonesish. I'm taking the septic chick with endocarditis. You can lose all the sleep you want, she still goes Home with me.
I glanced over the drips one more time. I've just added norepi. That's a whole bag full of bite me that says differently. So back off, sweetheart.
As with most true medical emergencies, I was too busy thinking ahead to realize just how much trouble my patient could have been in at that moment.
Now morning, like her fever, has broken.
The sun, for those that haven't noticed, gives off a beautiful red glow as it rises over Boot Hill. I got to see it from the rocking chair in my patient's room. Nurse Donna was finishing the night's vital sign flow sheet, politely pretending she hadn't heard me snoring away the past 15 minutes. I was trying to decide if that odor drifting into the room was someone's attempt at coffee or melana. Donna smiled politely at me as I rubbed the Sandman's crud out of my eyes. It was a very Chicago Hope kinda moment.
If I'd been playing a doctor on TV, I'd have made some reassuring comment about how my patient had made it through the night, and so she was now out of the woods. Uh‐huh. With Death sneaking up behind me, my bite‐me norepinephrine wasn't going to be weaned just yet.
Good morning, I greeted.
She seemed a bit put off that I had heard her sneaking in. But then, Death is not exactly graced with kitty‐cat feet.
You never turned your pager off. She said it like an accusation. The way my wife does.
Never do. An answer my wife doesn't appreciate, either. My patient's still with me, I said, managing to keep the victory dance out of my voice. Pressure is better, heart rate is lower, fever seems to have broken.
Death is patient, death is kind, she warned.
It took my postcall mind a moment to wrap itself around that one. Isn't that supposed to be Love? I asked her.
The shoulders of the robe shrugged. Love gets all the cool lines, she complained. What do I get? Be not proud, nothing's for sure but me and taxes, Yea, though I walk through the valley of the shadow of me, give me liberty or give me me. She turned to face me. I like that one, by the way.
Never fails to bring a tear to my eye. I'd give her that. She had lost the patient, I could afford to be gracious and throw her a bone. As long as said bone didn't belong to one of my patients.
Death was silent for a long moment. Well, I'm outta here, she finally decided. What about you?
I've got a few more hours. Rounds, orders, more rounds, discussions with families, likely more rounds
I thought I could hear a wry grin in her voice. Well, you be careful driving home, she suggested. It'd be a shame if you fell asleep at the wheel. And then ended up on my doorstep.
Damn.
She is not, however, without a certain, well, grim sense of humor.
The voice throws you at first. It's deceptively mild and calm, more Gwyneth Paltrow than James Earl Jones. To the best of my knowledge, however, Gwyneth Paltrow has never shown up in a patient's room wearing a hooded black robe and sporting a scythe.
Hmm, bad case of sepsis, huh? she asked.
I was still cleaning up from the central line, so I tried to get rid of her gracefully. Now's not really a good time. That line always seems to work with pesky administrators.
Death, however, would not be dissuaded. HIV positive for nearly 10 years now, flirting with CD4 counts in double digits, rising viral load the past 3 years. Picked a bad time to have her gallbladder go bad on her, didn't she?
Or a bad time to pick a surgeon who was less than fastidious about his sterile fields, I muttered.
Death, however, chose to ignore me. Ventilator, pressors, antibiotics. She reached for the girl's right hand, attached as it was to an arterial line blood pressure monitor, and somethingdamn, if it didn't look like a scarabfell out of the robe and then scrambled across my patient's chest, right next to my newly placed and oh‐so‐deftly sutured plastic catheter, ducking away from the light.
Hey, that was my sterile field, I complained. I, unlike the aforementioned surgeon, had been fastidious about it. I hoped the scarab was sterile, at least.
Splinter hemorrhages, Death noted with obvious glee, taking particular interest in the little streaks of blood under my patient's fingernails. Septic emboli. Death seems to have Gwnyeth Paltrow's laugh along with her voice. It's a light and airy thing, almost like a favorite wind chime. That laugh could almost make you think Death had a heart. Endocarditis, she said fondly. One of my favorites.
Almost make you think she had a heart.
Go away. I tried to look more busy than worried, but I didn't think she was buying it.
It's been a good month for me here in the ICU. Heck, you yourself have 4 kills. Why don't you throw in this one and call yourself an ace?
Very funny. Now get out. I would imagine it's best not to lose your temper with Death. Or with any of the other three Horsemen of the Apocalypse, for that matter. Didn't anyone ever tell you it's poor form to make fun of your host? You're in Donna Smith's house now, buddy. Don't be dissin' my nurse manager.
Come on, she pushed. She's pathetic, she's tired, she lives alone in a tiny room in a forgotten crack house that she pretends is her apartment, and her parents haven't taken her calls for years.
Donna? I asked. That was some serious disrespect.
Don't go all medical student on me. I was talking about your patient, she explained evenly. She'd be better off with me anyway. You know that.
She can be persuasive when she might be right. Death and I have been having similar conversations all the way back to my surgical rotation as a third‐year medical student. Although she has never been above making the easy score, she typically only makes serious plays for patients who, in all honesty, just might be better off heading with her beyond the vale. That's not my decision to make, I told Death, tweaking the pressors. I have this thing about systolic blood pressure less than room temperature. By being here, she's placed her life in my hands, I said, staring down into what almost looked like a lifeless face. Until and unless I have a compelling reason to do otherwise, I will do whatever is necessary to ensure she comes out of this thing as well as she can. I turned to look Death in those beady little eyes. Even go 15 rounds with you, if that's what it takes.
There was a moment of silence, during which I began to think that perhaps I had made my point. Rock, paper, scissors? Death asked with a hopeful lilt.
I sighed. Damn, but she was persistent. I don't gamble with death. That's something my mommy taught me, way back when I used to think I'd be able to fly if I jumped from that really tall tree on the hill.
Oh, come on. Your patient in the next bed did. Overdosed on Ativan with a fifth of Johnny Walker Black as a chaser? Now there's a lifestyle choice I can find myself endorsing.
I was out of witty comebacks. Everyone deserves another chance, I told her, my eyes fixed now on the hollowed, closed eyes of my patient in the ICU bed. Evenor maybe even especiallymy patient in the next bed. I don't know what it was about his life that had driven him to the lifestyle choices he had made. There's a part of every physician that thinks that he or she can make that difference in a patient's life. All too often, though, we see enough repeat business to learn, the hard way, that we rarely make the kind of differences we'd like to. With a little luck, and no small amount of medical diligence, both my patients would survive this hospitalization.
But would they survive their next?
Would there be a next?
Would this be the time they'd turn the corner?
I had to hope so, because I still consider myself far too young to be any more cynical than I already am.
What about your partner? Death asked suddenly.
No, I said with something I hoped sounded like authority. You can't have her either.
No, I meanhow about I work a deal with her? She's young, she's impressionable, she's idealisticwe could make it not your fault.
She's got enough to do; please just leave her alone.
The hood shook slowly back and forth, so I assumed she was shaking her head. Or her skull. Whichever. Look, you wouldn't even have to do anything, that sweet, seductive voice told me. Just head back to your call room for a few minutes. Turn off your pager.
I don't know why we're even having this conversation, I groaned. We both know that you're just a hallucination caused by way too little sleep and way too much caffeine. Last call night I caught myself discussing the relative merits of high‐frequency jet ventilation with Galen.
Galen, Death reflected, suddenly nostalgic. Highly overrated, a positive trait in a doctor if you don't mind my saying so. Has an ego that would make an orthopedist seem humble.
You're lying, I decided.
Yes, I am. And you're stalling. Her voice suddenly became a whole lot more James Earl Jonesish. I'm taking the septic chick with endocarditis. You can lose all the sleep you want, she still goes Home with me.
I glanced over the drips one more time. I've just added norepi. That's a whole bag full of bite me that says differently. So back off, sweetheart.
As with most true medical emergencies, I was too busy thinking ahead to realize just how much trouble my patient could have been in at that moment.
Now morning, like her fever, has broken.
The sun, for those that haven't noticed, gives off a beautiful red glow as it rises over Boot Hill. I got to see it from the rocking chair in my patient's room. Nurse Donna was finishing the night's vital sign flow sheet, politely pretending she hadn't heard me snoring away the past 15 minutes. I was trying to decide if that odor drifting into the room was someone's attempt at coffee or melana. Donna smiled politely at me as I rubbed the Sandman's crud out of my eyes. It was a very Chicago Hope kinda moment.
If I'd been playing a doctor on TV, I'd have made some reassuring comment about how my patient had made it through the night, and so she was now out of the woods. Uh‐huh. With Death sneaking up behind me, my bite‐me norepinephrine wasn't going to be weaned just yet.
Good morning, I greeted.
She seemed a bit put off that I had heard her sneaking in. But then, Death is not exactly graced with kitty‐cat feet.
You never turned your pager off. She said it like an accusation. The way my wife does.
Never do. An answer my wife doesn't appreciate, either. My patient's still with me, I said, managing to keep the victory dance out of my voice. Pressure is better, heart rate is lower, fever seems to have broken.
Death is patient, death is kind, she warned.
It took my postcall mind a moment to wrap itself around that one. Isn't that supposed to be Love? I asked her.
The shoulders of the robe shrugged. Love gets all the cool lines, she complained. What do I get? Be not proud, nothing's for sure but me and taxes, Yea, though I walk through the valley of the shadow of me, give me liberty or give me me. She turned to face me. I like that one, by the way.
Never fails to bring a tear to my eye. I'd give her that. She had lost the patient, I could afford to be gracious and throw her a bone. As long as said bone didn't belong to one of my patients.
Death was silent for a long moment. Well, I'm outta here, she finally decided. What about you?
I've got a few more hours. Rounds, orders, more rounds, discussions with families, likely more rounds
I thought I could hear a wry grin in her voice. Well, you be careful driving home, she suggested. It'd be a shame if you fell asleep at the wheel. And then ended up on my doorstep.
Damn.
She is not, however, without a certain, well, grim sense of humor.
The voice throws you at first. It's deceptively mild and calm, more Gwyneth Paltrow than James Earl Jones. To the best of my knowledge, however, Gwyneth Paltrow has never shown up in a patient's room wearing a hooded black robe and sporting a scythe.
Hmm, bad case of sepsis, huh? she asked.
I was still cleaning up from the central line, so I tried to get rid of her gracefully. Now's not really a good time. That line always seems to work with pesky administrators.
Death, however, would not be dissuaded. HIV positive for nearly 10 years now, flirting with CD4 counts in double digits, rising viral load the past 3 years. Picked a bad time to have her gallbladder go bad on her, didn't she?
Or a bad time to pick a surgeon who was less than fastidious about his sterile fields, I muttered.
Death, however, chose to ignore me. Ventilator, pressors, antibiotics. She reached for the girl's right hand, attached as it was to an arterial line blood pressure monitor, and somethingdamn, if it didn't look like a scarabfell out of the robe and then scrambled across my patient's chest, right next to my newly placed and oh‐so‐deftly sutured plastic catheter, ducking away from the light.
Hey, that was my sterile field, I complained. I, unlike the aforementioned surgeon, had been fastidious about it. I hoped the scarab was sterile, at least.
Splinter hemorrhages, Death noted with obvious glee, taking particular interest in the little streaks of blood under my patient's fingernails. Septic emboli. Death seems to have Gwnyeth Paltrow's laugh along with her voice. It's a light and airy thing, almost like a favorite wind chime. That laugh could almost make you think Death had a heart. Endocarditis, she said fondly. One of my favorites.
Almost make you think she had a heart.
Go away. I tried to look more busy than worried, but I didn't think she was buying it.
It's been a good month for me here in the ICU. Heck, you yourself have 4 kills. Why don't you throw in this one and call yourself an ace?
Very funny. Now get out. I would imagine it's best not to lose your temper with Death. Or with any of the other three Horsemen of the Apocalypse, for that matter. Didn't anyone ever tell you it's poor form to make fun of your host? You're in Donna Smith's house now, buddy. Don't be dissin' my nurse manager.
Come on, she pushed. She's pathetic, she's tired, she lives alone in a tiny room in a forgotten crack house that she pretends is her apartment, and her parents haven't taken her calls for years.
Donna? I asked. That was some serious disrespect.
Don't go all medical student on me. I was talking about your patient, she explained evenly. She'd be better off with me anyway. You know that.
She can be persuasive when she might be right. Death and I have been having similar conversations all the way back to my surgical rotation as a third‐year medical student. Although she has never been above making the easy score, she typically only makes serious plays for patients who, in all honesty, just might be better off heading with her beyond the vale. That's not my decision to make, I told Death, tweaking the pressors. I have this thing about systolic blood pressure less than room temperature. By being here, she's placed her life in my hands, I said, staring down into what almost looked like a lifeless face. Until and unless I have a compelling reason to do otherwise, I will do whatever is necessary to ensure she comes out of this thing as well as she can. I turned to look Death in those beady little eyes. Even go 15 rounds with you, if that's what it takes.
There was a moment of silence, during which I began to think that perhaps I had made my point. Rock, paper, scissors? Death asked with a hopeful lilt.
I sighed. Damn, but she was persistent. I don't gamble with death. That's something my mommy taught me, way back when I used to think I'd be able to fly if I jumped from that really tall tree on the hill.
Oh, come on. Your patient in the next bed did. Overdosed on Ativan with a fifth of Johnny Walker Black as a chaser? Now there's a lifestyle choice I can find myself endorsing.
I was out of witty comebacks. Everyone deserves another chance, I told her, my eyes fixed now on the hollowed, closed eyes of my patient in the ICU bed. Evenor maybe even especiallymy patient in the next bed. I don't know what it was about his life that had driven him to the lifestyle choices he had made. There's a part of every physician that thinks that he or she can make that difference in a patient's life. All too often, though, we see enough repeat business to learn, the hard way, that we rarely make the kind of differences we'd like to. With a little luck, and no small amount of medical diligence, both my patients would survive this hospitalization.
But would they survive their next?
Would there be a next?
Would this be the time they'd turn the corner?
I had to hope so, because I still consider myself far too young to be any more cynical than I already am.
What about your partner? Death asked suddenly.
No, I said with something I hoped sounded like authority. You can't have her either.
No, I meanhow about I work a deal with her? She's young, she's impressionable, she's idealisticwe could make it not your fault.
She's got enough to do; please just leave her alone.
The hood shook slowly back and forth, so I assumed she was shaking her head. Or her skull. Whichever. Look, you wouldn't even have to do anything, that sweet, seductive voice told me. Just head back to your call room for a few minutes. Turn off your pager.
I don't know why we're even having this conversation, I groaned. We both know that you're just a hallucination caused by way too little sleep and way too much caffeine. Last call night I caught myself discussing the relative merits of high‐frequency jet ventilation with Galen.
Galen, Death reflected, suddenly nostalgic. Highly overrated, a positive trait in a doctor if you don't mind my saying so. Has an ego that would make an orthopedist seem humble.
You're lying, I decided.
Yes, I am. And you're stalling. Her voice suddenly became a whole lot more James Earl Jonesish. I'm taking the septic chick with endocarditis. You can lose all the sleep you want, she still goes Home with me.
I glanced over the drips one more time. I've just added norepi. That's a whole bag full of bite me that says differently. So back off, sweetheart.
As with most true medical emergencies, I was too busy thinking ahead to realize just how much trouble my patient could have been in at that moment.
Now morning, like her fever, has broken.
The sun, for those that haven't noticed, gives off a beautiful red glow as it rises over Boot Hill. I got to see it from the rocking chair in my patient's room. Nurse Donna was finishing the night's vital sign flow sheet, politely pretending she hadn't heard me snoring away the past 15 minutes. I was trying to decide if that odor drifting into the room was someone's attempt at coffee or melana. Donna smiled politely at me as I rubbed the Sandman's crud out of my eyes. It was a very Chicago Hope kinda moment.
If I'd been playing a doctor on TV, I'd have made some reassuring comment about how my patient had made it through the night, and so she was now out of the woods. Uh‐huh. With Death sneaking up behind me, my bite‐me norepinephrine wasn't going to be weaned just yet.
Good morning, I greeted.
She seemed a bit put off that I had heard her sneaking in. But then, Death is not exactly graced with kitty‐cat feet.
You never turned your pager off. She said it like an accusation. The way my wife does.
Never do. An answer my wife doesn't appreciate, either. My patient's still with me, I said, managing to keep the victory dance out of my voice. Pressure is better, heart rate is lower, fever seems to have broken.
Death is patient, death is kind, she warned.
It took my postcall mind a moment to wrap itself around that one. Isn't that supposed to be Love? I asked her.
The shoulders of the robe shrugged. Love gets all the cool lines, she complained. What do I get? Be not proud, nothing's for sure but me and taxes, Yea, though I walk through the valley of the shadow of me, give me liberty or give me me. She turned to face me. I like that one, by the way.
Never fails to bring a tear to my eye. I'd give her that. She had lost the patient, I could afford to be gracious and throw her a bone. As long as said bone didn't belong to one of my patients.
Death was silent for a long moment. Well, I'm outta here, she finally decided. What about you?
I've got a few more hours. Rounds, orders, more rounds, discussions with families, likely more rounds
I thought I could hear a wry grin in her voice. Well, you be careful driving home, she suggested. It'd be a shame if you fell asleep at the wheel. And then ended up on my doorstep.
Damn.