User login
SHM Workshops on Health Care–Associated Infections and Antimicrobial Resistance / Bush‐Knapp et al.
In the United States, hospitalized patients are at risk of acquiring health careassociated infections that increase morbidity, mortality, length of hospital stay, and cost of care.1 If a health careassociated infection is caused by an antimicrobial‐resistant pathogen, treatment efforts may be further complicated.2, 3 With the decreasing effectiveness of antimicrobials and suboptimal adherence to certain infection control measures, new and multifaceted prevention strategies are necessary to address the problem of health careassociated infections and antimicrobial resistance.410
One strategy that hospitals can use to reduce the incidence of health careassociated infections and antimicrobial resistance is implementation of quality improvement programs. These programs require clinicians to employ techniques, such as root cause analysis (RCA), which investigates contributing factors to an event to prevent reoccurrence, and healthcare failure mode effects analysis (HFMEA), which applies a systematic method of identifying and preventing problems before they occur.1113 Programs and strategies such as these require leadership and adoption within the hospital. Because of their availability and specialized role in the hospital setting, hospitalists are in a unique position to promote and uphold quality improvement efforts.1417 Professional societies, health care organizations, and governmental agencies can play a role in engaging this group of physicians in improving the quality of patient care in hospitals by providing educational programs and materials.18
In 2004, the Society of Hospital Medicine (SHM) collaborated with the Centers for Disease Control and Prevention (CDC) to develop a quality improvement tool kit to reduce antimicrobial resistance and health careassociated infections. The tool kit was based on the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings (Campaign), an educational program targeted at clinicians.19 The SHM/CDC tool kit contained campaign materials, a set of slides about quality improvement, worksheets, and additional materials such as infection control policies and guidelines to supplement a 90‐minute workshop consisting of didactic lectures about antimicrobial resistance, quality improvement initiatives, RCA, and HFMEA; a lecture and case study about intravascular catheter‐related infections; and small‐group activity and discussion. The complete toolkit is now available online via the SHM Antimicrobial Resistance Resource Room at
The purpose of the workshop was to present the tool kit and increase hospitalists' knowledge and awareness about antimicrobial resistance, health careassociated infections, and quality improvement programs. We assessed the workshop participants' familiarity with the Campaign prior to the workshop, perceptions of antimicrobial resistance, knowledge gained as a result of the workshop, and opinions about the usefulness of the workshop.
METHODS
Data were collected from pretests and posttests administered to participants of one of the SHM workshops in May, June, or July 2005 in Denver, Colorado; Boston, Massachusetts; or Portland, Oregon. One SHM physician leader (D.D.D., coauthor of this article) presented all 3 workshops. The workshops were advertised by SHM using E‐mail to local chapter members. Individual sites used a variety of methods to encourage their hospitalists to attend, and participants were provided a complimentary dinner.
Prior to each workshop, participants completed a 10‐question pretest that had been pilot‐tested by hospitalists in other cities. The pretest assessed demographics; perceptions of the problem of antimicrobial resistance using a Likert scale; familiarity with the Campaign; and knowledge of common infection sites, RCA, HFMEA, and antimicrobial resistance prevention measures.
Immediately following each workshop, a 13‐question posttest was administered to participants. This posttest evaluated the workshop and materials using Likert scales, asked for suggestions for future programming using open‐ended questions, and repeated pretest questions to assess changes in perceptions and knowledge.
Data were entered into an Excel spreadsheet and analyzed using descriptive statistics and t tests to compare pre‐ and posttest changes in knowledge. Likert data assessing perceptions were dichotomized into strongly agree versus all other scale responses. Qualitative open‐ended responses were categorized by theme.
RESULTS
A total of 69 SHM members attended the workshops. Of the 69 participants, 65 completed the pretest, 53 completed the posttest, and 50 completed both the pre‐ and the posttests. Only participants who completed both the pretest and the posttest were included in the analyses (n = 21, Denver; n = 11, Boston; n = 18, Portland). Of the 50 participants who completed both the pre‐ and posttests, 44 (88%) classified themselves as hospitalists in practices ranging from 2 to more than 25 physicians. Participants averaged 9.2 years (range = 1‐27 years) in practice and 4.9 years (range = 1‐10 years) as practicing hospitalists, with no significant differences between the 3 groups. Only 17 participants (34%) were familiar with the Campaign prior to the workshop, and there was no significant variation between the 3 workshops. Those familiar with the Campaign had heard about or received the educational materials from colleagues (n = 5), their facilities (n = 4), professional journals (n = 4), medical conferences (n = 4), or the CDC or SHM websites (n = 4).
Overall, most participants strongly agreed with the statement that antimicrobial resistance was a problem nationally, institutionally, and within their individual practices (Table 1). These perceptions did not significantly differ between the pretest and the posttest. However, statistically significant differences were found when comparing perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels; more participants strongly agreed that antimicrobial resistance was a problem nationally than within their institutions (pretest, P = .01; posttest, P = .04) or within their practices (pretest, P < .0001; posttest, P = .01).
| Nationally | Institutionally | Within own practice | ||||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | |
| ||||||
| Denver (n = 21) | 100% | 100% | 86% | 95% | 67% | 86% |
| Portland (n = 18) | 83% | 94% | 67% | 78% | 67% | 78% |
| Boston (n = 11) | 91% | 82% | 91% | 82% | 91% | 82% |
| Average | 91% | 94% | 81% | 85% | 72% | 82% |
| P value | .28 | .18 | .06 | |||
On the knowledge‐based questions, the overall average test score was 48% on the pretest and 63% on the posttest (P < .0001), with scores varying by question (Table 2). For example, knowledge of quality improvement initiatives/HFMEA was low (an average of 10% correct on the pretest, 48% on the posttest) compared with knowledge about the key prevention strategies from the Campaign to Prevent Antimicrobial Resistance (average of 94% correct on the pretest, 98% on the posttest). Furthermore, scores also varied by workshop location. On the pretest, participants in Boston and Portland scored higher (both 53%) than Denver participants (40%). On the posttest, Portland participants scored the highest (78%) followed by Boston participants (64%) and then Denver participants (50%). Boston and Denver participants differed significantly on pretest knowledge score (P = .04) and Portland and Denver participants differed significantly on posttest knowledge score (P < .0001).
| Question Topic | Pretest average | Posttest average | Percent difference (P value)* |
|---|---|---|---|
| |||
| Quality improvement initiatives/HFMEA Which quality improvement initiative(s) must be performed yearly by all hospitals (JCAHO accreditation requirement)? | 10% | 48% | 38% (P < .0001) |
| Prevention of central venous catheter‐associated bloodstream infections: Key prevention steps for preventing central venous catheter‐associated bloodstream infections include all of the following except: | 62% | 88% | 26% (P = .0001) |
| RCA Which of the following is NOT true about root cause analysis? | 20% | 38% | 18% (P = .01) |
| Campaign to Prevent Antimicrobial Resistance The key prevention strategies from the Campaign to Prevent Antimicrobial Resistance include all of the following except: | 94% | 98% | 4% (P = .32) |
| Common body sites for healthcare‐associated infection: The most common site of hospital‐acquired (nosocomial) infection is: | 52% | 44% | 8% (P = .29) |
| Overall average | 48% | 63% | 15% (P < .0001) |
Overall, 43 participants (85%) rated the workshop as either very good or excellent. All but 1 participant (n = 49, 98%) would encourage a colleague to attend the workshop, giving reasons such as that the workshop outlined a major program in delivering good and safe care, offered great information on antimicrobial resistance and methods of quality improvement systems implementation, assisted in find[ing] new tools for improving hospital practice, and addressed a significant factor in hospitals related to morbidity [and] mortality. When asked for general comments about the workshop and suggestions for future improvements, participants requested more direction, more detail, more discussion, specific examples of antimicrobial resistance, and protocols and processes for implementing quality improvement programs. On a scale from 1 (not useful) to 5 (essential), participants rated the usefulness of each workshop segment: intravascular catheter‐related infections lecture and case study (x̄ = 4.3, range = 3‐5), quality improvement initiatives lecture (x̄ = 4.1, range = 2‐5), background on antimicrobial resistance (x̄ = 3.9, range = 2‐5), RCA lecture (x̄ = 3.9, range = 2‐5), HFMEA lecture (x̄ = 3.8, range = 2‐5), and small‐group discussion (x̄ = 3.4, range = 2‐5). These ratings did not vary significantly between the 3 groups.
CONCLUSIONS
To address antimicrobial resistance and health careassociated infections in the hospital setting, the SHM and CDC developed a tool kit and presented a quality improvement workshop to hospitalists in 3 U.S. cities. Overall, the participants scored significantly higher on the knowledge‐based questions on the posttest than on the pretest, indicating that knowledge improved as a result of the workshop. By providing a format that combined didactic lectures with case‐based education, small‐group activities, and discussion, the SHM workshop may have optimized its ability to increase knowledge, similar to the findings in previous research.2021
There were no significant differences between the 3 groups in years of practice, perceptions of the problem, and overall evaluation of the workshop. However, differences were found in knowledge gained as a result of the workshop. For example, the Denver group scored lower on the knowledge‐based questions than did the Boston group on the pretest and the Portland group on the posttest, indicating that knowledge and learning styles may differ by location. These differences may be attributed to variations in hospital environments, hospital‐based educational programs, or medical school and residency training. Differences like these may impact the effectiveness of a program and should be a consideration in the program development process, especially when a program is national in scope, like the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. In addition, more than 90% of participants correctly identified key prevention strategies of the Campaign, whereas only 34% were familiar with the Campaign itself prior to the workshop. This result may be a result of the key prevention strategies of the Campaign being derived from well‐established and ‐recognized evidence‐based best practices for patient safety and care.
Although knowledge changed as a result of the workshop, overall perceptions of the problem of antimicrobial resistance did not change significantly from pretest to posttest. It is possible this is because changes in perception require a different or more intensive educational approach. This result also may reflect the initial levels of agreement on the pretest, the measurement instrument itself, and/or the inability to detect differences because of the small number of participants.
Difference did exist in perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels. Antimicrobial resistance was perceived to be a greater problem on the national level than on the institutional and practice levels. Other studies also have found that clinicians more strongly agree that antimicrobial resistance is a problem nationally than within their institutions and practices.2224 When antimicrobial resistance is not perceived as a problem within institutions and practices, physicians may be less likely to overcome the barriers to following recommended infection prevention guidelines or to implementing quality improvement projects.4 Therefore, educational and intervention efforts like this workshop should address hospitalists' perceptions of the problem of antimicrobial resistance on the individual level as a first step in motivating them to engage in quality improvement.
Although participants' knowledge scores increased from pretest to posttest, gaps in knowledge remained, as indicated by the significantly improved but low overall posttest scores related to RCA and HFMEA. As hospitalists are in a unique position to promote quality improvement programs, these topic areas should be given more attention in future workshops and in training. Furthermore, by adding more specific questions related to each section of the workshop, associations among presentation style, knowledge gained, and perceived usefulness of each section could be evaluated. For example, the participants significantly increased their scores from pretest to posttest on the catheter‐related knowledge‐based question and rated the lecture and case study on intravascular catheter‐related infections as the most useful sections. Future research may explore these possible relationships to better guide selection of presentation styles and topics to ensure that participants gain knowledge and perceive the sections as useful. In addition, by addressing the feedback from participants, such as offering more detail, examples, and discussion, future workshops may have greater perceived usefulness and be better able to increase the knowledge and awareness of quality improvement programs for the prevention of health careassociated infections and antimicrobial resistance.
Although there were 3 workshops conducted in 3 areas across the United States, the sample size at each site was small, and results may not be representative of hospitalists at large. In addition, power calculations should be considered in future studies to increase the ability to better detect differences between and within groups. Another limitation of this study was that the limited data available and participant anonymity meant it was not possible to follow‐up with participants after the workshop to evaluate whether the knowledge they gained was sustained and/or whether they reported changes in practice. However, possession of knowledge and skills to inform practice does not mean that practice will change; therefore, follow‐up is necessary to determine if this workshop was effective in changing behaviors in the long term.25 Although the SHM workshop improved knowledge, more intensive educational strategies may be necessary to affect perceptions and improve the leadership skills required for implementation of quality improvement programs at an institutional level.
Overall, the SHM workshop was found to be a useful tool for increasing knowledge and outlining methods by which hospitalists can lead, coordinate, or participate in measures to prevent infections and improve patient safety. In addition, through the workshop, the SHM and the CDC have provided an example of how professional societies and government agencies can collaborate to address emerging issues in the health care setting.
- ,,.Impact of nosocomial infection on cost of illness and length of stay in intensive care units.Infect Control Hosp Epidemiol2005;26:281–287.
- .Implementation of strategies to control antimicrobial resistance.Chest.2001;119:405S–411S.
- ,,, et al.Society for Healthcare Epidemiology of America and Infectious Diseases Society of American Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals.Clin Infect Dis.1997;25:584–599.
- ,,, et al.Strategies to prevent and control the emergence and spread of antimicrobial‐resistant microorganisms in hospitals: a challenge to hospital leadership.JAMA.1996;275:234–240.
- Centers for Disease Control and Prevention.Guidelines for hand hygiene in health‐care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.MMWR Recomm Rep.2002;51:1–44.
- .Hospital Infection Control Practices Advisory Committee.Guideline for isolation precautions in hospitals.Infect Control Hosp Epidemiol.1996;17:53–80.
- ,,, et al.SHEA guideline for prevention nosocomial transmission of multidrug‐resistant strains of Staphylococcus aureus and Enterococcus.Infect Control Hosp Epidemiol.2003;24:362–386.
- .Improving adherence to hand hygiene practice: a multidisciplinary approach.Emerg Infect Dis.2001;7:234–240.
- ,,.Alcohol‐based handrub improves compliance with hand hygiene in intensive care units.Arch Intern Med.2002;162:1037–1043.
- ,,, et al.An organizational climate intervention associated with increased handwashing and decreased nosocomial infections.Behav Med.2000;26:14–22.
- ,.Getting to the root of the matter.AHRQ Web M 29:319–330.
- ,,.The Basics of FMEA.New York:Quality Resources;1996.
- .The hospitalist model of care: A positive influence on efficiency, quality of care, and outcomes.Crit Path Cardiol.2004;3:S5–S7.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- .The impact of hospitalists on medical education and the academic health systems.Ann Intern Med.1999;130:364–367.
- ,,, et al.Hospitalists' perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- ,,.Preventing the emergence of antimicrobial resistance: A call for action by clinicians, public health officials and patients.JAMA1997;278:944–945.
- Centers for Disease Control and Prevention. Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. 2005. Available at: URL: http://www.cdc.gov/drugresistance/healthcare/default.htm. Accessed November 8,2005.
- ,,, et al.Impact of formal continuing medical education: Do conferences, workshops, rounds and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,, et al.Physician preferences for continuing medical education with a focus on the topic of antimicrobial resistance: Society for Healthcare Epidemiology of America.Infect Control Hosp Epidemiol.2001;22:656–660.
- ,,, et al.Clinicians' perceptions of the problem of antimicrobial resistance in health care facilities.Arch Intern Med.2004;164:1662–1668.
- ,,, et al.Antibiotic resistance: a survey of physician perceptions.Arch Intern Med.2002;162:2210–2216.
- ,,, et al.Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the health belief model as a framework.Am J Infect Control.2004;33:175–181.
- .Educational theory into practice: Development of an infection control link nurse programme.Nurs Ed Pract.2001;1:35–41.
In the United States, hospitalized patients are at risk of acquiring health careassociated infections that increase morbidity, mortality, length of hospital stay, and cost of care.1 If a health careassociated infection is caused by an antimicrobial‐resistant pathogen, treatment efforts may be further complicated.2, 3 With the decreasing effectiveness of antimicrobials and suboptimal adherence to certain infection control measures, new and multifaceted prevention strategies are necessary to address the problem of health careassociated infections and antimicrobial resistance.410
One strategy that hospitals can use to reduce the incidence of health careassociated infections and antimicrobial resistance is implementation of quality improvement programs. These programs require clinicians to employ techniques, such as root cause analysis (RCA), which investigates contributing factors to an event to prevent reoccurrence, and healthcare failure mode effects analysis (HFMEA), which applies a systematic method of identifying and preventing problems before they occur.1113 Programs and strategies such as these require leadership and adoption within the hospital. Because of their availability and specialized role in the hospital setting, hospitalists are in a unique position to promote and uphold quality improvement efforts.1417 Professional societies, health care organizations, and governmental agencies can play a role in engaging this group of physicians in improving the quality of patient care in hospitals by providing educational programs and materials.18
In 2004, the Society of Hospital Medicine (SHM) collaborated with the Centers for Disease Control and Prevention (CDC) to develop a quality improvement tool kit to reduce antimicrobial resistance and health careassociated infections. The tool kit was based on the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings (Campaign), an educational program targeted at clinicians.19 The SHM/CDC tool kit contained campaign materials, a set of slides about quality improvement, worksheets, and additional materials such as infection control policies and guidelines to supplement a 90‐minute workshop consisting of didactic lectures about antimicrobial resistance, quality improvement initiatives, RCA, and HFMEA; a lecture and case study about intravascular catheter‐related infections; and small‐group activity and discussion. The complete toolkit is now available online via the SHM Antimicrobial Resistance Resource Room at
The purpose of the workshop was to present the tool kit and increase hospitalists' knowledge and awareness about antimicrobial resistance, health careassociated infections, and quality improvement programs. We assessed the workshop participants' familiarity with the Campaign prior to the workshop, perceptions of antimicrobial resistance, knowledge gained as a result of the workshop, and opinions about the usefulness of the workshop.
METHODS
Data were collected from pretests and posttests administered to participants of one of the SHM workshops in May, June, or July 2005 in Denver, Colorado; Boston, Massachusetts; or Portland, Oregon. One SHM physician leader (D.D.D., coauthor of this article) presented all 3 workshops. The workshops were advertised by SHM using E‐mail to local chapter members. Individual sites used a variety of methods to encourage their hospitalists to attend, and participants were provided a complimentary dinner.
Prior to each workshop, participants completed a 10‐question pretest that had been pilot‐tested by hospitalists in other cities. The pretest assessed demographics; perceptions of the problem of antimicrobial resistance using a Likert scale; familiarity with the Campaign; and knowledge of common infection sites, RCA, HFMEA, and antimicrobial resistance prevention measures.
Immediately following each workshop, a 13‐question posttest was administered to participants. This posttest evaluated the workshop and materials using Likert scales, asked for suggestions for future programming using open‐ended questions, and repeated pretest questions to assess changes in perceptions and knowledge.
Data were entered into an Excel spreadsheet and analyzed using descriptive statistics and t tests to compare pre‐ and posttest changes in knowledge. Likert data assessing perceptions were dichotomized into strongly agree versus all other scale responses. Qualitative open‐ended responses were categorized by theme.
RESULTS
A total of 69 SHM members attended the workshops. Of the 69 participants, 65 completed the pretest, 53 completed the posttest, and 50 completed both the pre‐ and the posttests. Only participants who completed both the pretest and the posttest were included in the analyses (n = 21, Denver; n = 11, Boston; n = 18, Portland). Of the 50 participants who completed both the pre‐ and posttests, 44 (88%) classified themselves as hospitalists in practices ranging from 2 to more than 25 physicians. Participants averaged 9.2 years (range = 1‐27 years) in practice and 4.9 years (range = 1‐10 years) as practicing hospitalists, with no significant differences between the 3 groups. Only 17 participants (34%) were familiar with the Campaign prior to the workshop, and there was no significant variation between the 3 workshops. Those familiar with the Campaign had heard about or received the educational materials from colleagues (n = 5), their facilities (n = 4), professional journals (n = 4), medical conferences (n = 4), or the CDC or SHM websites (n = 4).
Overall, most participants strongly agreed with the statement that antimicrobial resistance was a problem nationally, institutionally, and within their individual practices (Table 1). These perceptions did not significantly differ between the pretest and the posttest. However, statistically significant differences were found when comparing perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels; more participants strongly agreed that antimicrobial resistance was a problem nationally than within their institutions (pretest, P = .01; posttest, P = .04) or within their practices (pretest, P < .0001; posttest, P = .01).
| Nationally | Institutionally | Within own practice | ||||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | |
| ||||||
| Denver (n = 21) | 100% | 100% | 86% | 95% | 67% | 86% |
| Portland (n = 18) | 83% | 94% | 67% | 78% | 67% | 78% |
| Boston (n = 11) | 91% | 82% | 91% | 82% | 91% | 82% |
| Average | 91% | 94% | 81% | 85% | 72% | 82% |
| P value | .28 | .18 | .06 | |||
On the knowledge‐based questions, the overall average test score was 48% on the pretest and 63% on the posttest (P < .0001), with scores varying by question (Table 2). For example, knowledge of quality improvement initiatives/HFMEA was low (an average of 10% correct on the pretest, 48% on the posttest) compared with knowledge about the key prevention strategies from the Campaign to Prevent Antimicrobial Resistance (average of 94% correct on the pretest, 98% on the posttest). Furthermore, scores also varied by workshop location. On the pretest, participants in Boston and Portland scored higher (both 53%) than Denver participants (40%). On the posttest, Portland participants scored the highest (78%) followed by Boston participants (64%) and then Denver participants (50%). Boston and Denver participants differed significantly on pretest knowledge score (P = .04) and Portland and Denver participants differed significantly on posttest knowledge score (P < .0001).
| Question Topic | Pretest average | Posttest average | Percent difference (P value)* |
|---|---|---|---|
| |||
| Quality improvement initiatives/HFMEA Which quality improvement initiative(s) must be performed yearly by all hospitals (JCAHO accreditation requirement)? | 10% | 48% | 38% (P < .0001) |
| Prevention of central venous catheter‐associated bloodstream infections: Key prevention steps for preventing central venous catheter‐associated bloodstream infections include all of the following except: | 62% | 88% | 26% (P = .0001) |
| RCA Which of the following is NOT true about root cause analysis? | 20% | 38% | 18% (P = .01) |
| Campaign to Prevent Antimicrobial Resistance The key prevention strategies from the Campaign to Prevent Antimicrobial Resistance include all of the following except: | 94% | 98% | 4% (P = .32) |
| Common body sites for healthcare‐associated infection: The most common site of hospital‐acquired (nosocomial) infection is: | 52% | 44% | 8% (P = .29) |
| Overall average | 48% | 63% | 15% (P < .0001) |
Overall, 43 participants (85%) rated the workshop as either very good or excellent. All but 1 participant (n = 49, 98%) would encourage a colleague to attend the workshop, giving reasons such as that the workshop outlined a major program in delivering good and safe care, offered great information on antimicrobial resistance and methods of quality improvement systems implementation, assisted in find[ing] new tools for improving hospital practice, and addressed a significant factor in hospitals related to morbidity [and] mortality. When asked for general comments about the workshop and suggestions for future improvements, participants requested more direction, more detail, more discussion, specific examples of antimicrobial resistance, and protocols and processes for implementing quality improvement programs. On a scale from 1 (not useful) to 5 (essential), participants rated the usefulness of each workshop segment: intravascular catheter‐related infections lecture and case study (x̄ = 4.3, range = 3‐5), quality improvement initiatives lecture (x̄ = 4.1, range = 2‐5), background on antimicrobial resistance (x̄ = 3.9, range = 2‐5), RCA lecture (x̄ = 3.9, range = 2‐5), HFMEA lecture (x̄ = 3.8, range = 2‐5), and small‐group discussion (x̄ = 3.4, range = 2‐5). These ratings did not vary significantly between the 3 groups.
CONCLUSIONS
To address antimicrobial resistance and health careassociated infections in the hospital setting, the SHM and CDC developed a tool kit and presented a quality improvement workshop to hospitalists in 3 U.S. cities. Overall, the participants scored significantly higher on the knowledge‐based questions on the posttest than on the pretest, indicating that knowledge improved as a result of the workshop. By providing a format that combined didactic lectures with case‐based education, small‐group activities, and discussion, the SHM workshop may have optimized its ability to increase knowledge, similar to the findings in previous research.2021
There were no significant differences between the 3 groups in years of practice, perceptions of the problem, and overall evaluation of the workshop. However, differences were found in knowledge gained as a result of the workshop. For example, the Denver group scored lower on the knowledge‐based questions than did the Boston group on the pretest and the Portland group on the posttest, indicating that knowledge and learning styles may differ by location. These differences may be attributed to variations in hospital environments, hospital‐based educational programs, or medical school and residency training. Differences like these may impact the effectiveness of a program and should be a consideration in the program development process, especially when a program is national in scope, like the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. In addition, more than 90% of participants correctly identified key prevention strategies of the Campaign, whereas only 34% were familiar with the Campaign itself prior to the workshop. This result may be a result of the key prevention strategies of the Campaign being derived from well‐established and ‐recognized evidence‐based best practices for patient safety and care.
Although knowledge changed as a result of the workshop, overall perceptions of the problem of antimicrobial resistance did not change significantly from pretest to posttest. It is possible this is because changes in perception require a different or more intensive educational approach. This result also may reflect the initial levels of agreement on the pretest, the measurement instrument itself, and/or the inability to detect differences because of the small number of participants.
Difference did exist in perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels. Antimicrobial resistance was perceived to be a greater problem on the national level than on the institutional and practice levels. Other studies also have found that clinicians more strongly agree that antimicrobial resistance is a problem nationally than within their institutions and practices.2224 When antimicrobial resistance is not perceived as a problem within institutions and practices, physicians may be less likely to overcome the barriers to following recommended infection prevention guidelines or to implementing quality improvement projects.4 Therefore, educational and intervention efforts like this workshop should address hospitalists' perceptions of the problem of antimicrobial resistance on the individual level as a first step in motivating them to engage in quality improvement.
Although participants' knowledge scores increased from pretest to posttest, gaps in knowledge remained, as indicated by the significantly improved but low overall posttest scores related to RCA and HFMEA. As hospitalists are in a unique position to promote quality improvement programs, these topic areas should be given more attention in future workshops and in training. Furthermore, by adding more specific questions related to each section of the workshop, associations among presentation style, knowledge gained, and perceived usefulness of each section could be evaluated. For example, the participants significantly increased their scores from pretest to posttest on the catheter‐related knowledge‐based question and rated the lecture and case study on intravascular catheter‐related infections as the most useful sections. Future research may explore these possible relationships to better guide selection of presentation styles and topics to ensure that participants gain knowledge and perceive the sections as useful. In addition, by addressing the feedback from participants, such as offering more detail, examples, and discussion, future workshops may have greater perceived usefulness and be better able to increase the knowledge and awareness of quality improvement programs for the prevention of health careassociated infections and antimicrobial resistance.
Although there were 3 workshops conducted in 3 areas across the United States, the sample size at each site was small, and results may not be representative of hospitalists at large. In addition, power calculations should be considered in future studies to increase the ability to better detect differences between and within groups. Another limitation of this study was that the limited data available and participant anonymity meant it was not possible to follow‐up with participants after the workshop to evaluate whether the knowledge they gained was sustained and/or whether they reported changes in practice. However, possession of knowledge and skills to inform practice does not mean that practice will change; therefore, follow‐up is necessary to determine if this workshop was effective in changing behaviors in the long term.25 Although the SHM workshop improved knowledge, more intensive educational strategies may be necessary to affect perceptions and improve the leadership skills required for implementation of quality improvement programs at an institutional level.
Overall, the SHM workshop was found to be a useful tool for increasing knowledge and outlining methods by which hospitalists can lead, coordinate, or participate in measures to prevent infections and improve patient safety. In addition, through the workshop, the SHM and the CDC have provided an example of how professional societies and government agencies can collaborate to address emerging issues in the health care setting.
In the United States, hospitalized patients are at risk of acquiring health careassociated infections that increase morbidity, mortality, length of hospital stay, and cost of care.1 If a health careassociated infection is caused by an antimicrobial‐resistant pathogen, treatment efforts may be further complicated.2, 3 With the decreasing effectiveness of antimicrobials and suboptimal adherence to certain infection control measures, new and multifaceted prevention strategies are necessary to address the problem of health careassociated infections and antimicrobial resistance.410
One strategy that hospitals can use to reduce the incidence of health careassociated infections and antimicrobial resistance is implementation of quality improvement programs. These programs require clinicians to employ techniques, such as root cause analysis (RCA), which investigates contributing factors to an event to prevent reoccurrence, and healthcare failure mode effects analysis (HFMEA), which applies a systematic method of identifying and preventing problems before they occur.1113 Programs and strategies such as these require leadership and adoption within the hospital. Because of their availability and specialized role in the hospital setting, hospitalists are in a unique position to promote and uphold quality improvement efforts.1417 Professional societies, health care organizations, and governmental agencies can play a role in engaging this group of physicians in improving the quality of patient care in hospitals by providing educational programs and materials.18
In 2004, the Society of Hospital Medicine (SHM) collaborated with the Centers for Disease Control and Prevention (CDC) to develop a quality improvement tool kit to reduce antimicrobial resistance and health careassociated infections. The tool kit was based on the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings (Campaign), an educational program targeted at clinicians.19 The SHM/CDC tool kit contained campaign materials, a set of slides about quality improvement, worksheets, and additional materials such as infection control policies and guidelines to supplement a 90‐minute workshop consisting of didactic lectures about antimicrobial resistance, quality improvement initiatives, RCA, and HFMEA; a lecture and case study about intravascular catheter‐related infections; and small‐group activity and discussion. The complete toolkit is now available online via the SHM Antimicrobial Resistance Resource Room at
The purpose of the workshop was to present the tool kit and increase hospitalists' knowledge and awareness about antimicrobial resistance, health careassociated infections, and quality improvement programs. We assessed the workshop participants' familiarity with the Campaign prior to the workshop, perceptions of antimicrobial resistance, knowledge gained as a result of the workshop, and opinions about the usefulness of the workshop.
METHODS
Data were collected from pretests and posttests administered to participants of one of the SHM workshops in May, June, or July 2005 in Denver, Colorado; Boston, Massachusetts; or Portland, Oregon. One SHM physician leader (D.D.D., coauthor of this article) presented all 3 workshops. The workshops were advertised by SHM using E‐mail to local chapter members. Individual sites used a variety of methods to encourage their hospitalists to attend, and participants were provided a complimentary dinner.
Prior to each workshop, participants completed a 10‐question pretest that had been pilot‐tested by hospitalists in other cities. The pretest assessed demographics; perceptions of the problem of antimicrobial resistance using a Likert scale; familiarity with the Campaign; and knowledge of common infection sites, RCA, HFMEA, and antimicrobial resistance prevention measures.
Immediately following each workshop, a 13‐question posttest was administered to participants. This posttest evaluated the workshop and materials using Likert scales, asked for suggestions for future programming using open‐ended questions, and repeated pretest questions to assess changes in perceptions and knowledge.
Data were entered into an Excel spreadsheet and analyzed using descriptive statistics and t tests to compare pre‐ and posttest changes in knowledge. Likert data assessing perceptions were dichotomized into strongly agree versus all other scale responses. Qualitative open‐ended responses were categorized by theme.
RESULTS
A total of 69 SHM members attended the workshops. Of the 69 participants, 65 completed the pretest, 53 completed the posttest, and 50 completed both the pre‐ and the posttests. Only participants who completed both the pretest and the posttest were included in the analyses (n = 21, Denver; n = 11, Boston; n = 18, Portland). Of the 50 participants who completed both the pre‐ and posttests, 44 (88%) classified themselves as hospitalists in practices ranging from 2 to more than 25 physicians. Participants averaged 9.2 years (range = 1‐27 years) in practice and 4.9 years (range = 1‐10 years) as practicing hospitalists, with no significant differences between the 3 groups. Only 17 participants (34%) were familiar with the Campaign prior to the workshop, and there was no significant variation between the 3 workshops. Those familiar with the Campaign had heard about or received the educational materials from colleagues (n = 5), their facilities (n = 4), professional journals (n = 4), medical conferences (n = 4), or the CDC or SHM websites (n = 4).
Overall, most participants strongly agreed with the statement that antimicrobial resistance was a problem nationally, institutionally, and within their individual practices (Table 1). These perceptions did not significantly differ between the pretest and the posttest. However, statistically significant differences were found when comparing perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels; more participants strongly agreed that antimicrobial resistance was a problem nationally than within their institutions (pretest, P = .01; posttest, P = .04) or within their practices (pretest, P < .0001; posttest, P = .01).
| Nationally | Institutionally | Within own practice | ||||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | |
| ||||||
| Denver (n = 21) | 100% | 100% | 86% | 95% | 67% | 86% |
| Portland (n = 18) | 83% | 94% | 67% | 78% | 67% | 78% |
| Boston (n = 11) | 91% | 82% | 91% | 82% | 91% | 82% |
| Average | 91% | 94% | 81% | 85% | 72% | 82% |
| P value | .28 | .18 | .06 | |||
On the knowledge‐based questions, the overall average test score was 48% on the pretest and 63% on the posttest (P < .0001), with scores varying by question (Table 2). For example, knowledge of quality improvement initiatives/HFMEA was low (an average of 10% correct on the pretest, 48% on the posttest) compared with knowledge about the key prevention strategies from the Campaign to Prevent Antimicrobial Resistance (average of 94% correct on the pretest, 98% on the posttest). Furthermore, scores also varied by workshop location. On the pretest, participants in Boston and Portland scored higher (both 53%) than Denver participants (40%). On the posttest, Portland participants scored the highest (78%) followed by Boston participants (64%) and then Denver participants (50%). Boston and Denver participants differed significantly on pretest knowledge score (P = .04) and Portland and Denver participants differed significantly on posttest knowledge score (P < .0001).
| Question Topic | Pretest average | Posttest average | Percent difference (P value)* |
|---|---|---|---|
| |||
| Quality improvement initiatives/HFMEA Which quality improvement initiative(s) must be performed yearly by all hospitals (JCAHO accreditation requirement)? | 10% | 48% | 38% (P < .0001) |
| Prevention of central venous catheter‐associated bloodstream infections: Key prevention steps for preventing central venous catheter‐associated bloodstream infections include all of the following except: | 62% | 88% | 26% (P = .0001) |
| RCA Which of the following is NOT true about root cause analysis? | 20% | 38% | 18% (P = .01) |
| Campaign to Prevent Antimicrobial Resistance The key prevention strategies from the Campaign to Prevent Antimicrobial Resistance include all of the following except: | 94% | 98% | 4% (P = .32) |
| Common body sites for healthcare‐associated infection: The most common site of hospital‐acquired (nosocomial) infection is: | 52% | 44% | 8% (P = .29) |
| Overall average | 48% | 63% | 15% (P < .0001) |
Overall, 43 participants (85%) rated the workshop as either very good or excellent. All but 1 participant (n = 49, 98%) would encourage a colleague to attend the workshop, giving reasons such as that the workshop outlined a major program in delivering good and safe care, offered great information on antimicrobial resistance and methods of quality improvement systems implementation, assisted in find[ing] new tools for improving hospital practice, and addressed a significant factor in hospitals related to morbidity [and] mortality. When asked for general comments about the workshop and suggestions for future improvements, participants requested more direction, more detail, more discussion, specific examples of antimicrobial resistance, and protocols and processes for implementing quality improvement programs. On a scale from 1 (not useful) to 5 (essential), participants rated the usefulness of each workshop segment: intravascular catheter‐related infections lecture and case study (x̄ = 4.3, range = 3‐5), quality improvement initiatives lecture (x̄ = 4.1, range = 2‐5), background on antimicrobial resistance (x̄ = 3.9, range = 2‐5), RCA lecture (x̄ = 3.9, range = 2‐5), HFMEA lecture (x̄ = 3.8, range = 2‐5), and small‐group discussion (x̄ = 3.4, range = 2‐5). These ratings did not vary significantly between the 3 groups.
CONCLUSIONS
To address antimicrobial resistance and health careassociated infections in the hospital setting, the SHM and CDC developed a tool kit and presented a quality improvement workshop to hospitalists in 3 U.S. cities. Overall, the participants scored significantly higher on the knowledge‐based questions on the posttest than on the pretest, indicating that knowledge improved as a result of the workshop. By providing a format that combined didactic lectures with case‐based education, small‐group activities, and discussion, the SHM workshop may have optimized its ability to increase knowledge, similar to the findings in previous research.2021
There were no significant differences between the 3 groups in years of practice, perceptions of the problem, and overall evaluation of the workshop. However, differences were found in knowledge gained as a result of the workshop. For example, the Denver group scored lower on the knowledge‐based questions than did the Boston group on the pretest and the Portland group on the posttest, indicating that knowledge and learning styles may differ by location. These differences may be attributed to variations in hospital environments, hospital‐based educational programs, or medical school and residency training. Differences like these may impact the effectiveness of a program and should be a consideration in the program development process, especially when a program is national in scope, like the CDC's Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. In addition, more than 90% of participants correctly identified key prevention strategies of the Campaign, whereas only 34% were familiar with the Campaign itself prior to the workshop. This result may be a result of the key prevention strategies of the Campaign being derived from well‐established and ‐recognized evidence‐based best practices for patient safety and care.
Although knowledge changed as a result of the workshop, overall perceptions of the problem of antimicrobial resistance did not change significantly from pretest to posttest. It is possible this is because changes in perception require a different or more intensive educational approach. This result also may reflect the initial levels of agreement on the pretest, the measurement instrument itself, and/or the inability to detect differences because of the small number of participants.
Difference did exist in perceptions of the problem of antimicrobial resistance at the national, institutional, and practice levels. Antimicrobial resistance was perceived to be a greater problem on the national level than on the institutional and practice levels. Other studies also have found that clinicians more strongly agree that antimicrobial resistance is a problem nationally than within their institutions and practices.2224 When antimicrobial resistance is not perceived as a problem within institutions and practices, physicians may be less likely to overcome the barriers to following recommended infection prevention guidelines or to implementing quality improvement projects.4 Therefore, educational and intervention efforts like this workshop should address hospitalists' perceptions of the problem of antimicrobial resistance on the individual level as a first step in motivating them to engage in quality improvement.
Although participants' knowledge scores increased from pretest to posttest, gaps in knowledge remained, as indicated by the significantly improved but low overall posttest scores related to RCA and HFMEA. As hospitalists are in a unique position to promote quality improvement programs, these topic areas should be given more attention in future workshops and in training. Furthermore, by adding more specific questions related to each section of the workshop, associations among presentation style, knowledge gained, and perceived usefulness of each section could be evaluated. For example, the participants significantly increased their scores from pretest to posttest on the catheter‐related knowledge‐based question and rated the lecture and case study on intravascular catheter‐related infections as the most useful sections. Future research may explore these possible relationships to better guide selection of presentation styles and topics to ensure that participants gain knowledge and perceive the sections as useful. In addition, by addressing the feedback from participants, such as offering more detail, examples, and discussion, future workshops may have greater perceived usefulness and be better able to increase the knowledge and awareness of quality improvement programs for the prevention of health careassociated infections and antimicrobial resistance.
Although there were 3 workshops conducted in 3 areas across the United States, the sample size at each site was small, and results may not be representative of hospitalists at large. In addition, power calculations should be considered in future studies to increase the ability to better detect differences between and within groups. Another limitation of this study was that the limited data available and participant anonymity meant it was not possible to follow‐up with participants after the workshop to evaluate whether the knowledge they gained was sustained and/or whether they reported changes in practice. However, possession of knowledge and skills to inform practice does not mean that practice will change; therefore, follow‐up is necessary to determine if this workshop was effective in changing behaviors in the long term.25 Although the SHM workshop improved knowledge, more intensive educational strategies may be necessary to affect perceptions and improve the leadership skills required for implementation of quality improvement programs at an institutional level.
Overall, the SHM workshop was found to be a useful tool for increasing knowledge and outlining methods by which hospitalists can lead, coordinate, or participate in measures to prevent infections and improve patient safety. In addition, through the workshop, the SHM and the CDC have provided an example of how professional societies and government agencies can collaborate to address emerging issues in the health care setting.
- ,,.Impact of nosocomial infection on cost of illness and length of stay in intensive care units.Infect Control Hosp Epidemiol2005;26:281–287.
- .Implementation of strategies to control antimicrobial resistance.Chest.2001;119:405S–411S.
- ,,, et al.Society for Healthcare Epidemiology of America and Infectious Diseases Society of American Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals.Clin Infect Dis.1997;25:584–599.
- ,,, et al.Strategies to prevent and control the emergence and spread of antimicrobial‐resistant microorganisms in hospitals: a challenge to hospital leadership.JAMA.1996;275:234–240.
- Centers for Disease Control and Prevention.Guidelines for hand hygiene in health‐care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.MMWR Recomm Rep.2002;51:1–44.
- .Hospital Infection Control Practices Advisory Committee.Guideline for isolation precautions in hospitals.Infect Control Hosp Epidemiol.1996;17:53–80.
- ,,, et al.SHEA guideline for prevention nosocomial transmission of multidrug‐resistant strains of Staphylococcus aureus and Enterococcus.Infect Control Hosp Epidemiol.2003;24:362–386.
- .Improving adherence to hand hygiene practice: a multidisciplinary approach.Emerg Infect Dis.2001;7:234–240.
- ,,.Alcohol‐based handrub improves compliance with hand hygiene in intensive care units.Arch Intern Med.2002;162:1037–1043.
- ,,, et al.An organizational climate intervention associated with increased handwashing and decreased nosocomial infections.Behav Med.2000;26:14–22.
- ,.Getting to the root of the matter.AHRQ Web M 29:319–330.
- ,,.The Basics of FMEA.New York:Quality Resources;1996.
- .The hospitalist model of care: A positive influence on efficiency, quality of care, and outcomes.Crit Path Cardiol.2004;3:S5–S7.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- .The impact of hospitalists on medical education and the academic health systems.Ann Intern Med.1999;130:364–367.
- ,,, et al.Hospitalists' perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- ,,.Preventing the emergence of antimicrobial resistance: A call for action by clinicians, public health officials and patients.JAMA1997;278:944–945.
- Centers for Disease Control and Prevention. Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. 2005. Available at: URL: http://www.cdc.gov/drugresistance/healthcare/default.htm. Accessed November 8,2005.
- ,,, et al.Impact of formal continuing medical education: Do conferences, workshops, rounds and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,, et al.Physician preferences for continuing medical education with a focus on the topic of antimicrobial resistance: Society for Healthcare Epidemiology of America.Infect Control Hosp Epidemiol.2001;22:656–660.
- ,,, et al.Clinicians' perceptions of the problem of antimicrobial resistance in health care facilities.Arch Intern Med.2004;164:1662–1668.
- ,,, et al.Antibiotic resistance: a survey of physician perceptions.Arch Intern Med.2002;162:2210–2216.
- ,,, et al.Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the health belief model as a framework.Am J Infect Control.2004;33:175–181.
- .Educational theory into practice: Development of an infection control link nurse programme.Nurs Ed Pract.2001;1:35–41.
- ,,.Impact of nosocomial infection on cost of illness and length of stay in intensive care units.Infect Control Hosp Epidemiol2005;26:281–287.
- .Implementation of strategies to control antimicrobial resistance.Chest.2001;119:405S–411S.
- ,,, et al.Society for Healthcare Epidemiology of America and Infectious Diseases Society of American Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals.Clin Infect Dis.1997;25:584–599.
- ,,, et al.Strategies to prevent and control the emergence and spread of antimicrobial‐resistant microorganisms in hospitals: a challenge to hospital leadership.JAMA.1996;275:234–240.
- Centers for Disease Control and Prevention.Guidelines for hand hygiene in health‐care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.MMWR Recomm Rep.2002;51:1–44.
- .Hospital Infection Control Practices Advisory Committee.Guideline for isolation precautions in hospitals.Infect Control Hosp Epidemiol.1996;17:53–80.
- ,,, et al.SHEA guideline for prevention nosocomial transmission of multidrug‐resistant strains of Staphylococcus aureus and Enterococcus.Infect Control Hosp Epidemiol.2003;24:362–386.
- .Improving adherence to hand hygiene practice: a multidisciplinary approach.Emerg Infect Dis.2001;7:234–240.
- ,,.Alcohol‐based handrub improves compliance with hand hygiene in intensive care units.Arch Intern Med.2002;162:1037–1043.
- ,,, et al.An organizational climate intervention associated with increased handwashing and decreased nosocomial infections.Behav Med.2000;26:14–22.
- ,.Getting to the root of the matter.AHRQ Web M 29:319–330.
- ,,.The Basics of FMEA.New York:Quality Resources;1996.
- .The hospitalist model of care: A positive influence on efficiency, quality of care, and outcomes.Crit Path Cardiol.2004;3:S5–S7.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- .The impact of hospitalists on medical education and the academic health systems.Ann Intern Med.1999;130:364–367.
- ,,, et al.Hospitalists' perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- ,,.Preventing the emergence of antimicrobial resistance: A call for action by clinicians, public health officials and patients.JAMA1997;278:944–945.
- Centers for Disease Control and Prevention. Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. 2005. Available at: URL: http://www.cdc.gov/drugresistance/healthcare/default.htm. Accessed November 8,2005.
- ,,, et al.Impact of formal continuing medical education: Do conferences, workshops, rounds and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,, et al.Physician preferences for continuing medical education with a focus on the topic of antimicrobial resistance: Society for Healthcare Epidemiology of America.Infect Control Hosp Epidemiol.2001;22:656–660.
- ,,, et al.Clinicians' perceptions of the problem of antimicrobial resistance in health care facilities.Arch Intern Med.2004;164:1662–1668.
- ,,, et al.Antibiotic resistance: a survey of physician perceptions.Arch Intern Med.2002;162:2210–2216.
- ,,, et al.Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the health belief model as a framework.Am J Infect Control.2004;33:175–181.
- .Educational theory into practice: Development of an infection control link nurse programme.Nurs Ed Pract.2001;1:35–41.
Copyright © 2007 Society of Hospital Medicine
Consequences of Missed Opportunities
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
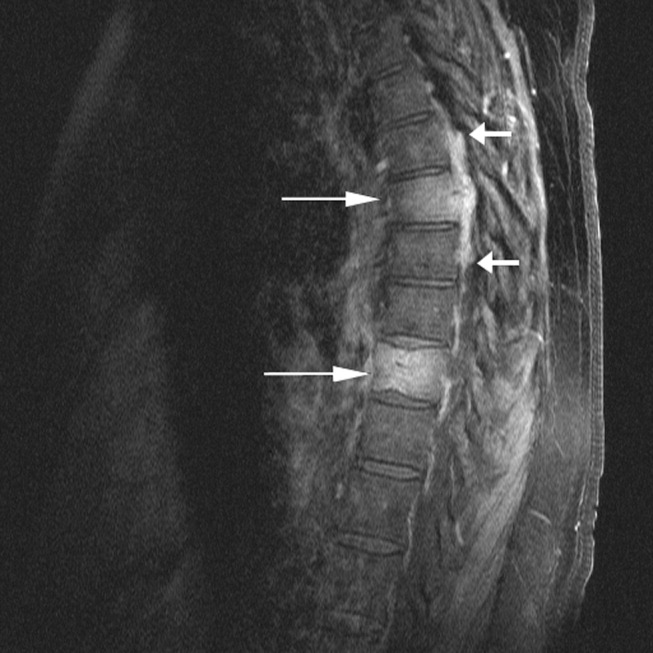
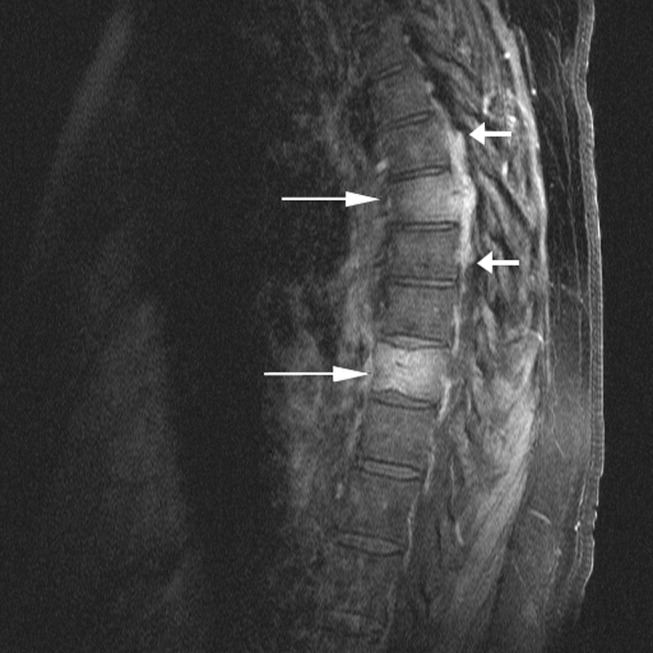
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).
A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).
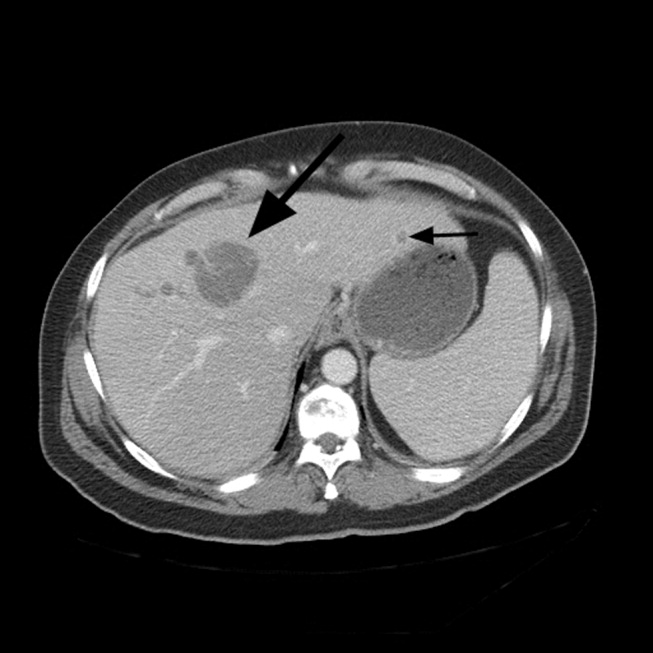
The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).

A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).

The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).

A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).

The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.
Management of Blood Pressure after Stroke
Hospitalists are on the front lines of care for patients with cerebrovascular accidents. After the first steps in acute stroke management occur in the emergency room, care is frequently transferred to the hospitalist. This review focuses on the evidence‐based management of blood pressure following acute ischemic stroke. Management of blood pressure after stroke is still controversial, and current consensus statement guidelines acknowledge that optimal treatment has not yet been established.1, 2 As such, it is essential to understand the changes in normal homeostatic physiologic processes that occur after stroke and their subsequent effects on neurologic function. Only then can the appropriate blood pressure target and antihypertensive regimen be chosen.
Physiology of Cerebral Perfusion
Once a stroke has occurred and perfusion to a section of brain tissue has been acutely compromised, systemic pressure tends to rise. This rise is presumably due in part to increased adrenergic tone and activation of the renin‐aldosterone system and potentially is part of Cushing's reflex in cases in which intracranial pressure is elevated.3 The increase in mean arterial pressure may represent a protective response. In the first major study on the topic, in 1981, on admission for stroke mean blood pressure was 163/90 mm Hg for patients without a history of hypertension and 214/118 mm Hg for patients who had been treated for hypertension previously.4 This rise in blood pressure in response to endogenous mechanisms is attenuated over the first 24 hours, and even without intervention, blood pressure tends to fall spontaneously over the next 10 days.47
Under normal circumstances, cerebral blood flow (CBF) is tightly autoregulated across a wide range of cerebral perfusion pressures by alteration in cerebrovascular resistance via arteriolar constriction.89 This allows CBF to remain constant even if cerebral perfusion pressure (CPP) fluctuates from 60 to 150 mm Hg.9 In patients with chronic hypertension, autoregulation works best with blood pressure in a higher range because of vascular smooth muscle hypertrophy and structural changes in the cerebral vessels (see Table 1).
| Cerebral blood flow (CBF) of 50‐70 mL/100 g/minnormal |
| CBF of 20‐50 mL/100 g/minreduced flow compensated for by increased oxygen extraction |
| CBF of 15‐20 mL/100 g/minneuronal quiescence |
| CBF < 15 mL/100 g/minneuronal death |
After an acute ischemic stroke, autoregulation is lost, and local CBF becomes linearly associated with cerebral perfusion pressure. Loss of autoregulation occurs because of several local factors as a result of the infarct. Acidosis and hypoxia in the region of the stroke lead to vasodilatation in the perfusing vessels.9 This may improve local circulation in collateral vessels as a response to the obstruction in the primary supplying artery, but the consequence of maximal vasodilatation is loss of the ability to autoregulate. Normal CPP is driven by the mean arterial pressure minus the intracranial pressure. However, if intracranial vessels have lost the ability to accommodate changes in perfusion pressure, then blood flow to the area of injury becomes linearly correlated with mean arterial pressure.
Surrounding a central core of irreversible necrosis may be a zone of at‐risk tissue that is susceptible to reduction below the threshold of viability in response to any decrement in systemic mean blood pressure.11 This critical concept in stroke management is referred to as the peri‐infarct penumbra (see Fig. 1 and Table 2).
| Mean arterial pressure (MAP) = ⅔ diastolic blood pressure (DBP) + ⅓ systolic blood pressure (SBP) |
| Cerebral perfusion pressure (CPP) = mean arterial pressure (MAP) intracranial pressure (ICP) |
| Cerebral blood flow (CBF) = cerebral perfusion pressure (CPP)/cerebrovascular resistance (CVR) |
Blood Pressure Management after Stroke
The presence of a systemic blood pressure‐dependent peri‐infarct penumbra that might be compromised by blood pressure reduction and thus extend the infarct is the principal argument for allowing permissive hypertension. This is bolstered by the observation that decreases in blood pressure in the first 24 hours are associated with a significant risk of poor neurologic outcome.6 In their an observational study Vlcek et al. found that a greater than 25% drop in diastolic blood pressure (DBP) was associated with a 4‐fold risk of severe disability.5 In a 2003 observational study Oliveria et al. found that reduction in systolic blood pressure (SBP) in the first 24 hours was independently associated with poor outcomes, with a doubling of the risk for poor outcomes for every 10% drop in systolic blood pressure.6 In both these studies, the worsened outcome did not depend on whether the drop was spontaneous or induced by medications. Case reports suggest that large drops in blood pressure can be catastrophic and that even moderate lowering of blood pressure after acute stroke can be associated with clinical deterioration.8, 12
The primary argument for lowering blood pressure after an acute stroke hinges on secondary prevention of new ischemic events, minimization of cerebral edema, and prevention of hemorrhagic conversion. It has long been recognized that hypertension itself is a risk factor for stroke13 and that reduction in chronic hypertension is part of secondary prevention for cerebrovascular accidents.14 Further, hypertension is the most common risk factor for intracerebral hemorrhage, and it would stand to reason that damage to the brain parenchyma from ischemic stroke would increase the risk of pressure‐induced bleeding in an acute setting.15
Because there are compelling theoretical arguments both for and against lowering blood pressure after an acute stroke, it is necessary to look at the results of randomized clinical trials for guidance in weighing the risks and benefits. The overall goal of blood pressure management is to maximize perfusion to the ischemic penumbra while minimizing the hypertensive risk of hemorrhagic transformation.
Lisk et al. conducted a small randomized trial in 1993 of antihypertensive therapy versus placebo after ischemic stroke looking at SPECT perfusion and found lower CBF if mean arterial pressure (MAP) dropped more than 16%.16 This is in contrast with the results of a 1997 randomized trial of perindopril after cerebrovascular accident that found no decrease in Doppler CBF despite a 10% decrease in blood pressure in the active treatment group.17 Neither study was powered to detect significant differences in clinical outcome.
Early randomized controlled trials of antihypertensive agents after acute stroke investigated neuroprotection via mechanisms other than the antihypertensive effect. Nimodipine, a dihydropyridine derivative thought to prevent neuronal death via blockade of calcium channels, has been studied extensively.1820 A meta‐analysis of the 9 early trials of nimodipine, from 1988 to 1992, suggested that nimodipine was potentially beneficial in neurologic score and functional outcome only if used within the first 12 hours and that it could be harmful if started after 24 hours.21 Two additional studies were done in 1994 using both intravenous and oral nimodipine formulations. They demonstrated worsened neurologic function and higher mortality, respectively, which was hypothesized to be a result of the detrimental hemodynamic effects of nimodipine.18, 22
The BEST trial used beta‐blockers in the early period after acute stroke and failed to find benefit, whereas the FIST trial had similar results with the calcium channel blocker flunarizine.23, 24 The ACCESS trial in 2003 was a prospective, randomized, controlled trial using oral candesartan in the first 24 hours after stroke.25 It did find improved mortality in the candesartan arm after 1 year, yet there were no significant differences in blood pressure between candesartan and placebo. Thus, the improved outcome was presumed to be a result of mechanisms other than antihypertensive effect.
Some significant caveats should be kept in mind when interpreting the results of these studies. None of the trials was designed to titrate blood pressure to a prespecified goal in a prospective randomized fashion. It is also difficult to tease out the effect of the active medical intervention from the effect of spontaneous drops in blood pressure, and many trials did not find a significant difference in blood pressure between the medication and placebo groups. A large randomized trial to evaluate interventions to a predefined blood pressure target is needed.
The Cochrane Stroke Group reviewed 32 trials involving 5368 patients and concluded there was not enough evidence to reliably evaluate the effect of altering blood pressure on outcomes.3
Recommendations for Treatment of Hypertension in Acute Stroke
The decision of when to initiate antihypertensive therapy has not been clearly delineated. However, most experts agree that blood pressure targets need to take into account whether thrombolytics are used. The available data provide little evidence that lowering blood pressure decreases adverse events; however, there is some evidence that lowering blood pressure can worsen outcomes by expanding the area of ischemia. Thus, in treating most acute ischemic strokes, antihypertensive therapy can and should be withheld. The exception to this is stroke in a patient with comorbid hypertensive organ damage such as myocardial infarction, aortic dissection, acute hypertensive renal failure, pulmonary edema, or encephalopathy. The consensus statement of the American Stroke Association is to withhold treatment until blood pressure exceeds 220/120 mm Hg,1 roughly corresponding to a MAP of 150 mm Hg, the normal upper limit of cerebral autoregulation. Treatment optimally should be titratable in order to avoid overcorrection, preferably with minimal cerebral venodilatation effect. The parenteral agents labetolol, fenoldopam, nicardipine, and nitroprusside are most commonly used. A disadvantage of nitrates or nitroprusside is that their venodilating effect may raise intracranial pressure. Clonidine may induce central nervous system depression, which can complicate interpretation of the mental status of a patient with an acute neurologic event. Sublingual nifedipine should be avoided because of its well‐documented tendency to cause overcorrection of blood pressure.26 No large direct comparison trial has evaluated which of these antihypertensive agents is superior in clinical outcomes. For the hospitalist, choice of antihypertensive agent should be driven by a need for rapid control of blood pressure to target without overcorrection (see Table 3). Issues such as intracranial pressure, heart rate, and comorbidities may also play a role in choice of medication.
| Not eligible for thrombolytic therapy | |
|---|---|
| |
| Systolic < 220 or diastolic < 120 | Observe unless other end‐organ involvement (aortic dissection, acute myocardial infarction, pulmonary edema, hypertensive encephalopathy) |
| Systolic > 220 or diastolic 121‐140 | Aim for 10%‐15% reduction in blood pressure |
| Labetalol 10‐20 mg IV over 1‐2 minutes; repeat or double every 10 minutes; maximum 300 mg | |
| or | |
| Nicardipine 5 mg/hour IV; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| or | |
| Nitroprusside 0.5 g/kg/minute IV; titrate to goal | |
| Diastolic > 140 | Aim for 10%‐15% reduction in blood pressure |
| Nitroprusside 0.5 g/kg/minute IV; titrate to desired blood pressure | |
| Eligible for thrombolytic therapy | |
| Pretreatment | |
| Systolic > 185 or diastolic > 110 | Labetalol 10‐20mg IV |
| May repeat 1 or Nitropaste 1‐2 inches | |
| During and after tPA | |
| Systolic 180‐230 or Diastolic 105‐120 | Labetalol 10 mg |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute | |
| Systolic > 230 or diastolic 121‐140 | Labetalol 10mg IV |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute or | |
| Nicardipine 5 mg/hour; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| If not at goal with labetalol or nicardipine, consider nitroprusside | |
| Diastolic>140 | Nitroprusside 0.5 g/kg/minute IV infusion; titrate to desired blood pressure |
The risk of hemorrhagic conversion is greater when thrombolysis is used for the treatment of cerebrovascular accident, in the NINDS trial rising from 0.6% in patients receiving a placebo to 6.4% in patients receiving tPA.27 Given that the goal of thrombolysis is to restore perfusion through the previously blocked vessel and that the risk of hemorrhage rises after thrombolysis, the balance between maintaining CPP and decreasing the risk of bleeding shifts toward maintaining a lower blood pressure postthrombolysis. The NINDS thrombolytic trial required patients to have a blood pressure less than 185/110 mm Hg to be included.27 Once a thrombolytic had been administered, the permitted maximum blood pressure dropped to 180/105 mm Hg. Blood pressure needs to be monitored closely, initially every 15 minutes for 2 hours, then every 30 minutes for the next 6 hours, and then hourly until the end of the first 24 hours. After this, blood pressure monitoring intervals can be more spaced out depending on the need for active antihypertensive therapy.28 However, arterial punctures for invasive monitoring are not recommended if thrombolytics are administered. Review of the experience of the NINDS trial suggests that approximately one third of patients receiving thrombolytics will require pharmacologic therapy to reach the recommended blood pressure goals in the first 24 hours.28
Although guidelines for the management of blood pressure after cerebrovascular accident from major organizations such as the American Stroke Association and the European Stroke Initiative are published and widely quoted in reviews of stroke management, physician adherence to these guidelines is poor.1, 2933 A 2002 review of prescribing practices in a Canadian hospital found excessive reduction of blood pressure in 60% of patients where nitroglycerin was used, and sublingual nifedipine was still the second most commonly prescribed medication.34 A similar 2004 study by Lindenauer et al. found that only 26% of patients who had antihypertensive medication initiated in the hospital met consensus guidelines for therapy.35
Induced Hypertension
Hypotension should be avoided after acute stroke. Observational studies have demonstrated an increased mortality rate of patients who present with hypotension.42 Patients with acute ischemic stroke and loss of autoregulation may have impaired tolerance to even mild levels of hypotension.8 First‐line therapy for hypotension after stroke is volume resuscitation and optimization of cardiac function by correcting arrhythmias.1 If this should fail, vasopressor support is advisable to avoid ongoing hypotension and cerebral hypoperfusion.
The same physiologic arguments that favor permissively managing high blood pressure and avoiding hypotension raise the question of whether inducing hypertension through the use of vasopressors might improve outcomes of stroke. This hypothesis has been bolstered by animal studies and imaging data that suggest improved perfusion to the area of injury with vasopressor‐induced hypertension.3638 However, there are concerns that this strategy may increase edema and hemorrhage and create the potential for vasopressor‐induced myocardial ischemia or arrhythmias. Although the results of small trials have appeared promising, particularly for patients with large vessel stenosis, at this point induced hypertension is still considered experimental until the results of larger randomized, controlled trials provide greater support for this procedure.1, 3941
Chronic Blood Pressure Control for Secondary Prevention of Stroke
Multiple trials have demonstrated that interventions to treat chronic hypertension can reduce the rate of future strokes.14, 43, 44 The PROGRESS trial demonstrated that blood pressure reduction with combination therapy decreased stroke recurrence by 43%.43 Clearly long‐term blood pressure control is a vital component of secondary prevention of stroke. Based on the results of the PROGRESS and ACCESS trials, it is suggested that angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers, potentially with additional diuretic therapy, are beneficial in the treatment of chronic hypertension following cerebrovascular accident.25, 42 The issue of optimal medication class is not settled. Recommendations are to treat chronically elevated blood pressure to lower and maintain it at less than 140/80 mm Hg. Because there is no single threshold level of blood pressure beyond which the risk of cerebrovascular disease increases, reduction below this cut point may still offer incremental benefit.13, 45, 46 The timing of initiation of therapy to reach secondary‐prevention goals is not addressed in current guidelines, although some authors recommend waiting days to weeks after an acute stroke before starting therapy.1, 37 For a patient who remains hypertensive at discharge, it is incumbent on the hospitalist to communicate the plan for initiation of antihypertensive agents to the patient and the primary care physician in order to ensure that this critical secondary prevention measure is addressed.
Areas of Ambiguity and Need for Future Research
Within an evidence‐guided practice, there are still many areas for which the evidence to date does not answer important clinical questions about patient management. Whether a patient's prestroke baseline blood pressure should be considered in individualizing blood pressure goals is unclear, as is the question of whether to continue or hold previous chronic antihypertensive therapy. Given the many stroke subtypes, from tiny lacunar infarcts with little collateralization to massive malignant middle‐cerebral infarctions, should blood pressure management be individualized according to the likely size and duration of the peri‐infarct penumbra? Research is needed to determine whether reducing blood pressure to a specific target will improve outcome and the optimal timing of therapy to achieve secondary prevention goals. Finally, using the concept of the penumbra as the theoretical physiologic basis for current blood pressure recommendations, there exists a potential for rapidly evolving neuroimaging techniques to help define the presence, size, and duration of the penumbra to guide these decisions.
CONCLUSIONS
Management of blood pressure after acute ischemic stroke differs from that of many other hypertensive conditions because of the need to preserve perfusion of the peri‐infarct penumbra. Evidence to date suggests little benefit and potential harm from acutely lowering blood pressure after stroke, although there have not been large randomized trials examining outcomes when blood pressure is lowered to a prespecified goal. Current consensus guidelines suggest that blood pressure may be managed permissively up to 220/120 mm Hg when thrombolysis is not administered and to 180/105 mm Hg when thrombolysis is performed. Data suggest that low‐dose antihypertensive therapy such as ACE inhibitors or angiotensin receptor blockers may be safe in the acute setting if pressure is reduced by less than 10%‐15%.17, 25 However, the evidence is not sufficiently strong for this practice to be routinely recommended. At this point, it is recommended that hypotension be aggressively treated; however, induced hypertension remains a promising but unproven therapy. Evidence is strong that long‐term blood pressure control is key to secondary prevention of stroke, but the timing of its initiation remains poorly determined.
- ,,, et al.Guidelines for the early management of patients with ischemic stroke.Stroke.2003;34:1056–1083.
- ,,,.Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update.Stroke.2005;36:916–921.
- Blood Pressure in Acute Stroke Collaboration (BASC).Vasoactive drugs for acute strokeCochrane Database Syst Rev.2004;1:CD002839.
- ,.Blood pressure after stroke.JAMA.1981;246:2177–2180.
- ,,,,,.Association between course of blood pressure within the first 24 hours and functional recovery after acute ischemic stroke.Ann Emerg Med.2003;42:619–626.
- ,,,,,.Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset.Neurology.2003;61:1047–1050.
- ,,,, et al.Blood pressure during the first minutes of focal cerebral ischemia.Ann Emerg Med.1993;22:1438–1443.
- ,,,,.Stroke precipitated by moderate blood pressure reduction.J Emerg Med.2000;19:339–346.
- .Hemodynamics and metabolism in ischemic cerebrovascular disease.Neurologic Clinics.1992;10(1):31–48.
- ,.The 5 Ps of acute ischemic stroke treatment: parenchyma, pipes, perfusion, penumbra, and prevention of complications.South Med J.2003;96:336–341.
- .Viability Thresholds and the Penumbra of Focal Ischemia.Annals of Neurology.1994;36:557–565.
- ,,.Hazards of therapy for excessive hypertension in acute stroke.Acta Med Scand.1980;207:253–257.
- Prospective Studies Collaboration.Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies.Lancet.2002;360:1903–1913.
- ,,,.Secondary prevention of ischemic cerebrovascular disease. What is the evidence?Angiology.2005;56:539–552.
- ,,.Intracerebral hemorrhage.Emerg Med Clin North Am.2002;20:631–655.
- ,,, et al.Should hypertension be treated after acute stroke?Arch Neurol.1993;50:855–862.
- ,,.Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke.Stroke.1997;28:580–583.
- ,,,,.Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke.Cerebrovasc Dis.1994;4:204–210.
- The American Nimodipine Study Group.Clinical trial of nimodipine in acute ischemic stroke.Stroke.1992;23(1):3–8.
- ,,, et al.Placebo‐controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction.Stroke.1990;21:1023–1028.
- ,,, et al.Meta‐analysis of oral nimodipine trials in acute ischemic stroke.Cerebrovasc Dis.1994;4:197–203.
- ,,, et al.A randomized, double‐blind, placebo‐controlled trial of nimodipine in acute ischemic hemispheric stroke.Stroke.1994;25:1348–1353.
- ,,,.Low dose B blockade in acute stroke (“BEST” trial): an evaluationBMJ.1988;296:737–741.
- ,,, et al.Flunarizine in stroke treatment (FIST): a double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands.Acta Neurol Scand.1996;93:56–60.
- ,,, et al.The ACCESS study: Evaluation of acute candesartan cilexetil therapy in stroke survivors.Stroke.2003;34:1699–1703.
- .FDA gives Calcium channel blockers clean bill of health but warns of short‐acting nifedipine hazards.JAMA.1996;275:1638.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Hypertension and its treatment in the NINDS rt‐PA Stroke Trial.Stroke.1998;29:1504–1509.
- ,.Optimizing blood pressure in neurologic emergencies.Neurocrit Care.2004;1:287–299.
- .Management of acute stroke.South M J.2003;96:380–385.
- .Management of acute stroke.Lancet Neurol.2002;1(1):41–50.
- ,.Acute ischemic stroke: emergent evaluation and management.Emerg Med Clin North Am.2002;20:609–630.
- ,.Post‐emergency department management of stroke.Emerg Med Clin North Am.2002;20:687–702.
- ,,.Blood pressure management in acute stroke: comparison of current guidelines with prescribing patterns.Can J Neurol Sci.2002;29(2):125–131.
- ,,,,,.Use of antihypertensive agents in the management of patients with acute ischemic stroke.Neurology.2004;63:318–323.
- ,,,.Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats.Surg Neurol.1996;46:229–234.
- ,,,.Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke.Stroke.2002;33:998–1004.
- ,,,.Induced hypertension improves cerebral blood flow in acute ischemic stroke.Neurology.2005;64:1979.
- ,,,,.A pilot study of drug induced hypertension for treatment of acute stroke.Neurology.2001;56:1210–1213.
- ,,,,,.Pharmacologic elevation of blood pressure in acute stroke: clinical effects and safety.Stroke.1997;28:2133–2138.
- ,,,,.Feasibility and safety of norephinephrine‐induced arterial hypertension in acute ischemic stroke.Neurology.2004;62:1193–1195.
- ,,,,.Initial emergency department blood pressure as predictor of survival after acute ischemic stroke.Neurology.2005;65:1179–1183.
- The PROGRESS Collaborative Group.Randomized trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke of transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke.JAMA.2000;284:465–471.
- ,.Treatment of chronic hypertension for the prevention of stroke.South Med J.2003;96:359–362.
- ,,,.Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study.JAMA.1996;276:1269–1278.
Hospitalists are on the front lines of care for patients with cerebrovascular accidents. After the first steps in acute stroke management occur in the emergency room, care is frequently transferred to the hospitalist. This review focuses on the evidence‐based management of blood pressure following acute ischemic stroke. Management of blood pressure after stroke is still controversial, and current consensus statement guidelines acknowledge that optimal treatment has not yet been established.1, 2 As such, it is essential to understand the changes in normal homeostatic physiologic processes that occur after stroke and their subsequent effects on neurologic function. Only then can the appropriate blood pressure target and antihypertensive regimen be chosen.
Physiology of Cerebral Perfusion
Once a stroke has occurred and perfusion to a section of brain tissue has been acutely compromised, systemic pressure tends to rise. This rise is presumably due in part to increased adrenergic tone and activation of the renin‐aldosterone system and potentially is part of Cushing's reflex in cases in which intracranial pressure is elevated.3 The increase in mean arterial pressure may represent a protective response. In the first major study on the topic, in 1981, on admission for stroke mean blood pressure was 163/90 mm Hg for patients without a history of hypertension and 214/118 mm Hg for patients who had been treated for hypertension previously.4 This rise in blood pressure in response to endogenous mechanisms is attenuated over the first 24 hours, and even without intervention, blood pressure tends to fall spontaneously over the next 10 days.47
Under normal circumstances, cerebral blood flow (CBF) is tightly autoregulated across a wide range of cerebral perfusion pressures by alteration in cerebrovascular resistance via arteriolar constriction.89 This allows CBF to remain constant even if cerebral perfusion pressure (CPP) fluctuates from 60 to 150 mm Hg.9 In patients with chronic hypertension, autoregulation works best with blood pressure in a higher range because of vascular smooth muscle hypertrophy and structural changes in the cerebral vessels (see Table 1).
| Cerebral blood flow (CBF) of 50‐70 mL/100 g/minnormal |
| CBF of 20‐50 mL/100 g/minreduced flow compensated for by increased oxygen extraction |
| CBF of 15‐20 mL/100 g/minneuronal quiescence |
| CBF < 15 mL/100 g/minneuronal death |
After an acute ischemic stroke, autoregulation is lost, and local CBF becomes linearly associated with cerebral perfusion pressure. Loss of autoregulation occurs because of several local factors as a result of the infarct. Acidosis and hypoxia in the region of the stroke lead to vasodilatation in the perfusing vessels.9 This may improve local circulation in collateral vessels as a response to the obstruction in the primary supplying artery, but the consequence of maximal vasodilatation is loss of the ability to autoregulate. Normal CPP is driven by the mean arterial pressure minus the intracranial pressure. However, if intracranial vessels have lost the ability to accommodate changes in perfusion pressure, then blood flow to the area of injury becomes linearly correlated with mean arterial pressure.
Surrounding a central core of irreversible necrosis may be a zone of at‐risk tissue that is susceptible to reduction below the threshold of viability in response to any decrement in systemic mean blood pressure.11 This critical concept in stroke management is referred to as the peri‐infarct penumbra (see Fig. 1 and Table 2).

| Mean arterial pressure (MAP) = ⅔ diastolic blood pressure (DBP) + ⅓ systolic blood pressure (SBP) |
| Cerebral perfusion pressure (CPP) = mean arterial pressure (MAP) intracranial pressure (ICP) |
| Cerebral blood flow (CBF) = cerebral perfusion pressure (CPP)/cerebrovascular resistance (CVR) |
Blood Pressure Management after Stroke
The presence of a systemic blood pressure‐dependent peri‐infarct penumbra that might be compromised by blood pressure reduction and thus extend the infarct is the principal argument for allowing permissive hypertension. This is bolstered by the observation that decreases in blood pressure in the first 24 hours are associated with a significant risk of poor neurologic outcome.6 In their an observational study Vlcek et al. found that a greater than 25% drop in diastolic blood pressure (DBP) was associated with a 4‐fold risk of severe disability.5 In a 2003 observational study Oliveria et al. found that reduction in systolic blood pressure (SBP) in the first 24 hours was independently associated with poor outcomes, with a doubling of the risk for poor outcomes for every 10% drop in systolic blood pressure.6 In both these studies, the worsened outcome did not depend on whether the drop was spontaneous or induced by medications. Case reports suggest that large drops in blood pressure can be catastrophic and that even moderate lowering of blood pressure after acute stroke can be associated with clinical deterioration.8, 12
The primary argument for lowering blood pressure after an acute stroke hinges on secondary prevention of new ischemic events, minimization of cerebral edema, and prevention of hemorrhagic conversion. It has long been recognized that hypertension itself is a risk factor for stroke13 and that reduction in chronic hypertension is part of secondary prevention for cerebrovascular accidents.14 Further, hypertension is the most common risk factor for intracerebral hemorrhage, and it would stand to reason that damage to the brain parenchyma from ischemic stroke would increase the risk of pressure‐induced bleeding in an acute setting.15
Because there are compelling theoretical arguments both for and against lowering blood pressure after an acute stroke, it is necessary to look at the results of randomized clinical trials for guidance in weighing the risks and benefits. The overall goal of blood pressure management is to maximize perfusion to the ischemic penumbra while minimizing the hypertensive risk of hemorrhagic transformation.
Lisk et al. conducted a small randomized trial in 1993 of antihypertensive therapy versus placebo after ischemic stroke looking at SPECT perfusion and found lower CBF if mean arterial pressure (MAP) dropped more than 16%.16 This is in contrast with the results of a 1997 randomized trial of perindopril after cerebrovascular accident that found no decrease in Doppler CBF despite a 10% decrease in blood pressure in the active treatment group.17 Neither study was powered to detect significant differences in clinical outcome.
Early randomized controlled trials of antihypertensive agents after acute stroke investigated neuroprotection via mechanisms other than the antihypertensive effect. Nimodipine, a dihydropyridine derivative thought to prevent neuronal death via blockade of calcium channels, has been studied extensively.1820 A meta‐analysis of the 9 early trials of nimodipine, from 1988 to 1992, suggested that nimodipine was potentially beneficial in neurologic score and functional outcome only if used within the first 12 hours and that it could be harmful if started after 24 hours.21 Two additional studies were done in 1994 using both intravenous and oral nimodipine formulations. They demonstrated worsened neurologic function and higher mortality, respectively, which was hypothesized to be a result of the detrimental hemodynamic effects of nimodipine.18, 22
The BEST trial used beta‐blockers in the early period after acute stroke and failed to find benefit, whereas the FIST trial had similar results with the calcium channel blocker flunarizine.23, 24 The ACCESS trial in 2003 was a prospective, randomized, controlled trial using oral candesartan in the first 24 hours after stroke.25 It did find improved mortality in the candesartan arm after 1 year, yet there were no significant differences in blood pressure between candesartan and placebo. Thus, the improved outcome was presumed to be a result of mechanisms other than antihypertensive effect.
Some significant caveats should be kept in mind when interpreting the results of these studies. None of the trials was designed to titrate blood pressure to a prespecified goal in a prospective randomized fashion. It is also difficult to tease out the effect of the active medical intervention from the effect of spontaneous drops in blood pressure, and many trials did not find a significant difference in blood pressure between the medication and placebo groups. A large randomized trial to evaluate interventions to a predefined blood pressure target is needed.
The Cochrane Stroke Group reviewed 32 trials involving 5368 patients and concluded there was not enough evidence to reliably evaluate the effect of altering blood pressure on outcomes.3
Recommendations for Treatment of Hypertension in Acute Stroke
The decision of when to initiate antihypertensive therapy has not been clearly delineated. However, most experts agree that blood pressure targets need to take into account whether thrombolytics are used. The available data provide little evidence that lowering blood pressure decreases adverse events; however, there is some evidence that lowering blood pressure can worsen outcomes by expanding the area of ischemia. Thus, in treating most acute ischemic strokes, antihypertensive therapy can and should be withheld. The exception to this is stroke in a patient with comorbid hypertensive organ damage such as myocardial infarction, aortic dissection, acute hypertensive renal failure, pulmonary edema, or encephalopathy. The consensus statement of the American Stroke Association is to withhold treatment until blood pressure exceeds 220/120 mm Hg,1 roughly corresponding to a MAP of 150 mm Hg, the normal upper limit of cerebral autoregulation. Treatment optimally should be titratable in order to avoid overcorrection, preferably with minimal cerebral venodilatation effect. The parenteral agents labetolol, fenoldopam, nicardipine, and nitroprusside are most commonly used. A disadvantage of nitrates or nitroprusside is that their venodilating effect may raise intracranial pressure. Clonidine may induce central nervous system depression, which can complicate interpretation of the mental status of a patient with an acute neurologic event. Sublingual nifedipine should be avoided because of its well‐documented tendency to cause overcorrection of blood pressure.26 No large direct comparison trial has evaluated which of these antihypertensive agents is superior in clinical outcomes. For the hospitalist, choice of antihypertensive agent should be driven by a need for rapid control of blood pressure to target without overcorrection (see Table 3). Issues such as intracranial pressure, heart rate, and comorbidities may also play a role in choice of medication.
| Not eligible for thrombolytic therapy | |
|---|---|
| |
| Systolic < 220 or diastolic < 120 | Observe unless other end‐organ involvement (aortic dissection, acute myocardial infarction, pulmonary edema, hypertensive encephalopathy) |
| Systolic > 220 or diastolic 121‐140 | Aim for 10%‐15% reduction in blood pressure |
| Labetalol 10‐20 mg IV over 1‐2 minutes; repeat or double every 10 minutes; maximum 300 mg | |
| or | |
| Nicardipine 5 mg/hour IV; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| or | |
| Nitroprusside 0.5 g/kg/minute IV; titrate to goal | |
| Diastolic > 140 | Aim for 10%‐15% reduction in blood pressure |
| Nitroprusside 0.5 g/kg/minute IV; titrate to desired blood pressure | |
| Eligible for thrombolytic therapy | |
| Pretreatment | |
| Systolic > 185 or diastolic > 110 | Labetalol 10‐20mg IV |
| May repeat 1 or Nitropaste 1‐2 inches | |
| During and after tPA | |
| Systolic 180‐230 or Diastolic 105‐120 | Labetalol 10 mg |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute | |
| Systolic > 230 or diastolic 121‐140 | Labetalol 10mg IV |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute or | |
| Nicardipine 5 mg/hour; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| If not at goal with labetalol or nicardipine, consider nitroprusside | |
| Diastolic>140 | Nitroprusside 0.5 g/kg/minute IV infusion; titrate to desired blood pressure |
The risk of hemorrhagic conversion is greater when thrombolysis is used for the treatment of cerebrovascular accident, in the NINDS trial rising from 0.6% in patients receiving a placebo to 6.4% in patients receiving tPA.27 Given that the goal of thrombolysis is to restore perfusion through the previously blocked vessel and that the risk of hemorrhage rises after thrombolysis, the balance between maintaining CPP and decreasing the risk of bleeding shifts toward maintaining a lower blood pressure postthrombolysis. The NINDS thrombolytic trial required patients to have a blood pressure less than 185/110 mm Hg to be included.27 Once a thrombolytic had been administered, the permitted maximum blood pressure dropped to 180/105 mm Hg. Blood pressure needs to be monitored closely, initially every 15 minutes for 2 hours, then every 30 minutes for the next 6 hours, and then hourly until the end of the first 24 hours. After this, blood pressure monitoring intervals can be more spaced out depending on the need for active antihypertensive therapy.28 However, arterial punctures for invasive monitoring are not recommended if thrombolytics are administered. Review of the experience of the NINDS trial suggests that approximately one third of patients receiving thrombolytics will require pharmacologic therapy to reach the recommended blood pressure goals in the first 24 hours.28
Although guidelines for the management of blood pressure after cerebrovascular accident from major organizations such as the American Stroke Association and the European Stroke Initiative are published and widely quoted in reviews of stroke management, physician adherence to these guidelines is poor.1, 2933 A 2002 review of prescribing practices in a Canadian hospital found excessive reduction of blood pressure in 60% of patients where nitroglycerin was used, and sublingual nifedipine was still the second most commonly prescribed medication.34 A similar 2004 study by Lindenauer et al. found that only 26% of patients who had antihypertensive medication initiated in the hospital met consensus guidelines for therapy.35
Induced Hypertension
Hypotension should be avoided after acute stroke. Observational studies have demonstrated an increased mortality rate of patients who present with hypotension.42 Patients with acute ischemic stroke and loss of autoregulation may have impaired tolerance to even mild levels of hypotension.8 First‐line therapy for hypotension after stroke is volume resuscitation and optimization of cardiac function by correcting arrhythmias.1 If this should fail, vasopressor support is advisable to avoid ongoing hypotension and cerebral hypoperfusion.
The same physiologic arguments that favor permissively managing high blood pressure and avoiding hypotension raise the question of whether inducing hypertension through the use of vasopressors might improve outcomes of stroke. This hypothesis has been bolstered by animal studies and imaging data that suggest improved perfusion to the area of injury with vasopressor‐induced hypertension.3638 However, there are concerns that this strategy may increase edema and hemorrhage and create the potential for vasopressor‐induced myocardial ischemia or arrhythmias. Although the results of small trials have appeared promising, particularly for patients with large vessel stenosis, at this point induced hypertension is still considered experimental until the results of larger randomized, controlled trials provide greater support for this procedure.1, 3941
Chronic Blood Pressure Control for Secondary Prevention of Stroke
Multiple trials have demonstrated that interventions to treat chronic hypertension can reduce the rate of future strokes.14, 43, 44 The PROGRESS trial demonstrated that blood pressure reduction with combination therapy decreased stroke recurrence by 43%.43 Clearly long‐term blood pressure control is a vital component of secondary prevention of stroke. Based on the results of the PROGRESS and ACCESS trials, it is suggested that angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers, potentially with additional diuretic therapy, are beneficial in the treatment of chronic hypertension following cerebrovascular accident.25, 42 The issue of optimal medication class is not settled. Recommendations are to treat chronically elevated blood pressure to lower and maintain it at less than 140/80 mm Hg. Because there is no single threshold level of blood pressure beyond which the risk of cerebrovascular disease increases, reduction below this cut point may still offer incremental benefit.13, 45, 46 The timing of initiation of therapy to reach secondary‐prevention goals is not addressed in current guidelines, although some authors recommend waiting days to weeks after an acute stroke before starting therapy.1, 37 For a patient who remains hypertensive at discharge, it is incumbent on the hospitalist to communicate the plan for initiation of antihypertensive agents to the patient and the primary care physician in order to ensure that this critical secondary prevention measure is addressed.
Areas of Ambiguity and Need for Future Research
Within an evidence‐guided practice, there are still many areas for which the evidence to date does not answer important clinical questions about patient management. Whether a patient's prestroke baseline blood pressure should be considered in individualizing blood pressure goals is unclear, as is the question of whether to continue or hold previous chronic antihypertensive therapy. Given the many stroke subtypes, from tiny lacunar infarcts with little collateralization to massive malignant middle‐cerebral infarctions, should blood pressure management be individualized according to the likely size and duration of the peri‐infarct penumbra? Research is needed to determine whether reducing blood pressure to a specific target will improve outcome and the optimal timing of therapy to achieve secondary prevention goals. Finally, using the concept of the penumbra as the theoretical physiologic basis for current blood pressure recommendations, there exists a potential for rapidly evolving neuroimaging techniques to help define the presence, size, and duration of the penumbra to guide these decisions.
CONCLUSIONS
Management of blood pressure after acute ischemic stroke differs from that of many other hypertensive conditions because of the need to preserve perfusion of the peri‐infarct penumbra. Evidence to date suggests little benefit and potential harm from acutely lowering blood pressure after stroke, although there have not been large randomized trials examining outcomes when blood pressure is lowered to a prespecified goal. Current consensus guidelines suggest that blood pressure may be managed permissively up to 220/120 mm Hg when thrombolysis is not administered and to 180/105 mm Hg when thrombolysis is performed. Data suggest that low‐dose antihypertensive therapy such as ACE inhibitors or angiotensin receptor blockers may be safe in the acute setting if pressure is reduced by less than 10%‐15%.17, 25 However, the evidence is not sufficiently strong for this practice to be routinely recommended. At this point, it is recommended that hypotension be aggressively treated; however, induced hypertension remains a promising but unproven therapy. Evidence is strong that long‐term blood pressure control is key to secondary prevention of stroke, but the timing of its initiation remains poorly determined.
Hospitalists are on the front lines of care for patients with cerebrovascular accidents. After the first steps in acute stroke management occur in the emergency room, care is frequently transferred to the hospitalist. This review focuses on the evidence‐based management of blood pressure following acute ischemic stroke. Management of blood pressure after stroke is still controversial, and current consensus statement guidelines acknowledge that optimal treatment has not yet been established.1, 2 As such, it is essential to understand the changes in normal homeostatic physiologic processes that occur after stroke and their subsequent effects on neurologic function. Only then can the appropriate blood pressure target and antihypertensive regimen be chosen.
Physiology of Cerebral Perfusion
Once a stroke has occurred and perfusion to a section of brain tissue has been acutely compromised, systemic pressure tends to rise. This rise is presumably due in part to increased adrenergic tone and activation of the renin‐aldosterone system and potentially is part of Cushing's reflex in cases in which intracranial pressure is elevated.3 The increase in mean arterial pressure may represent a protective response. In the first major study on the topic, in 1981, on admission for stroke mean blood pressure was 163/90 mm Hg for patients without a history of hypertension and 214/118 mm Hg for patients who had been treated for hypertension previously.4 This rise in blood pressure in response to endogenous mechanisms is attenuated over the first 24 hours, and even without intervention, blood pressure tends to fall spontaneously over the next 10 days.47
Under normal circumstances, cerebral blood flow (CBF) is tightly autoregulated across a wide range of cerebral perfusion pressures by alteration in cerebrovascular resistance via arteriolar constriction.89 This allows CBF to remain constant even if cerebral perfusion pressure (CPP) fluctuates from 60 to 150 mm Hg.9 In patients with chronic hypertension, autoregulation works best with blood pressure in a higher range because of vascular smooth muscle hypertrophy and structural changes in the cerebral vessels (see Table 1).
| Cerebral blood flow (CBF) of 50‐70 mL/100 g/minnormal |
| CBF of 20‐50 mL/100 g/minreduced flow compensated for by increased oxygen extraction |
| CBF of 15‐20 mL/100 g/minneuronal quiescence |
| CBF < 15 mL/100 g/minneuronal death |
After an acute ischemic stroke, autoregulation is lost, and local CBF becomes linearly associated with cerebral perfusion pressure. Loss of autoregulation occurs because of several local factors as a result of the infarct. Acidosis and hypoxia in the region of the stroke lead to vasodilatation in the perfusing vessels.9 This may improve local circulation in collateral vessels as a response to the obstruction in the primary supplying artery, but the consequence of maximal vasodilatation is loss of the ability to autoregulate. Normal CPP is driven by the mean arterial pressure minus the intracranial pressure. However, if intracranial vessels have lost the ability to accommodate changes in perfusion pressure, then blood flow to the area of injury becomes linearly correlated with mean arterial pressure.
Surrounding a central core of irreversible necrosis may be a zone of at‐risk tissue that is susceptible to reduction below the threshold of viability in response to any decrement in systemic mean blood pressure.11 This critical concept in stroke management is referred to as the peri‐infarct penumbra (see Fig. 1 and Table 2).

| Mean arterial pressure (MAP) = ⅔ diastolic blood pressure (DBP) + ⅓ systolic blood pressure (SBP) |
| Cerebral perfusion pressure (CPP) = mean arterial pressure (MAP) intracranial pressure (ICP) |
| Cerebral blood flow (CBF) = cerebral perfusion pressure (CPP)/cerebrovascular resistance (CVR) |
Blood Pressure Management after Stroke
The presence of a systemic blood pressure‐dependent peri‐infarct penumbra that might be compromised by blood pressure reduction and thus extend the infarct is the principal argument for allowing permissive hypertension. This is bolstered by the observation that decreases in blood pressure in the first 24 hours are associated with a significant risk of poor neurologic outcome.6 In their an observational study Vlcek et al. found that a greater than 25% drop in diastolic blood pressure (DBP) was associated with a 4‐fold risk of severe disability.5 In a 2003 observational study Oliveria et al. found that reduction in systolic blood pressure (SBP) in the first 24 hours was independently associated with poor outcomes, with a doubling of the risk for poor outcomes for every 10% drop in systolic blood pressure.6 In both these studies, the worsened outcome did not depend on whether the drop was spontaneous or induced by medications. Case reports suggest that large drops in blood pressure can be catastrophic and that even moderate lowering of blood pressure after acute stroke can be associated with clinical deterioration.8, 12
The primary argument for lowering blood pressure after an acute stroke hinges on secondary prevention of new ischemic events, minimization of cerebral edema, and prevention of hemorrhagic conversion. It has long been recognized that hypertension itself is a risk factor for stroke13 and that reduction in chronic hypertension is part of secondary prevention for cerebrovascular accidents.14 Further, hypertension is the most common risk factor for intracerebral hemorrhage, and it would stand to reason that damage to the brain parenchyma from ischemic stroke would increase the risk of pressure‐induced bleeding in an acute setting.15
Because there are compelling theoretical arguments both for and against lowering blood pressure after an acute stroke, it is necessary to look at the results of randomized clinical trials for guidance in weighing the risks and benefits. The overall goal of blood pressure management is to maximize perfusion to the ischemic penumbra while minimizing the hypertensive risk of hemorrhagic transformation.
Lisk et al. conducted a small randomized trial in 1993 of antihypertensive therapy versus placebo after ischemic stroke looking at SPECT perfusion and found lower CBF if mean arterial pressure (MAP) dropped more than 16%.16 This is in contrast with the results of a 1997 randomized trial of perindopril after cerebrovascular accident that found no decrease in Doppler CBF despite a 10% decrease in blood pressure in the active treatment group.17 Neither study was powered to detect significant differences in clinical outcome.
Early randomized controlled trials of antihypertensive agents after acute stroke investigated neuroprotection via mechanisms other than the antihypertensive effect. Nimodipine, a dihydropyridine derivative thought to prevent neuronal death via blockade of calcium channels, has been studied extensively.1820 A meta‐analysis of the 9 early trials of nimodipine, from 1988 to 1992, suggested that nimodipine was potentially beneficial in neurologic score and functional outcome only if used within the first 12 hours and that it could be harmful if started after 24 hours.21 Two additional studies were done in 1994 using both intravenous and oral nimodipine formulations. They demonstrated worsened neurologic function and higher mortality, respectively, which was hypothesized to be a result of the detrimental hemodynamic effects of nimodipine.18, 22
The BEST trial used beta‐blockers in the early period after acute stroke and failed to find benefit, whereas the FIST trial had similar results with the calcium channel blocker flunarizine.23, 24 The ACCESS trial in 2003 was a prospective, randomized, controlled trial using oral candesartan in the first 24 hours after stroke.25 It did find improved mortality in the candesartan arm after 1 year, yet there were no significant differences in blood pressure between candesartan and placebo. Thus, the improved outcome was presumed to be a result of mechanisms other than antihypertensive effect.
Some significant caveats should be kept in mind when interpreting the results of these studies. None of the trials was designed to titrate blood pressure to a prespecified goal in a prospective randomized fashion. It is also difficult to tease out the effect of the active medical intervention from the effect of spontaneous drops in blood pressure, and many trials did not find a significant difference in blood pressure between the medication and placebo groups. A large randomized trial to evaluate interventions to a predefined blood pressure target is needed.
The Cochrane Stroke Group reviewed 32 trials involving 5368 patients and concluded there was not enough evidence to reliably evaluate the effect of altering blood pressure on outcomes.3
Recommendations for Treatment of Hypertension in Acute Stroke
The decision of when to initiate antihypertensive therapy has not been clearly delineated. However, most experts agree that blood pressure targets need to take into account whether thrombolytics are used. The available data provide little evidence that lowering blood pressure decreases adverse events; however, there is some evidence that lowering blood pressure can worsen outcomes by expanding the area of ischemia. Thus, in treating most acute ischemic strokes, antihypertensive therapy can and should be withheld. The exception to this is stroke in a patient with comorbid hypertensive organ damage such as myocardial infarction, aortic dissection, acute hypertensive renal failure, pulmonary edema, or encephalopathy. The consensus statement of the American Stroke Association is to withhold treatment until blood pressure exceeds 220/120 mm Hg,1 roughly corresponding to a MAP of 150 mm Hg, the normal upper limit of cerebral autoregulation. Treatment optimally should be titratable in order to avoid overcorrection, preferably with minimal cerebral venodilatation effect. The parenteral agents labetolol, fenoldopam, nicardipine, and nitroprusside are most commonly used. A disadvantage of nitrates or nitroprusside is that their venodilating effect may raise intracranial pressure. Clonidine may induce central nervous system depression, which can complicate interpretation of the mental status of a patient with an acute neurologic event. Sublingual nifedipine should be avoided because of its well‐documented tendency to cause overcorrection of blood pressure.26 No large direct comparison trial has evaluated which of these antihypertensive agents is superior in clinical outcomes. For the hospitalist, choice of antihypertensive agent should be driven by a need for rapid control of blood pressure to target without overcorrection (see Table 3). Issues such as intracranial pressure, heart rate, and comorbidities may also play a role in choice of medication.
| Not eligible for thrombolytic therapy | |
|---|---|
| |
| Systolic < 220 or diastolic < 120 | Observe unless other end‐organ involvement (aortic dissection, acute myocardial infarction, pulmonary edema, hypertensive encephalopathy) |
| Systolic > 220 or diastolic 121‐140 | Aim for 10%‐15% reduction in blood pressure |
| Labetalol 10‐20 mg IV over 1‐2 minutes; repeat or double every 10 minutes; maximum 300 mg | |
| or | |
| Nicardipine 5 mg/hour IV; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| or | |
| Nitroprusside 0.5 g/kg/minute IV; titrate to goal | |
| Diastolic > 140 | Aim for 10%‐15% reduction in blood pressure |
| Nitroprusside 0.5 g/kg/minute IV; titrate to desired blood pressure | |
| Eligible for thrombolytic therapy | |
| Pretreatment | |
| Systolic > 185 or diastolic > 110 | Labetalol 10‐20mg IV |
| May repeat 1 or Nitropaste 1‐2 inches | |
| During and after tPA | |
| Systolic 180‐230 or Diastolic 105‐120 | Labetalol 10 mg |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute | |
| Systolic > 230 or diastolic 121‐140 | Labetalol 10mg IV |
| May repeat or double labetalol every 10‐20 minutes to a maximum dose of 300 mg or give bolus and start drip at 2‐8 mg/minute or | |
| Nicardipine 5 mg/hour; titrate by 2.5 mg every 5 minutes; maximum 15 mg/hour | |
| If not at goal with labetalol or nicardipine, consider nitroprusside | |
| Diastolic>140 | Nitroprusside 0.5 g/kg/minute IV infusion; titrate to desired blood pressure |
The risk of hemorrhagic conversion is greater when thrombolysis is used for the treatment of cerebrovascular accident, in the NINDS trial rising from 0.6% in patients receiving a placebo to 6.4% in patients receiving tPA.27 Given that the goal of thrombolysis is to restore perfusion through the previously blocked vessel and that the risk of hemorrhage rises after thrombolysis, the balance between maintaining CPP and decreasing the risk of bleeding shifts toward maintaining a lower blood pressure postthrombolysis. The NINDS thrombolytic trial required patients to have a blood pressure less than 185/110 mm Hg to be included.27 Once a thrombolytic had been administered, the permitted maximum blood pressure dropped to 180/105 mm Hg. Blood pressure needs to be monitored closely, initially every 15 minutes for 2 hours, then every 30 minutes for the next 6 hours, and then hourly until the end of the first 24 hours. After this, blood pressure monitoring intervals can be more spaced out depending on the need for active antihypertensive therapy.28 However, arterial punctures for invasive monitoring are not recommended if thrombolytics are administered. Review of the experience of the NINDS trial suggests that approximately one third of patients receiving thrombolytics will require pharmacologic therapy to reach the recommended blood pressure goals in the first 24 hours.28
Although guidelines for the management of blood pressure after cerebrovascular accident from major organizations such as the American Stroke Association and the European Stroke Initiative are published and widely quoted in reviews of stroke management, physician adherence to these guidelines is poor.1, 2933 A 2002 review of prescribing practices in a Canadian hospital found excessive reduction of blood pressure in 60% of patients where nitroglycerin was used, and sublingual nifedipine was still the second most commonly prescribed medication.34 A similar 2004 study by Lindenauer et al. found that only 26% of patients who had antihypertensive medication initiated in the hospital met consensus guidelines for therapy.35
Induced Hypertension
Hypotension should be avoided after acute stroke. Observational studies have demonstrated an increased mortality rate of patients who present with hypotension.42 Patients with acute ischemic stroke and loss of autoregulation may have impaired tolerance to even mild levels of hypotension.8 First‐line therapy for hypotension after stroke is volume resuscitation and optimization of cardiac function by correcting arrhythmias.1 If this should fail, vasopressor support is advisable to avoid ongoing hypotension and cerebral hypoperfusion.
The same physiologic arguments that favor permissively managing high blood pressure and avoiding hypotension raise the question of whether inducing hypertension through the use of vasopressors might improve outcomes of stroke. This hypothesis has been bolstered by animal studies and imaging data that suggest improved perfusion to the area of injury with vasopressor‐induced hypertension.3638 However, there are concerns that this strategy may increase edema and hemorrhage and create the potential for vasopressor‐induced myocardial ischemia or arrhythmias. Although the results of small trials have appeared promising, particularly for patients with large vessel stenosis, at this point induced hypertension is still considered experimental until the results of larger randomized, controlled trials provide greater support for this procedure.1, 3941
Chronic Blood Pressure Control for Secondary Prevention of Stroke
Multiple trials have demonstrated that interventions to treat chronic hypertension can reduce the rate of future strokes.14, 43, 44 The PROGRESS trial demonstrated that blood pressure reduction with combination therapy decreased stroke recurrence by 43%.43 Clearly long‐term blood pressure control is a vital component of secondary prevention of stroke. Based on the results of the PROGRESS and ACCESS trials, it is suggested that angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers, potentially with additional diuretic therapy, are beneficial in the treatment of chronic hypertension following cerebrovascular accident.25, 42 The issue of optimal medication class is not settled. Recommendations are to treat chronically elevated blood pressure to lower and maintain it at less than 140/80 mm Hg. Because there is no single threshold level of blood pressure beyond which the risk of cerebrovascular disease increases, reduction below this cut point may still offer incremental benefit.13, 45, 46 The timing of initiation of therapy to reach secondary‐prevention goals is not addressed in current guidelines, although some authors recommend waiting days to weeks after an acute stroke before starting therapy.1, 37 For a patient who remains hypertensive at discharge, it is incumbent on the hospitalist to communicate the plan for initiation of antihypertensive agents to the patient and the primary care physician in order to ensure that this critical secondary prevention measure is addressed.
Areas of Ambiguity and Need for Future Research
Within an evidence‐guided practice, there are still many areas for which the evidence to date does not answer important clinical questions about patient management. Whether a patient's prestroke baseline blood pressure should be considered in individualizing blood pressure goals is unclear, as is the question of whether to continue or hold previous chronic antihypertensive therapy. Given the many stroke subtypes, from tiny lacunar infarcts with little collateralization to massive malignant middle‐cerebral infarctions, should blood pressure management be individualized according to the likely size and duration of the peri‐infarct penumbra? Research is needed to determine whether reducing blood pressure to a specific target will improve outcome and the optimal timing of therapy to achieve secondary prevention goals. Finally, using the concept of the penumbra as the theoretical physiologic basis for current blood pressure recommendations, there exists a potential for rapidly evolving neuroimaging techniques to help define the presence, size, and duration of the penumbra to guide these decisions.
CONCLUSIONS
Management of blood pressure after acute ischemic stroke differs from that of many other hypertensive conditions because of the need to preserve perfusion of the peri‐infarct penumbra. Evidence to date suggests little benefit and potential harm from acutely lowering blood pressure after stroke, although there have not been large randomized trials examining outcomes when blood pressure is lowered to a prespecified goal. Current consensus guidelines suggest that blood pressure may be managed permissively up to 220/120 mm Hg when thrombolysis is not administered and to 180/105 mm Hg when thrombolysis is performed. Data suggest that low‐dose antihypertensive therapy such as ACE inhibitors or angiotensin receptor blockers may be safe in the acute setting if pressure is reduced by less than 10%‐15%.17, 25 However, the evidence is not sufficiently strong for this practice to be routinely recommended. At this point, it is recommended that hypotension be aggressively treated; however, induced hypertension remains a promising but unproven therapy. Evidence is strong that long‐term blood pressure control is key to secondary prevention of stroke, but the timing of its initiation remains poorly determined.
- ,,, et al.Guidelines for the early management of patients with ischemic stroke.Stroke.2003;34:1056–1083.
- ,,,.Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update.Stroke.2005;36:916–921.
- Blood Pressure in Acute Stroke Collaboration (BASC).Vasoactive drugs for acute strokeCochrane Database Syst Rev.2004;1:CD002839.
- ,.Blood pressure after stroke.JAMA.1981;246:2177–2180.
- ,,,,,.Association between course of blood pressure within the first 24 hours and functional recovery after acute ischemic stroke.Ann Emerg Med.2003;42:619–626.
- ,,,,,.Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset.Neurology.2003;61:1047–1050.
- ,,,, et al.Blood pressure during the first minutes of focal cerebral ischemia.Ann Emerg Med.1993;22:1438–1443.
- ,,,,.Stroke precipitated by moderate blood pressure reduction.J Emerg Med.2000;19:339–346.
- .Hemodynamics and metabolism in ischemic cerebrovascular disease.Neurologic Clinics.1992;10(1):31–48.
- ,.The 5 Ps of acute ischemic stroke treatment: parenchyma, pipes, perfusion, penumbra, and prevention of complications.South Med J.2003;96:336–341.
- .Viability Thresholds and the Penumbra of Focal Ischemia.Annals of Neurology.1994;36:557–565.
- ,,.Hazards of therapy for excessive hypertension in acute stroke.Acta Med Scand.1980;207:253–257.
- Prospective Studies Collaboration.Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies.Lancet.2002;360:1903–1913.
- ,,,.Secondary prevention of ischemic cerebrovascular disease. What is the evidence?Angiology.2005;56:539–552.
- ,,.Intracerebral hemorrhage.Emerg Med Clin North Am.2002;20:631–655.
- ,,, et al.Should hypertension be treated after acute stroke?Arch Neurol.1993;50:855–862.
- ,,.Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke.Stroke.1997;28:580–583.
- ,,,,.Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke.Cerebrovasc Dis.1994;4:204–210.
- The American Nimodipine Study Group.Clinical trial of nimodipine in acute ischemic stroke.Stroke.1992;23(1):3–8.
- ,,, et al.Placebo‐controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction.Stroke.1990;21:1023–1028.
- ,,, et al.Meta‐analysis of oral nimodipine trials in acute ischemic stroke.Cerebrovasc Dis.1994;4:197–203.
- ,,, et al.A randomized, double‐blind, placebo‐controlled trial of nimodipine in acute ischemic hemispheric stroke.Stroke.1994;25:1348–1353.
- ,,,.Low dose B blockade in acute stroke (“BEST” trial): an evaluationBMJ.1988;296:737–741.
- ,,, et al.Flunarizine in stroke treatment (FIST): a double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands.Acta Neurol Scand.1996;93:56–60.
- ,,, et al.The ACCESS study: Evaluation of acute candesartan cilexetil therapy in stroke survivors.Stroke.2003;34:1699–1703.
- .FDA gives Calcium channel blockers clean bill of health but warns of short‐acting nifedipine hazards.JAMA.1996;275:1638.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Hypertension and its treatment in the NINDS rt‐PA Stroke Trial.Stroke.1998;29:1504–1509.
- ,.Optimizing blood pressure in neurologic emergencies.Neurocrit Care.2004;1:287–299.
- .Management of acute stroke.South M J.2003;96:380–385.
- .Management of acute stroke.Lancet Neurol.2002;1(1):41–50.
- ,.Acute ischemic stroke: emergent evaluation and management.Emerg Med Clin North Am.2002;20:609–630.
- ,.Post‐emergency department management of stroke.Emerg Med Clin North Am.2002;20:687–702.
- ,,.Blood pressure management in acute stroke: comparison of current guidelines with prescribing patterns.Can J Neurol Sci.2002;29(2):125–131.
- ,,,,,.Use of antihypertensive agents in the management of patients with acute ischemic stroke.Neurology.2004;63:318–323.
- ,,,.Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats.Surg Neurol.1996;46:229–234.
- ,,,.Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke.Stroke.2002;33:998–1004.
- ,,,.Induced hypertension improves cerebral blood flow in acute ischemic stroke.Neurology.2005;64:1979.
- ,,,,.A pilot study of drug induced hypertension for treatment of acute stroke.Neurology.2001;56:1210–1213.
- ,,,,,.Pharmacologic elevation of blood pressure in acute stroke: clinical effects and safety.Stroke.1997;28:2133–2138.
- ,,,,.Feasibility and safety of norephinephrine‐induced arterial hypertension in acute ischemic stroke.Neurology.2004;62:1193–1195.
- ,,,,.Initial emergency department blood pressure as predictor of survival after acute ischemic stroke.Neurology.2005;65:1179–1183.
- The PROGRESS Collaborative Group.Randomized trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke of transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke.JAMA.2000;284:465–471.
- ,.Treatment of chronic hypertension for the prevention of stroke.South Med J.2003;96:359–362.
- ,,,.Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study.JAMA.1996;276:1269–1278.
- ,,, et al.Guidelines for the early management of patients with ischemic stroke.Stroke.2003;34:1056–1083.
- ,,,.Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update.Stroke.2005;36:916–921.
- Blood Pressure in Acute Stroke Collaboration (BASC).Vasoactive drugs for acute strokeCochrane Database Syst Rev.2004;1:CD002839.
- ,.Blood pressure after stroke.JAMA.1981;246:2177–2180.
- ,,,,,.Association between course of blood pressure within the first 24 hours and functional recovery after acute ischemic stroke.Ann Emerg Med.2003;42:619–626.
- ,,,,,.Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset.Neurology.2003;61:1047–1050.
- ,,,, et al.Blood pressure during the first minutes of focal cerebral ischemia.Ann Emerg Med.1993;22:1438–1443.
- ,,,,.Stroke precipitated by moderate blood pressure reduction.J Emerg Med.2000;19:339–346.
- .Hemodynamics and metabolism in ischemic cerebrovascular disease.Neurologic Clinics.1992;10(1):31–48.
- ,.The 5 Ps of acute ischemic stroke treatment: parenchyma, pipes, perfusion, penumbra, and prevention of complications.South Med J.2003;96:336–341.
- .Viability Thresholds and the Penumbra of Focal Ischemia.Annals of Neurology.1994;36:557–565.
- ,,.Hazards of therapy for excessive hypertension in acute stroke.Acta Med Scand.1980;207:253–257.
- Prospective Studies Collaboration.Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies.Lancet.2002;360:1903–1913.
- ,,,.Secondary prevention of ischemic cerebrovascular disease. What is the evidence?Angiology.2005;56:539–552.
- ,,.Intracerebral hemorrhage.Emerg Med Clin North Am.2002;20:631–655.
- ,,, et al.Should hypertension be treated after acute stroke?Arch Neurol.1993;50:855–862.
- ,,.Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke.Stroke.1997;28:580–583.
- ,,,,.Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke.Cerebrovasc Dis.1994;4:204–210.
- The American Nimodipine Study Group.Clinical trial of nimodipine in acute ischemic stroke.Stroke.1992;23(1):3–8.
- ,,, et al.Placebo‐controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction.Stroke.1990;21:1023–1028.
- ,,, et al.Meta‐analysis of oral nimodipine trials in acute ischemic stroke.Cerebrovasc Dis.1994;4:197–203.
- ,,, et al.A randomized, double‐blind, placebo‐controlled trial of nimodipine in acute ischemic hemispheric stroke.Stroke.1994;25:1348–1353.
- ,,,.Low dose B blockade in acute stroke (“BEST” trial): an evaluationBMJ.1988;296:737–741.
- ,,, et al.Flunarizine in stroke treatment (FIST): a double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands.Acta Neurol Scand.1996;93:56–60.
- ,,, et al.The ACCESS study: Evaluation of acute candesartan cilexetil therapy in stroke survivors.Stroke.2003;34:1699–1703.
- .FDA gives Calcium channel blockers clean bill of health but warns of short‐acting nifedipine hazards.JAMA.1996;275:1638.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Hypertension and its treatment in the NINDS rt‐PA Stroke Trial.Stroke.1998;29:1504–1509.
- ,.Optimizing blood pressure in neurologic emergencies.Neurocrit Care.2004;1:287–299.
- .Management of acute stroke.South M J.2003;96:380–385.
- .Management of acute stroke.Lancet Neurol.2002;1(1):41–50.
- ,.Acute ischemic stroke: emergent evaluation and management.Emerg Med Clin North Am.2002;20:609–630.
- ,.Post‐emergency department management of stroke.Emerg Med Clin North Am.2002;20:687–702.
- ,,.Blood pressure management in acute stroke: comparison of current guidelines with prescribing patterns.Can J Neurol Sci.2002;29(2):125–131.
- ,,,,,.Use of antihypertensive agents in the management of patients with acute ischemic stroke.Neurology.2004;63:318–323.
- ,,,.Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats.Surg Neurol.1996;46:229–234.
- ,,,.Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke.Stroke.2002;33:998–1004.
- ,,,.Induced hypertension improves cerebral blood flow in acute ischemic stroke.Neurology.2005;64:1979.
- ,,,,.A pilot study of drug induced hypertension for treatment of acute stroke.Neurology.2001;56:1210–1213.
- ,,,,,.Pharmacologic elevation of blood pressure in acute stroke: clinical effects and safety.Stroke.1997;28:2133–2138.
- ,,,,.Feasibility and safety of norephinephrine‐induced arterial hypertension in acute ischemic stroke.Neurology.2004;62:1193–1195.
- ,,,,.Initial emergency department blood pressure as predictor of survival after acute ischemic stroke.Neurology.2005;65:1179–1183.
- The PROGRESS Collaborative Group.Randomized trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke of transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke.JAMA.2000;284:465–471.
- ,.Treatment of chronic hypertension for the prevention of stroke.South Med J.2003;96:359–362.
- ,,,.Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study.JAMA.1996;276:1269–1278.
Suppurative complications and upper airway obstruction in infectious mononucleosis
A 17‐year‐old female patient presented to the emergency department reporting having fever, sore throat, and pain with swallowing for several days. The result of her rapid strep screen was negative. She had an elevated white blood cell count, mildly elevated AST and ALT levels, and a positive result from a heterophile antibody test (BBL Monoslide). She was diagnosed with infectious mononucleosis. Given her inability to tolerate oral fluids, she was admitted to the hospital for intravenous hydration. After 3 days of receiving methylprednisolone intravenously, she had worsening throat pain, progressive neck swelling, difficulty handling her secretions, and new respiratory symptoms. During the examination, she was sitting upright in bed in moderate respiratory distress. She had kissing, exudative tonsils with palatal and uvular edema. Examination of her neck showed significantly enlarged anterior and posterior cervical lymph nodes without fluctuance. Her lung exam revealed subcostal retractions with transmitted upper airway sounds but good aeration. The edge of her liver and spleen tip were palpable.
Because of the rapid progression of symptoms while on medical therapy, computed tomography (CT) of the neck was performed. Sagittal reconstructions showed adenoidal hypertrophy compromising her nasopharynx, and massive tonsillar enlargement causing nearly complete obstruction of her oropharyngeal airway (Fig. 1), with airway narrowing to less than 2.5 mm in axial images. Bilateral low‐density lesions within the paratonsillar regions were suggestive of abscesses and retropharyngeal soft‐tissue swelling was consistent with phlegmon (Fig. 2). The patient was taken to the operating room for an emergent tonsillectomy. Bilateral peritonsillar abscesses were drained, pus was sent for culture, and her tonsils were excised. Cultures from the abscesses grew Streptococcus milleri. The patient was discharged home 2 days later to complete a 2‐week course of oral clindamycin.
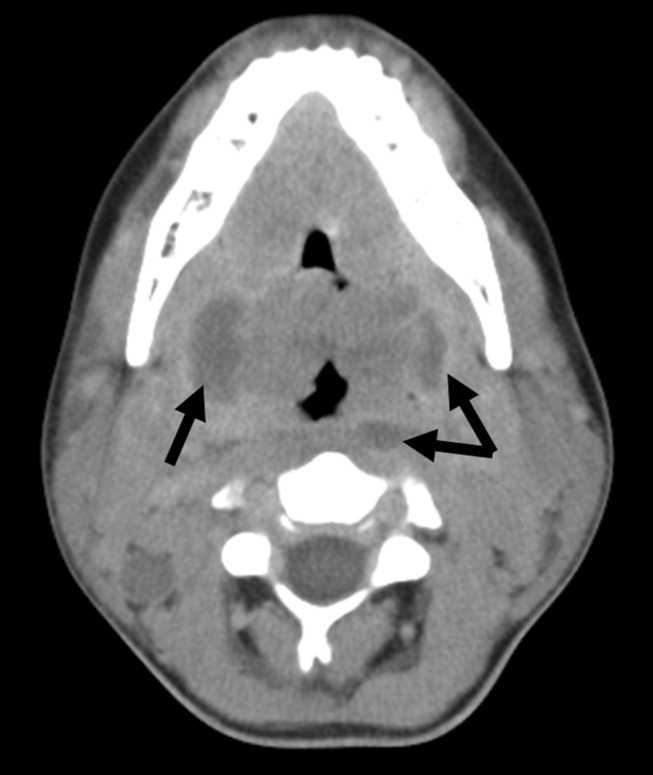
Most patients with infectious mononucleosis (IM) have a benign, self‐limited course. However, a wide range of severe complications have been described including airway obstruction, splenic rupture, meningoencephalitis, Guillain‐Barr syndrome, peritonsillar abscess, and hemolytic anemia.1, 2 Upper‐airway obstruction results from lymphoid hyperplasia throughout Waldeyer's ring, with associated soft‐tissue edema. As many as 25% of patients hospitalized for IM will have some degree of airway obstruction.2, 3 Peritonsillar abscesses (PTAs) occur in approximately 1% of hospitalized patients with IM and may further obstruct the airway.4 Most commonly, peritonsillar abscesses are polymicrobial, having both aerobic and anaerobic bacteria. In a study of young adults with peritonsillar abscesses from all causes, Streptococcus pyogenes was the most common aerobe, found in nearly half of isolates; Streptococcus milleri, the bacterium isolated from our patient, was the second most common organism, found in approximately 25% of these abscesses.5 One prior case of airway obstruction from IM complicated by bilateral peritonsillar abscesses has been reported6; however, this patient was not reported to have concomitant retropharyngeal infection, as was noted in our patient.
Guidelines suggest that patients with mild, uncomplicated IM should be managed with supportive care alone; current recommendations are that steroids be prescribed only for specific complications of IM, including upper‐airway obstruction.7 Protocols for steroid regimens include initial prednisone doses ranging from 20 to 80 mg/day, with most advocating that they be tapered off over 1‐2 weeks.7, 8
Some of the controversy about the routine use of steroids in IM is related to concern for potential infectious complications associated with immunosuppression. In a small case series, Handler et al. proposed that there is an association between steroid therapy and the development of peritonsillar abscesses.9 However, this has not been tested in controlled trials, and the results of more recent studies do not support an increased likelihood of PTA in patients with infectious mononucleosis treated with corticosteroids.10, 11 Therefore, given that it has documented benefits and no proven adverse consequences, steroid therapy is uniformly recommended for patients with upper‐airway obstruction secondary to infectious mononucleosis. However, use of steroids also mandates careful monitoring for signs or symptoms suggestive of secondary bacterial infection.
For patients whose symptoms progress despite medical management including steroid therapy, surgical intervention may be required. Both tracheostomy and tonsillectomy during acute infection, sometimes referred to as hot tonsillectomy, have been reported as surgical options for airway obstruction in IM, the latter having emerged as the preferred treatment.3 Such treatment allows for drainage of any infectious collections, as well as removal of obstructing lymphoid tissue as indicated.
In conclusion, enlargement of tonsils and adenoids with associated edema in infectious mononucleosis can lead to upper‐airway obstruction. Patients with evidence of such obstruction should be treated with a tapering course of corticosteroids. Peritonsillar abscesses and deep neck infections are also severe complications of IM and can cause further respiratory compromise. In cases where medical therapy is not effective, such as with our patient, evaluation for peritonsillar abscess and need for possible acute tonsillectomy may be required.
- .Acute complications of Epstein‐Barr virus infectious mononucleosis.Curr Opin Pediatr.2000;12(3):263–268.
- ,.Complications of infection with Epstein‐Barr virus during childhood: a study of children admitted to the hospital.Pediatr Infect Dis.1984;3:304–307.
- ,.The management of severe infectious mononucleosis tonsillitis and upper airway obstruction.J Laryngol Otol.2001;115:973–977.
- ,,.Otolaryngological complications in infectious mononucleosis.J Laryngol Otol.1984;98:999–1001.
- ,,.Bacteriologic findings in peritonsillar abscesses in young adults.Clin Infect Dis.1993;16(suppl 4):S292–S298.
- ,.Infectious mononucleosis and bilateral peritonsillar abscesses resulting in airway obstruction.J Laryngol Otol.1998;112:1186–1188.
- ,,, et al.Guidelines for the use of systemic glucocorticosteroids in the management of selected infections.Working Group on Steroid Use, Antimicrobial Agents Committee, Infectious Diseases Society of America.J Infect Dis.1992;165(1):1–13.
- ,Narula AA Steroids for airway problems in glandular fever.J Laryngol Otol.1987;10:673–675.
- ,.Peritonsillar abscess: a complication of corticosteroid treatment in infectious mononucleosis.Int J Pediatr Otorhinolaryngol.1979;1(3):265–268.
- ,,.Corticosteroids and peritonsillar abscess formation in infectious mononucleosis.J Laryngol Otol.2004;118:459–461.
- ,,.Otolaryngologic Clinical Patterns in Pediatric Infectious Mononucleosis.Am J Otolaryngol.1996;17:397–400.
A 17‐year‐old female patient presented to the emergency department reporting having fever, sore throat, and pain with swallowing for several days. The result of her rapid strep screen was negative. She had an elevated white blood cell count, mildly elevated AST and ALT levels, and a positive result from a heterophile antibody test (BBL Monoslide). She was diagnosed with infectious mononucleosis. Given her inability to tolerate oral fluids, she was admitted to the hospital for intravenous hydration. After 3 days of receiving methylprednisolone intravenously, she had worsening throat pain, progressive neck swelling, difficulty handling her secretions, and new respiratory symptoms. During the examination, she was sitting upright in bed in moderate respiratory distress. She had kissing, exudative tonsils with palatal and uvular edema. Examination of her neck showed significantly enlarged anterior and posterior cervical lymph nodes without fluctuance. Her lung exam revealed subcostal retractions with transmitted upper airway sounds but good aeration. The edge of her liver and spleen tip were palpable.
Because of the rapid progression of symptoms while on medical therapy, computed tomography (CT) of the neck was performed. Sagittal reconstructions showed adenoidal hypertrophy compromising her nasopharynx, and massive tonsillar enlargement causing nearly complete obstruction of her oropharyngeal airway (Fig. 1), with airway narrowing to less than 2.5 mm in axial images. Bilateral low‐density lesions within the paratonsillar regions were suggestive of abscesses and retropharyngeal soft‐tissue swelling was consistent with phlegmon (Fig. 2). The patient was taken to the operating room for an emergent tonsillectomy. Bilateral peritonsillar abscesses were drained, pus was sent for culture, and her tonsils were excised. Cultures from the abscesses grew Streptococcus milleri. The patient was discharged home 2 days later to complete a 2‐week course of oral clindamycin.


Most patients with infectious mononucleosis (IM) have a benign, self‐limited course. However, a wide range of severe complications have been described including airway obstruction, splenic rupture, meningoencephalitis, Guillain‐Barr syndrome, peritonsillar abscess, and hemolytic anemia.1, 2 Upper‐airway obstruction results from lymphoid hyperplasia throughout Waldeyer's ring, with associated soft‐tissue edema. As many as 25% of patients hospitalized for IM will have some degree of airway obstruction.2, 3 Peritonsillar abscesses (PTAs) occur in approximately 1% of hospitalized patients with IM and may further obstruct the airway.4 Most commonly, peritonsillar abscesses are polymicrobial, having both aerobic and anaerobic bacteria. In a study of young adults with peritonsillar abscesses from all causes, Streptococcus pyogenes was the most common aerobe, found in nearly half of isolates; Streptococcus milleri, the bacterium isolated from our patient, was the second most common organism, found in approximately 25% of these abscesses.5 One prior case of airway obstruction from IM complicated by bilateral peritonsillar abscesses has been reported6; however, this patient was not reported to have concomitant retropharyngeal infection, as was noted in our patient.
Guidelines suggest that patients with mild, uncomplicated IM should be managed with supportive care alone; current recommendations are that steroids be prescribed only for specific complications of IM, including upper‐airway obstruction.7 Protocols for steroid regimens include initial prednisone doses ranging from 20 to 80 mg/day, with most advocating that they be tapered off over 1‐2 weeks.7, 8
Some of the controversy about the routine use of steroids in IM is related to concern for potential infectious complications associated with immunosuppression. In a small case series, Handler et al. proposed that there is an association between steroid therapy and the development of peritonsillar abscesses.9 However, this has not been tested in controlled trials, and the results of more recent studies do not support an increased likelihood of PTA in patients with infectious mononucleosis treated with corticosteroids.10, 11 Therefore, given that it has documented benefits and no proven adverse consequences, steroid therapy is uniformly recommended for patients with upper‐airway obstruction secondary to infectious mononucleosis. However, use of steroids also mandates careful monitoring for signs or symptoms suggestive of secondary bacterial infection.
For patients whose symptoms progress despite medical management including steroid therapy, surgical intervention may be required. Both tracheostomy and tonsillectomy during acute infection, sometimes referred to as hot tonsillectomy, have been reported as surgical options for airway obstruction in IM, the latter having emerged as the preferred treatment.3 Such treatment allows for drainage of any infectious collections, as well as removal of obstructing lymphoid tissue as indicated.
In conclusion, enlargement of tonsils and adenoids with associated edema in infectious mononucleosis can lead to upper‐airway obstruction. Patients with evidence of such obstruction should be treated with a tapering course of corticosteroids. Peritonsillar abscesses and deep neck infections are also severe complications of IM and can cause further respiratory compromise. In cases where medical therapy is not effective, such as with our patient, evaluation for peritonsillar abscess and need for possible acute tonsillectomy may be required.
A 17‐year‐old female patient presented to the emergency department reporting having fever, sore throat, and pain with swallowing for several days. The result of her rapid strep screen was negative. She had an elevated white blood cell count, mildly elevated AST and ALT levels, and a positive result from a heterophile antibody test (BBL Monoslide). She was diagnosed with infectious mononucleosis. Given her inability to tolerate oral fluids, she was admitted to the hospital for intravenous hydration. After 3 days of receiving methylprednisolone intravenously, she had worsening throat pain, progressive neck swelling, difficulty handling her secretions, and new respiratory symptoms. During the examination, she was sitting upright in bed in moderate respiratory distress. She had kissing, exudative tonsils with palatal and uvular edema. Examination of her neck showed significantly enlarged anterior and posterior cervical lymph nodes without fluctuance. Her lung exam revealed subcostal retractions with transmitted upper airway sounds but good aeration. The edge of her liver and spleen tip were palpable.
Because of the rapid progression of symptoms while on medical therapy, computed tomography (CT) of the neck was performed. Sagittal reconstructions showed adenoidal hypertrophy compromising her nasopharynx, and massive tonsillar enlargement causing nearly complete obstruction of her oropharyngeal airway (Fig. 1), with airway narrowing to less than 2.5 mm in axial images. Bilateral low‐density lesions within the paratonsillar regions were suggestive of abscesses and retropharyngeal soft‐tissue swelling was consistent with phlegmon (Fig. 2). The patient was taken to the operating room for an emergent tonsillectomy. Bilateral peritonsillar abscesses were drained, pus was sent for culture, and her tonsils were excised. Cultures from the abscesses grew Streptococcus milleri. The patient was discharged home 2 days later to complete a 2‐week course of oral clindamycin.


Most patients with infectious mononucleosis (IM) have a benign, self‐limited course. However, a wide range of severe complications have been described including airway obstruction, splenic rupture, meningoencephalitis, Guillain‐Barr syndrome, peritonsillar abscess, and hemolytic anemia.1, 2 Upper‐airway obstruction results from lymphoid hyperplasia throughout Waldeyer's ring, with associated soft‐tissue edema. As many as 25% of patients hospitalized for IM will have some degree of airway obstruction.2, 3 Peritonsillar abscesses (PTAs) occur in approximately 1% of hospitalized patients with IM and may further obstruct the airway.4 Most commonly, peritonsillar abscesses are polymicrobial, having both aerobic and anaerobic bacteria. In a study of young adults with peritonsillar abscesses from all causes, Streptococcus pyogenes was the most common aerobe, found in nearly half of isolates; Streptococcus milleri, the bacterium isolated from our patient, was the second most common organism, found in approximately 25% of these abscesses.5 One prior case of airway obstruction from IM complicated by bilateral peritonsillar abscesses has been reported6; however, this patient was not reported to have concomitant retropharyngeal infection, as was noted in our patient.
Guidelines suggest that patients with mild, uncomplicated IM should be managed with supportive care alone; current recommendations are that steroids be prescribed only for specific complications of IM, including upper‐airway obstruction.7 Protocols for steroid regimens include initial prednisone doses ranging from 20 to 80 mg/day, with most advocating that they be tapered off over 1‐2 weeks.7, 8
Some of the controversy about the routine use of steroids in IM is related to concern for potential infectious complications associated with immunosuppression. In a small case series, Handler et al. proposed that there is an association between steroid therapy and the development of peritonsillar abscesses.9 However, this has not been tested in controlled trials, and the results of more recent studies do not support an increased likelihood of PTA in patients with infectious mononucleosis treated with corticosteroids.10, 11 Therefore, given that it has documented benefits and no proven adverse consequences, steroid therapy is uniformly recommended for patients with upper‐airway obstruction secondary to infectious mononucleosis. However, use of steroids also mandates careful monitoring for signs or symptoms suggestive of secondary bacterial infection.
For patients whose symptoms progress despite medical management including steroid therapy, surgical intervention may be required. Both tracheostomy and tonsillectomy during acute infection, sometimes referred to as hot tonsillectomy, have been reported as surgical options for airway obstruction in IM, the latter having emerged as the preferred treatment.3 Such treatment allows for drainage of any infectious collections, as well as removal of obstructing lymphoid tissue as indicated.
In conclusion, enlargement of tonsils and adenoids with associated edema in infectious mononucleosis can lead to upper‐airway obstruction. Patients with evidence of such obstruction should be treated with a tapering course of corticosteroids. Peritonsillar abscesses and deep neck infections are also severe complications of IM and can cause further respiratory compromise. In cases where medical therapy is not effective, such as with our patient, evaluation for peritonsillar abscess and need for possible acute tonsillectomy may be required.
- .Acute complications of Epstein‐Barr virus infectious mononucleosis.Curr Opin Pediatr.2000;12(3):263–268.
- ,.Complications of infection with Epstein‐Barr virus during childhood: a study of children admitted to the hospital.Pediatr Infect Dis.1984;3:304–307.
- ,.The management of severe infectious mononucleosis tonsillitis and upper airway obstruction.J Laryngol Otol.2001;115:973–977.
- ,,.Otolaryngological complications in infectious mononucleosis.J Laryngol Otol.1984;98:999–1001.
- ,,.Bacteriologic findings in peritonsillar abscesses in young adults.Clin Infect Dis.1993;16(suppl 4):S292–S298.
- ,.Infectious mononucleosis and bilateral peritonsillar abscesses resulting in airway obstruction.J Laryngol Otol.1998;112:1186–1188.
- ,,, et al.Guidelines for the use of systemic glucocorticosteroids in the management of selected infections.Working Group on Steroid Use, Antimicrobial Agents Committee, Infectious Diseases Society of America.J Infect Dis.1992;165(1):1–13.
- ,Narula AA Steroids for airway problems in glandular fever.J Laryngol Otol.1987;10:673–675.
- ,.Peritonsillar abscess: a complication of corticosteroid treatment in infectious mononucleosis.Int J Pediatr Otorhinolaryngol.1979;1(3):265–268.
- ,,.Corticosteroids and peritonsillar abscess formation in infectious mononucleosis.J Laryngol Otol.2004;118:459–461.
- ,,.Otolaryngologic Clinical Patterns in Pediatric Infectious Mononucleosis.Am J Otolaryngol.1996;17:397–400.
- .Acute complications of Epstein‐Barr virus infectious mononucleosis.Curr Opin Pediatr.2000;12(3):263–268.
- ,.Complications of infection with Epstein‐Barr virus during childhood: a study of children admitted to the hospital.Pediatr Infect Dis.1984;3:304–307.
- ,.The management of severe infectious mononucleosis tonsillitis and upper airway obstruction.J Laryngol Otol.2001;115:973–977.
- ,,.Otolaryngological complications in infectious mononucleosis.J Laryngol Otol.1984;98:999–1001.
- ,,.Bacteriologic findings in peritonsillar abscesses in young adults.Clin Infect Dis.1993;16(suppl 4):S292–S298.
- ,.Infectious mononucleosis and bilateral peritonsillar abscesses resulting in airway obstruction.J Laryngol Otol.1998;112:1186–1188.
- ,,, et al.Guidelines for the use of systemic glucocorticosteroids in the management of selected infections.Working Group on Steroid Use, Antimicrobial Agents Committee, Infectious Diseases Society of America.J Infect Dis.1992;165(1):1–13.
- ,Narula AA Steroids for airway problems in glandular fever.J Laryngol Otol.1987;10:673–675.
- ,.Peritonsillar abscess: a complication of corticosteroid treatment in infectious mononucleosis.Int J Pediatr Otorhinolaryngol.1979;1(3):265–268.
- ,,.Corticosteroids and peritonsillar abscess formation in infectious mononucleosis.J Laryngol Otol.2004;118:459–461.
- ,,.Otolaryngologic Clinical Patterns in Pediatric Infectious Mononucleosis.Am J Otolaryngol.1996;17:397–400.
Hypoglycemia in Hospitalized Patients / Garg et al.
Glycemic control in hospitalized patients is receiving greater attention. The American Diabetes Association and the American College of Endocrinology recently issued a joint consensus statement on the need to implement tight blood glucose (BG) control in hospitalized patients.1, 2 The Joint Commission on Accreditation of Healthcare Organizations (JACHO) has developed an Advanced Inpatient Diabetes Care Certification Program for hospitals. However, despite all these efforts, it has been difficult to change how well glucose is controlled.3 A major hurdle in implementing glycemic control strategies is the prevalent fear of hypoglycemia among hospital staff. Although there are multiple protocols for insulin treatment,47 guidelines for the prevention and treatment of hypoglycemia are lacking. Once a hypoglycemic episode has occurred, reducing the dosage of diabetes medications may reduce subsequent episodes. This study was conducted to assess whether diabetes medications were decreased following an episode of hypoglycemia that led to treatment with intravenous (IV) dextrose.
METHODS
Data were collected by the Diabetes Subcommittee of the Pharmacy and Therapeutics Committee as part of a quality improvement initiative. Hypoglycemic episodes were identified by computerized orders for 50% dextrose solution. All orders in a 1‐month period (June 2006) were collected. Characteristics of patients experiencing these episodes were identified from the electronic medical records (EMR). The following data were collected: age, sex, history of diabetes, serum creatinine, diabetes medications at time of hypoglycemia, blood glucose at time of hypoglycemia, and all BG values in the 24 hours before hypoglycemia. BG values included those obtained in the laboratory as well as those obtained by bedside blood glucose testing. Treatment changes made right when the hypoglycemic episode occurred (immediate) and within 24 hours of the hypoglycemic episode (subsequent) were evaluated by 2 diabetes specialists, a board‐certified endocrinologist and a nurse‐practitioner working on the diabetes management service. The 2 practitioners regularly work together, but the data were evaluated independently. Because there are no specific guidelines, the appropriateness of change in treatment was based on general guidelines and experience. For example, if hypoglycemia developed while a patient was on insulin infusion therapy, it was appropriate to stop the drip when the episode of hypoglycemia occurred and to restart it at a lower rate according to the insulin infusion protocol. No subsequent changes would have been made in a situation such as this, and it was deemed appropriate. However, if a patient developed hypoglycemia while on subcutaneous (SC) insulin and then insulin was either completely discontinued or no change was made in subsequent orders, it was deemed inappropriate. The 2 diabetes specialists agreed in 87% of cases (kappa = 0.68, 95% CI 0.53‐0.84). In the 13% of cases in which the diabetes specialists had different opinions, they conferred to reach agreement. In patients with more than 1 episode, data related to the first episode were evaluated. Data are presented as means with SDs.
RESULTS
The EMR contained information on time of episode of hypoglycemia and medication changes for 52 patients, all of whom were in the study. Patient characteristics and mean blood glucose level are shown in Table 1. All patients were being treated with insulin when the episode of hypoglycemia occurred: 9 were on intravenous (IV) insulin alone, 3 on IV and subcutaneous (SC) insulin, 30 on scheduled SC insulin, and 10 on sliding‐scale SC insulin alone. Three patients were prescribed sulfonylurea drugs in addition to insulin. Insulin dosage of all 52 patients was held at the time of the hypoglycemic episode. Diabetes specialists agreed with this decision 100% of the time. Only 21 patients (40%) subsequently had reductions made in their treatment dosage, and diabetes specialists agreed with the changes made for 11 of these patients (52%). Thirty‐one patients (60%) had no changes made to their treatment, and diabetes specialists agreed with that decision for 10 of these patients (32%). When diabetes specialists disagreed with a decision, they would have decreased the insulin dose or changed the regimen in a different way. Details on the changes in treatment and whether diabetes specialists agreed with the changes are shown in Table 2. Twenty‐four hours after an episode of hypoglycemia, mean blood glucose of patients whose providers had made changes was 190.7 87.9 mg/dL and that of patients whose providers had not made changes was 122.6 43.2 mg/dL (P = NS). The mean BG of patients for whom the diabetologists agreed with the decision was 110.7 90.3 mg/dL, and that of patients for whom they disagreed with the decision was 139.7 42.8 mg/dL (P = NS).
| Number of patients | 52 |
| Age (years) | 64.8 15.8 |
| Sex (male:female), n | 29:23 |
| Preexisting diabetes, n (%) | |
| No diabetes | 17 (33%) |
| Type 1 diabetes | 9 (17%) |
| Type 2 diabetes | 26 (50%) |
| Serum creatinine (mg/dL) | 2.1 1.9 |
| Serum creatinine 2 mg/dL, n (%) | 21 (40%) |
| BG at time of hypoglycemia (mg/dL) | 52.1 9.3 |
| Mean BG during 24 hours before hypoglycemic episode (mg/dL) | 137.5 57.0 |
| Mean BG during 24 hours after hypoglycemic episode (mg/dL) | 112 74.7 |
| Change | Number of patients receiving change | Number of patients for whom diabetes specialists agreed with change, n (%) |
|---|---|---|
| Basal insulin decreased | 6 | 6 (100%) |
| Basal insulin stopped | 2 | 0 (0%) |
| IV insulin changed to scheduled SC insulin | 2 | 1 (50%) |
| IV insulin to SC sliding‐scale insulin | 1 | 0 (0%) |
| Change in sliding‐scale insulin dose | 3 | 1 (33%) |
| Sliding‐scale insulin stopped | 1 | 1 (100%) |
| IV insulin started | 1 | 1 (100%) |
| Sulfonylurea stopped | 1 | 1 (100%) |
| Scheduled insulin changed to sliding scale | 1 | 0 (0%) |
| Insulin discontinued | 3 | 0 (0%) |
| No change | 31 | 10 (32%) |
DISCUSSION
These results suggest that treatment modification following an episode of hypoglycemia may be suboptimal. These data provide no information about the clinical circumstances leading to the choice of treatment with IV dextrose, as opposed to oral glucose or glucagon. Presumably, dextrose was chosen for many patients whom the physician considered to require the most urgent treatment. Appropriately, immediate treatment with insulin was held for all patients. On the other hand, 60% of the patients continued to receive the same insulin dose 24 hours after the hypoglycemic episode. Diabetes specialists judged continuation of the same dose as inappropriate in two thirds of the cases. Even when changes in treatment were made, those changes were judged suboptimal in half the cases. Blood glucose level 24 hours after an episode of hypoglycemia reflects these problems. These findings suggest that opportunities to prevent hypoglycemic episodes in the future are frequently missed. Lack of knowledge and/or guidelines for adjusting insulin dose following an episode of hypoglycemia seemed to have led to suboptimal changes for most patients.
Overall incidence of hypoglycemia (<60 mg/dL) among patients with diabetes admitted to a hospital has been reported to be 23%.8 In patients receiving continuous intravenous insulin infusion, the incidence of hypoglycemia has been variously reported as from 1.2% to 18.7%.9, 10 All insulin infusion protocols have guidelines for the immediate treatment of hypoglycemia and recommend steps to prevent further episodes. Although many hospitals have protocols for immediate action when hypoglycemia occurs (eg, hold insulin, give juice or dextrose), to our knowledge, no specific guidelines exist for adjustment of subcutaneous insulin following an episode of hypoglycemia. The vast majority of patients in a hospital are treated with SC insulin as opposed to IV insulin, and fear of hypoglycemia is a major barrier to intensified therapy. If widely applied, standardized protocols have the potential to be effective in preventing hypoglycemia.9
A limitation of our study was that it was a retrospective data analysis. We did not look at changes in clinical condition, in nutrition, and in other medications that might have led to the episode of hypoglycemia and affected the decision about which antidiabetic medications to treat with. Data on further episodes of hypoglycemia were also not available.
In conclusion, we have shown that treatment changes after an episode of hypoglycemia are chaotic and may be suboptimal. Standardized protocols may be helpful for making effective changes and potentially can reduce the risk of further episodes of hypoglycemia.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control.Endocr Pract.2006;12:458–468.
- ,,.Current state of inpatient diabetes burden and care, and goal of the conference.Endocr Pract.2006;12(suppl 3, sddendum):1–10.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,,,.Evaluation of an intensive insulin protocol for septic patients in a medical intensive care unit.Crit Care Med.2006;34:2974–2978.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
Glycemic control in hospitalized patients is receiving greater attention. The American Diabetes Association and the American College of Endocrinology recently issued a joint consensus statement on the need to implement tight blood glucose (BG) control in hospitalized patients.1, 2 The Joint Commission on Accreditation of Healthcare Organizations (JACHO) has developed an Advanced Inpatient Diabetes Care Certification Program for hospitals. However, despite all these efforts, it has been difficult to change how well glucose is controlled.3 A major hurdle in implementing glycemic control strategies is the prevalent fear of hypoglycemia among hospital staff. Although there are multiple protocols for insulin treatment,47 guidelines for the prevention and treatment of hypoglycemia are lacking. Once a hypoglycemic episode has occurred, reducing the dosage of diabetes medications may reduce subsequent episodes. This study was conducted to assess whether diabetes medications were decreased following an episode of hypoglycemia that led to treatment with intravenous (IV) dextrose.
METHODS
Data were collected by the Diabetes Subcommittee of the Pharmacy and Therapeutics Committee as part of a quality improvement initiative. Hypoglycemic episodes were identified by computerized orders for 50% dextrose solution. All orders in a 1‐month period (June 2006) were collected. Characteristics of patients experiencing these episodes were identified from the electronic medical records (EMR). The following data were collected: age, sex, history of diabetes, serum creatinine, diabetes medications at time of hypoglycemia, blood glucose at time of hypoglycemia, and all BG values in the 24 hours before hypoglycemia. BG values included those obtained in the laboratory as well as those obtained by bedside blood glucose testing. Treatment changes made right when the hypoglycemic episode occurred (immediate) and within 24 hours of the hypoglycemic episode (subsequent) were evaluated by 2 diabetes specialists, a board‐certified endocrinologist and a nurse‐practitioner working on the diabetes management service. The 2 practitioners regularly work together, but the data were evaluated independently. Because there are no specific guidelines, the appropriateness of change in treatment was based on general guidelines and experience. For example, if hypoglycemia developed while a patient was on insulin infusion therapy, it was appropriate to stop the drip when the episode of hypoglycemia occurred and to restart it at a lower rate according to the insulin infusion protocol. No subsequent changes would have been made in a situation such as this, and it was deemed appropriate. However, if a patient developed hypoglycemia while on subcutaneous (SC) insulin and then insulin was either completely discontinued or no change was made in subsequent orders, it was deemed inappropriate. The 2 diabetes specialists agreed in 87% of cases (kappa = 0.68, 95% CI 0.53‐0.84). In the 13% of cases in which the diabetes specialists had different opinions, they conferred to reach agreement. In patients with more than 1 episode, data related to the first episode were evaluated. Data are presented as means with SDs.
RESULTS
The EMR contained information on time of episode of hypoglycemia and medication changes for 52 patients, all of whom were in the study. Patient characteristics and mean blood glucose level are shown in Table 1. All patients were being treated with insulin when the episode of hypoglycemia occurred: 9 were on intravenous (IV) insulin alone, 3 on IV and subcutaneous (SC) insulin, 30 on scheduled SC insulin, and 10 on sliding‐scale SC insulin alone. Three patients were prescribed sulfonylurea drugs in addition to insulin. Insulin dosage of all 52 patients was held at the time of the hypoglycemic episode. Diabetes specialists agreed with this decision 100% of the time. Only 21 patients (40%) subsequently had reductions made in their treatment dosage, and diabetes specialists agreed with the changes made for 11 of these patients (52%). Thirty‐one patients (60%) had no changes made to their treatment, and diabetes specialists agreed with that decision for 10 of these patients (32%). When diabetes specialists disagreed with a decision, they would have decreased the insulin dose or changed the regimen in a different way. Details on the changes in treatment and whether diabetes specialists agreed with the changes are shown in Table 2. Twenty‐four hours after an episode of hypoglycemia, mean blood glucose of patients whose providers had made changes was 190.7 87.9 mg/dL and that of patients whose providers had not made changes was 122.6 43.2 mg/dL (P = NS). The mean BG of patients for whom the diabetologists agreed with the decision was 110.7 90.3 mg/dL, and that of patients for whom they disagreed with the decision was 139.7 42.8 mg/dL (P = NS).
| Number of patients | 52 |
| Age (years) | 64.8 15.8 |
| Sex (male:female), n | 29:23 |
| Preexisting diabetes, n (%) | |
| No diabetes | 17 (33%) |
| Type 1 diabetes | 9 (17%) |
| Type 2 diabetes | 26 (50%) |
| Serum creatinine (mg/dL) | 2.1 1.9 |
| Serum creatinine 2 mg/dL, n (%) | 21 (40%) |
| BG at time of hypoglycemia (mg/dL) | 52.1 9.3 |
| Mean BG during 24 hours before hypoglycemic episode (mg/dL) | 137.5 57.0 |
| Mean BG during 24 hours after hypoglycemic episode (mg/dL) | 112 74.7 |
| Change | Number of patients receiving change | Number of patients for whom diabetes specialists agreed with change, n (%) |
|---|---|---|
| Basal insulin decreased | 6 | 6 (100%) |
| Basal insulin stopped | 2 | 0 (0%) |
| IV insulin changed to scheduled SC insulin | 2 | 1 (50%) |
| IV insulin to SC sliding‐scale insulin | 1 | 0 (0%) |
| Change in sliding‐scale insulin dose | 3 | 1 (33%) |
| Sliding‐scale insulin stopped | 1 | 1 (100%) |
| IV insulin started | 1 | 1 (100%) |
| Sulfonylurea stopped | 1 | 1 (100%) |
| Scheduled insulin changed to sliding scale | 1 | 0 (0%) |
| Insulin discontinued | 3 | 0 (0%) |
| No change | 31 | 10 (32%) |
DISCUSSION
These results suggest that treatment modification following an episode of hypoglycemia may be suboptimal. These data provide no information about the clinical circumstances leading to the choice of treatment with IV dextrose, as opposed to oral glucose or glucagon. Presumably, dextrose was chosen for many patients whom the physician considered to require the most urgent treatment. Appropriately, immediate treatment with insulin was held for all patients. On the other hand, 60% of the patients continued to receive the same insulin dose 24 hours after the hypoglycemic episode. Diabetes specialists judged continuation of the same dose as inappropriate in two thirds of the cases. Even when changes in treatment were made, those changes were judged suboptimal in half the cases. Blood glucose level 24 hours after an episode of hypoglycemia reflects these problems. These findings suggest that opportunities to prevent hypoglycemic episodes in the future are frequently missed. Lack of knowledge and/or guidelines for adjusting insulin dose following an episode of hypoglycemia seemed to have led to suboptimal changes for most patients.
Overall incidence of hypoglycemia (<60 mg/dL) among patients with diabetes admitted to a hospital has been reported to be 23%.8 In patients receiving continuous intravenous insulin infusion, the incidence of hypoglycemia has been variously reported as from 1.2% to 18.7%.9, 10 All insulin infusion protocols have guidelines for the immediate treatment of hypoglycemia and recommend steps to prevent further episodes. Although many hospitals have protocols for immediate action when hypoglycemia occurs (eg, hold insulin, give juice or dextrose), to our knowledge, no specific guidelines exist for adjustment of subcutaneous insulin following an episode of hypoglycemia. The vast majority of patients in a hospital are treated with SC insulin as opposed to IV insulin, and fear of hypoglycemia is a major barrier to intensified therapy. If widely applied, standardized protocols have the potential to be effective in preventing hypoglycemia.9
A limitation of our study was that it was a retrospective data analysis. We did not look at changes in clinical condition, in nutrition, and in other medications that might have led to the episode of hypoglycemia and affected the decision about which antidiabetic medications to treat with. Data on further episodes of hypoglycemia were also not available.
In conclusion, we have shown that treatment changes after an episode of hypoglycemia are chaotic and may be suboptimal. Standardized protocols may be helpful for making effective changes and potentially can reduce the risk of further episodes of hypoglycemia.
Glycemic control in hospitalized patients is receiving greater attention. The American Diabetes Association and the American College of Endocrinology recently issued a joint consensus statement on the need to implement tight blood glucose (BG) control in hospitalized patients.1, 2 The Joint Commission on Accreditation of Healthcare Organizations (JACHO) has developed an Advanced Inpatient Diabetes Care Certification Program for hospitals. However, despite all these efforts, it has been difficult to change how well glucose is controlled.3 A major hurdle in implementing glycemic control strategies is the prevalent fear of hypoglycemia among hospital staff. Although there are multiple protocols for insulin treatment,47 guidelines for the prevention and treatment of hypoglycemia are lacking. Once a hypoglycemic episode has occurred, reducing the dosage of diabetes medications may reduce subsequent episodes. This study was conducted to assess whether diabetes medications were decreased following an episode of hypoglycemia that led to treatment with intravenous (IV) dextrose.
METHODS
Data were collected by the Diabetes Subcommittee of the Pharmacy and Therapeutics Committee as part of a quality improvement initiative. Hypoglycemic episodes were identified by computerized orders for 50% dextrose solution. All orders in a 1‐month period (June 2006) were collected. Characteristics of patients experiencing these episodes were identified from the electronic medical records (EMR). The following data were collected: age, sex, history of diabetes, serum creatinine, diabetes medications at time of hypoglycemia, blood glucose at time of hypoglycemia, and all BG values in the 24 hours before hypoglycemia. BG values included those obtained in the laboratory as well as those obtained by bedside blood glucose testing. Treatment changes made right when the hypoglycemic episode occurred (immediate) and within 24 hours of the hypoglycemic episode (subsequent) were evaluated by 2 diabetes specialists, a board‐certified endocrinologist and a nurse‐practitioner working on the diabetes management service. The 2 practitioners regularly work together, but the data were evaluated independently. Because there are no specific guidelines, the appropriateness of change in treatment was based on general guidelines and experience. For example, if hypoglycemia developed while a patient was on insulin infusion therapy, it was appropriate to stop the drip when the episode of hypoglycemia occurred and to restart it at a lower rate according to the insulin infusion protocol. No subsequent changes would have been made in a situation such as this, and it was deemed appropriate. However, if a patient developed hypoglycemia while on subcutaneous (SC) insulin and then insulin was either completely discontinued or no change was made in subsequent orders, it was deemed inappropriate. The 2 diabetes specialists agreed in 87% of cases (kappa = 0.68, 95% CI 0.53‐0.84). In the 13% of cases in which the diabetes specialists had different opinions, they conferred to reach agreement. In patients with more than 1 episode, data related to the first episode were evaluated. Data are presented as means with SDs.
RESULTS
The EMR contained information on time of episode of hypoglycemia and medication changes for 52 patients, all of whom were in the study. Patient characteristics and mean blood glucose level are shown in Table 1. All patients were being treated with insulin when the episode of hypoglycemia occurred: 9 were on intravenous (IV) insulin alone, 3 on IV and subcutaneous (SC) insulin, 30 on scheduled SC insulin, and 10 on sliding‐scale SC insulin alone. Three patients were prescribed sulfonylurea drugs in addition to insulin. Insulin dosage of all 52 patients was held at the time of the hypoglycemic episode. Diabetes specialists agreed with this decision 100% of the time. Only 21 patients (40%) subsequently had reductions made in their treatment dosage, and diabetes specialists agreed with the changes made for 11 of these patients (52%). Thirty‐one patients (60%) had no changes made to their treatment, and diabetes specialists agreed with that decision for 10 of these patients (32%). When diabetes specialists disagreed with a decision, they would have decreased the insulin dose or changed the regimen in a different way. Details on the changes in treatment and whether diabetes specialists agreed with the changes are shown in Table 2. Twenty‐four hours after an episode of hypoglycemia, mean blood glucose of patients whose providers had made changes was 190.7 87.9 mg/dL and that of patients whose providers had not made changes was 122.6 43.2 mg/dL (P = NS). The mean BG of patients for whom the diabetologists agreed with the decision was 110.7 90.3 mg/dL, and that of patients for whom they disagreed with the decision was 139.7 42.8 mg/dL (P = NS).
| Number of patients | 52 |
| Age (years) | 64.8 15.8 |
| Sex (male:female), n | 29:23 |
| Preexisting diabetes, n (%) | |
| No diabetes | 17 (33%) |
| Type 1 diabetes | 9 (17%) |
| Type 2 diabetes | 26 (50%) |
| Serum creatinine (mg/dL) | 2.1 1.9 |
| Serum creatinine 2 mg/dL, n (%) | 21 (40%) |
| BG at time of hypoglycemia (mg/dL) | 52.1 9.3 |
| Mean BG during 24 hours before hypoglycemic episode (mg/dL) | 137.5 57.0 |
| Mean BG during 24 hours after hypoglycemic episode (mg/dL) | 112 74.7 |
| Change | Number of patients receiving change | Number of patients for whom diabetes specialists agreed with change, n (%) |
|---|---|---|
| Basal insulin decreased | 6 | 6 (100%) |
| Basal insulin stopped | 2 | 0 (0%) |
| IV insulin changed to scheduled SC insulin | 2 | 1 (50%) |
| IV insulin to SC sliding‐scale insulin | 1 | 0 (0%) |
| Change in sliding‐scale insulin dose | 3 | 1 (33%) |
| Sliding‐scale insulin stopped | 1 | 1 (100%) |
| IV insulin started | 1 | 1 (100%) |
| Sulfonylurea stopped | 1 | 1 (100%) |
| Scheduled insulin changed to sliding scale | 1 | 0 (0%) |
| Insulin discontinued | 3 | 0 (0%) |
| No change | 31 | 10 (32%) |
DISCUSSION
These results suggest that treatment modification following an episode of hypoglycemia may be suboptimal. These data provide no information about the clinical circumstances leading to the choice of treatment with IV dextrose, as opposed to oral glucose or glucagon. Presumably, dextrose was chosen for many patients whom the physician considered to require the most urgent treatment. Appropriately, immediate treatment with insulin was held for all patients. On the other hand, 60% of the patients continued to receive the same insulin dose 24 hours after the hypoglycemic episode. Diabetes specialists judged continuation of the same dose as inappropriate in two thirds of the cases. Even when changes in treatment were made, those changes were judged suboptimal in half the cases. Blood glucose level 24 hours after an episode of hypoglycemia reflects these problems. These findings suggest that opportunities to prevent hypoglycemic episodes in the future are frequently missed. Lack of knowledge and/or guidelines for adjusting insulin dose following an episode of hypoglycemia seemed to have led to suboptimal changes for most patients.
Overall incidence of hypoglycemia (<60 mg/dL) among patients with diabetes admitted to a hospital has been reported to be 23%.8 In patients receiving continuous intravenous insulin infusion, the incidence of hypoglycemia has been variously reported as from 1.2% to 18.7%.9, 10 All insulin infusion protocols have guidelines for the immediate treatment of hypoglycemia and recommend steps to prevent further episodes. Although many hospitals have protocols for immediate action when hypoglycemia occurs (eg, hold insulin, give juice or dextrose), to our knowledge, no specific guidelines exist for adjustment of subcutaneous insulin following an episode of hypoglycemia. The vast majority of patients in a hospital are treated with SC insulin as opposed to IV insulin, and fear of hypoglycemia is a major barrier to intensified therapy. If widely applied, standardized protocols have the potential to be effective in preventing hypoglycemia.9
A limitation of our study was that it was a retrospective data analysis. We did not look at changes in clinical condition, in nutrition, and in other medications that might have led to the episode of hypoglycemia and affected the decision about which antidiabetic medications to treat with. Data on further episodes of hypoglycemia were also not available.
In conclusion, we have shown that treatment changes after an episode of hypoglycemia are chaotic and may be suboptimal. Standardized protocols may be helpful for making effective changes and potentially can reduce the risk of further episodes of hypoglycemia.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control.Endocr Pract.2006;12:458–468.
- ,,.Current state of inpatient diabetes burden and care, and goal of the conference.Endocr Pract.2006;12(suppl 3, sddendum):1–10.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,,,.Evaluation of an intensive insulin protocol for septic patients in a medical intensive care unit.Crit Care Med.2006;34:2974–2978.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control.Endocr Pract.2006;12:458–468.
- ,,.Current state of inpatient diabetes burden and care, and goal of the conference.Endocr Pract.2006;12(suppl 3, sddendum):1–10.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,,,.Evaluation of an intensive insulin protocol for septic patients in a medical intensive care unit.Crit Care Med.2006;34:2974–2978.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
Referral for CT Pulmonary Angiography
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.
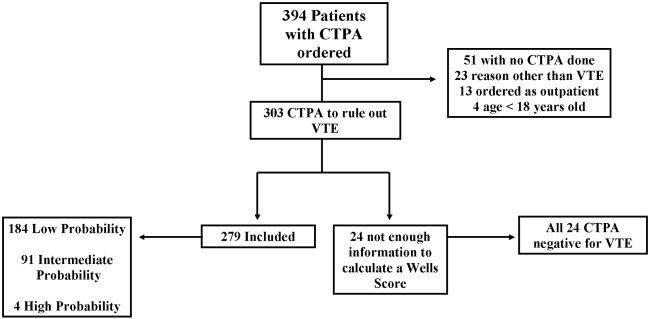
| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |
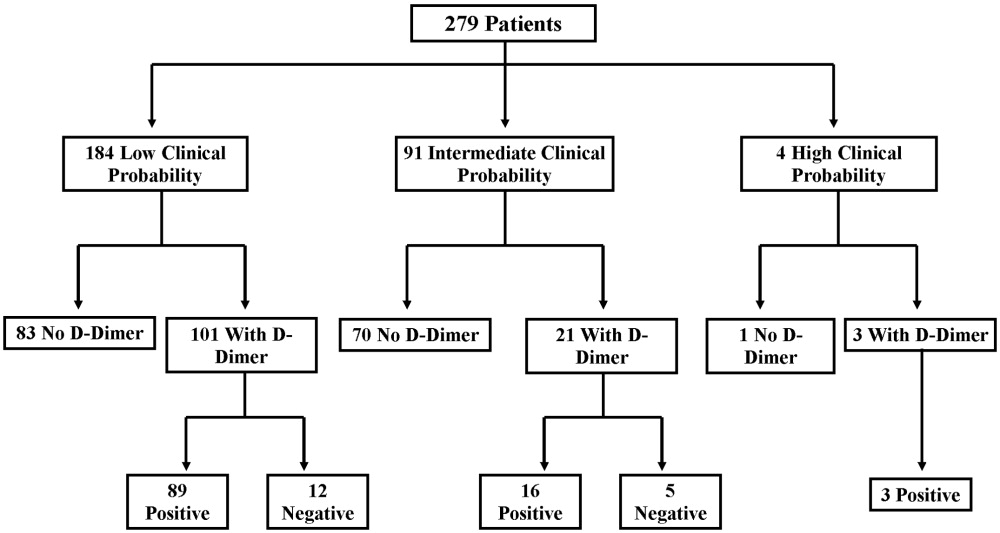
There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.
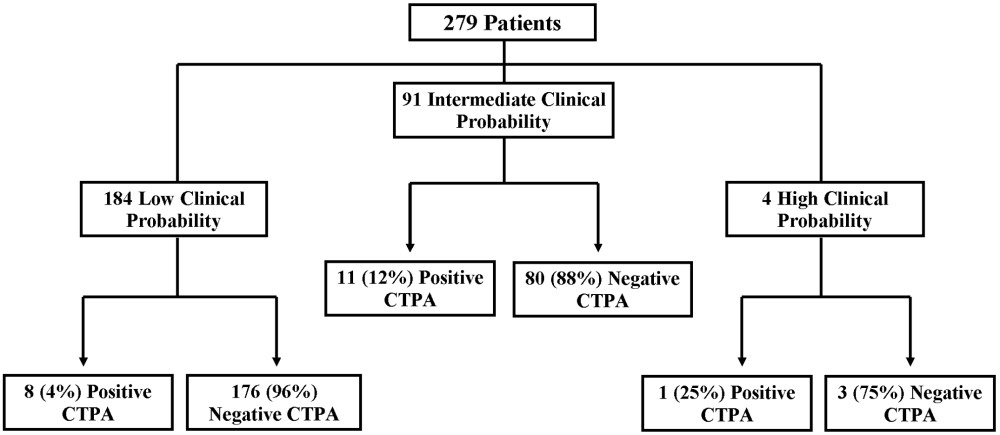
DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
Statins/Beta‐Blockers and Mortality after Vascular Surgery
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).
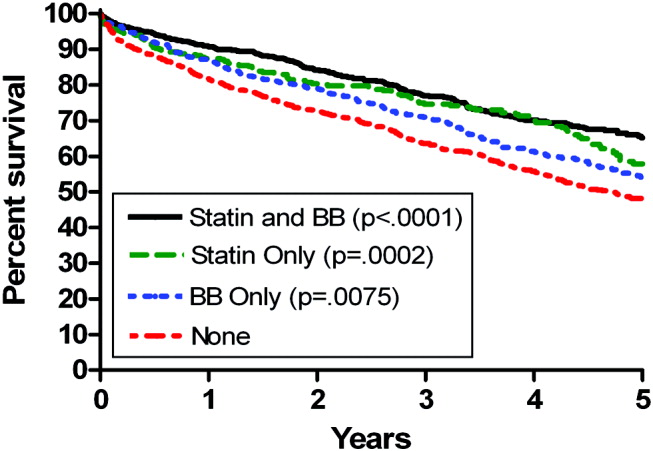
Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).
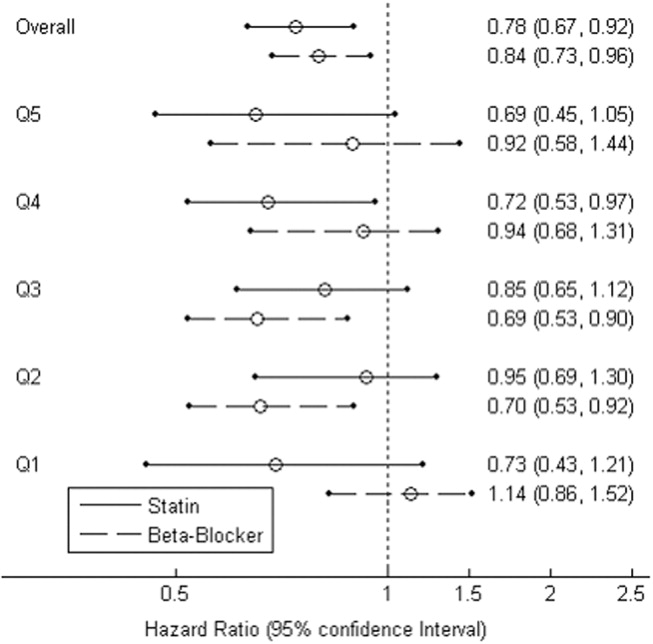
Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).

Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).

Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
Vascular surgery has higher morbidity and mortality than other noncardiac surgeries. Despite the identification of vascular surgery as higher risk, 30‐day mortality for this surgery has remained at 3%10%. Few studies have examined longer‐term outcomes, but higher mortality rates have been reported, for example, 10%30% 6 months after surgery, 20%40% 1 year after surgery, and 30%50% 5 years after surgery.15 Postoperative adverse events have been found to be highly correlated with perioperative ischemia and infarction.68 Perioperative beta‐blockers have been widely studied and have been shown to benefit patients undergoing noncardiac surgery generally and vascular surgery specifically.9, 10 However, 2 recent trials of perioperative beta‐blockers in noncardiac and vascular surgery patients failed to show an association with 18‐month and 30‐day postoperative morbidity and mortality, respectively.11, 12 In addition, the authors of a recent meta‐analysis of perioperative beta‐blockers suggested more studies were needed.13 Furthermore, there have been promising new data on the use of perioperative statins.1418 Finally, as a recent clinical trial of revascularization before vascular surgery did not demonstrate an advantage over medical management, the identification of which perioperative medicines improve postoperative outcomes and in what combinations becomes even more important.19 We sought to ascertain if the ambulatory use of statins and/or beta‐blockers within 30 days of surgery was associated with a reduction in long‐term mortality.
METHODS
Setting and Subjects
We conducted a retrospective cohort study using a regional Department of Veterans Affairs (VA) administrative and relational database, the Consumer Health Information and Performance Sets (CHIPs), which automatically extracts data from electronic medical records of all facilities in the Veterans Integrated Services Network 20, which encompasses Alaska, Washington, Oregon, and Idaho. CHIPs contains information on both outpatient and inpatient environments, and a record is generated for every contact a patient makes with the VA health care system, which includes picking up prescription medications, laboratory values, demographic information, International Classification of Diseases, 9th Revision (ICD‐9), codes, and vital status. In addition, we used the Beneficiary Identification and Records Locator Subsystem database, which is the national VA death index and includes Social Security Administration data that has been shown to be 90%95% complete for assessing vital status.20
Data for all patients who had vascular surgery at 5 VA medical centers in the region from January 1998 to March 2005 was ascertained. If a patient had a second operation within 2 years of the first, the patient was censored at the date of the second operation. A patient was defined as taking a statin or beta‐blocker if a prescription for either of these medications had been picked up within 30 days before or after surgery. The IRB at the Portland VA Medical Center approved the study with a waiver of informed consent.
Data Elements
For every patient we noted the type of vascular surgery (carotid, aortic, lower extremity bypass, or lower extremity amputation), age, sex, comorbid conditions (hypertension, cerebrovascular disease, cancer, diabetes, hyperlipidemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], coronary artery disease [CAD], heart failure), tobacco use, ethnicity, nutritional status (serum albumin), and medication use, defined as filling a prescription within 30 days before surgery (insulin, aspirin, angiotensin‐converting enzyme [ACE] inhibitor, and clonidine). Each patient was assigned a revised cardiac risk index (RCRI) score.21 For each the risk factors: use of insulin, CAD, heart failure, cerebrovascular disease, CKD, and high‐risk surgery (intrathoracic, intraperitoneal, or suprainguinal vascular procedures) 1 point was assigned. These variables were defined according to ICD‐9 codes. CKD was defined as either an ICD‐9 code for CKD or a serum creatinine > 2 mg/dL. Patients were identified by the index vascular surgery using ICD‐9 codes in the CHIPs database, and both inpatient and outpatient data were extracted.
Statistical Analysis
All patients were censored at the point of last contact up to 5 years after surgery to focus on more clinically relevant long‐term outcomes possibly associated with vascular surgery. We conducted 3 separate analyses: (1) statin exposure regardless of beta‐blocker exposure; (2) beta‐blocker exposure regardless of statin exposure, and; (3) combined exposure to statins and beta‐blockers.
Propensity score methods were used to adjust for imbalance in the baseline characteristics between statin users and nonusers, beta‐blocker users and nonusers, and combination statin and beta‐blocker users and nonusers.22, 23 The range of the propensity score distribution was similar in drug users and nonusers in the individual analyses. There was sufficient overlap between the 2 groups in each stratum. To derive propensity scores for the individual drug analyses, statin use and beta‐blocker use were modeled independently with the demographic and clinical variables using stepwise logistic regression with a relaxed entry criterion of = 0.20. Only 1 variable (hyperlipidemia) remained significantly different between statin users and nonusers, and it was included in the subsequent analyses as a potential confounder. The variable albumin had 511 missing values. To keep this variable in the propensity scores, the missing values were replaced by the predicted values of albumin from the multiple linear regression model that included the other demographic variables. The propensity scores were grouped into quintiles and used as a stratification variable in the subsequent analyses. To confirm that the propensity score method reduced the imbalances, the demographic and clinical characteristics of statin and beta‐blocker users and nonusers and combination users and nonusers were compared using Cochran‐Mantel‐Haenzel tests with the respective propensity score as a stratification variable.
For the combined use of both study drugs, we performed univariate analysis with adjustment only for RCRI (as this was a powerful predictor of mortality in our dataset; Table 1) as well as a propensity score analysis in an exploratory manner. There have been limited applications of propensity score methods to multiple treatment groups. Similar to that in the study by Huang et al.,24 we developed a multinomial baseline response logit model to obtain 3 separate propensity scores (statin only vs. none, beta‐blocker only vs. none, and both vs. none). Because of the limited sample size, the data were stratified according to the median split of each propensity score. Each score had similar ranges for each treatment group. All but 5 variables (CAD, hypertension, hyperlipidemia, ACE inhibitor use, and type of surgery) were balanced after accounting for strata. These 5 variables were then included in the final stratified Cox regression model as potential confounders.
| Variable | Level | N (%) Overall N = 3062 | Hazard ratio (95% CI) | Chi‐square P value |
|---|---|---|---|---|
| ||||
| Age in years, median (IQR) | 67 (5974) | 1.04 (1.04, 1.05)a | <.0001 | |
| Sex | Female | 45 (1) | 0.89 (0.53, 1.51) | .6704 |
| Male | 3017 (99) | 1 | 1.0000 | |
| Preoperative medical conditions | HTN | 2415 (79) | 1.32 (1.13, 1.55) | .0006 |
| CVA/TIA | 589 (19) | 1.05 (0.90, 1.22) | .5753 | |
| CA | 679 (22) | 1.55 (1.36, 1.78) | <.0001 | |
| DM | 1474 (48) | 1.75 (1.54, 1.98) | <.0001 | |
| Lipid | 872 (28) | 0.84 (0.74, 0.97) | .0187 | |
| COPD | 913 (30) | 1.68 (1.48, 1.90) | <.0001 | |
| CAD | 1491 (49) | 1.46 (1.29, 1.66) | <.0001 | |
| CHF | 747 (24) | 2.44 (2.15, 2.77) | <.0001 | |
| CKD | 443 (14) | 2.32 (2.00, 2.69) | <.0001 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 2.73 (2.28, 3.28) | <.0001 |
| Albumin 3.5 | 596 (23) | 2.70 (2.35, 3.10) | <.0001 | |
| Medication use | Aspirin | 1789 (58) | 1.10 (0.97, 1.25) | .1389 |
| ACE inhibitor | 1250 (41) | 0.93 (0.82, 1.06) | .2894 | |
| Insulin | 478 (16) | 1.31 (1.12, 1.54) | .0007 | |
| Clonidine | 115 (4) | 1.68 (1.29, 2.20) | .0001 | |
| Perioperative medication | Statinb | 1346 (44) | 0.66 (0.58, 0.75) | <.0001 |
| Beta‐blockerc | 1617 (53) | 0.74 (0.66, 0.84) | <.0001 | |
| Statin only | 414 (14) | 0.69 (0.56, 0.84) | .0002 | |
| Beta‐blocker only | 685 (22) | 0.81 (0.69, 0.95) | .0079 | |
| Statin and beta‐blocker | 932 (30) | 0.57 (0.49, 0.67) | <.0001 | |
| Noned | 1031 (34) | 1 | 1.0000 | |
| Type of surgery | Aorta | 232 (8) | 1.34 (1.01, 1.77) | <.0001 |
| Carotid | 875 (29) | 1 | ||
| Amputation | 867 (28) | 2.80 (2.36, 3.32) | ||
| Bypass | 1088 (36) | 1.57 (1.32, 1.87) | ||
| RCRI | 0 | 1223 (40) | 1 | <.0001 |
| 1 | 1005 (33) | 1.33 (1.13, 1.55) | ||
| 2 | 598 (20) | 2.22 (1.88, 2.62) | ||
| 3 | 200 (7) | 3.16 (2.54, 3.93) | ||
| 4 | 36 (1) | 4.82 (3.15, 7.37) | ||
| Year surgery occurred | 1998 | 544 (18) | 1 | .6509 |
| 1999 | 463 (15) | 0.91 (0.75, 1.10) | ||
| 2000 | 420 (14) | 0.93 (0.77, 1.13) | ||
| 2001 | 407 (13) | 0.93 (0.75, 1.14) | ||
| 2002 | 374 (12) | 1.12 (0.90, 1.40) | ||
| 2003 | 371 (12) | 1.15 (0.90, 1.47) | ||
| 2004 | 407 (13) | 0.97 (0.72, 1.31) | ||
| 2005 | 76 (3) | 0.68 (0.28, 1.65) | ||
| Tobacco user | Yes | 971 (32) | 0.90 (0.76, 1.08) | .4762 |
| No | 649 (21) | 1 | ||
| Null | 1442 (47) | 0.96 (0.81, 1.13) | ||
| Ethnicity | White | 563 (18) | 1 | .0366 |
| Other | 39 (1) | 0.98 (0.55, 1.76) | ||
| Unknown | 2460 (80) | 1.24 (1.05, 1.46) | ||
To comment on patient‐specific risk by stratification with the RCRI, we used a fixed time point of the 2‐year mortality estimated from the Cox regression model to analyze use of study drugs singly or in combination compared with use of neither.
Chi‐square tests were used to categorize and compare demographic and clinical characteristics of statin users and nonusers, of beta‐blocker users and nonusers, and combination users and nonusers. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Stratified or unstratified Cox regression was used to estimate the hazard ratios of statins and beta‐blockers, with or without adjustment for the propensity score. All analyses were performed using SAS (Statistical Analysis System) software, version 9.1.
RESULTS
Patient Characteristics
The study included 3062 patients whose median age was 67 (interquartile range, 5974; Table 1). Ninety‐nine percent of the study patients were men. Overall, ambulatory use of statins and beta‐blockers was found in 44% and 53% of patients, respectively, and combination use occurred in 30%. Sixty‐one percent of patients had an RCRI of 1 or greater; among them 71% were statin users (Table 2), 68% were beta‐blocker users (Table 3), and 75% were combination users (Table 4). Sixty‐four percent of surgeries were either lower extremity bypass or amputation, 29% were carotid, and 8% aortic. Median follow‐up for all patients was 2.7 years (interquartile range, 1.24.6). Of the whole study cohort, 53% and 62% filled a prescription for a statin or beta‐blocker within 1 year of surgery, respectively, and 58% and 67% filled a prescription within 2 years of surgery, respectively. Overall mortality at 30 days was 3%, at 1 year 14%, and at 2 years 22%.
| Variable, N (%) | Level | Overall (N = 3062) | Statin users (N = 1346 [44]) | Statin nonusers (N = 1716 [56]) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 66 (5973) | 68 (6075) | <.0001 | .9934 | |
| Sex | Female | 45 (1) | 15 (1) | 30 (2) | .1480 | .7822 |
| Male | 3017 (99) | 1331 (99) | 1686 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1176 (87) | 1239 (72) | <.0001 | .2984 |
| CVA/TIA | 589 (19) | 328 (24) | 261 (15) | <.0001 | .3935 | |
| CA | 679 (22) | 307 (23) | 372 (22) | .4550 | .8404 | |
| DM | 1474 (48) | 666 (49) | 808 (47) | .1883 | .5504 | |
| Lipid | 872 (28) | 629 (47) | 243 (14) | <.0001 | .0246 | |
| COPD | 913 (30) | 411 (31) | 502 (29) | .4419 | .8435 | |
| CAD | 1491 (49) | 837 (62) | 654 (38) | <.0001 | .4720 | |
| CHF | 747 (24) | 370 (27) | 377 (22) | .0004 | .4839 | |
| CKD | 443 (14) | 208 (15) | 235 (14) | .1698 | .9990 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 101 (8) | 128 (7) | .9629 | .6911 |
| Albumin 3.5 | 596 (23) | 191 (16) | 405 (30) | <.0001 | .5917 | |
| Medication use | Aspirin | 1789 (58) | 904 (67) | 885 (52) | <.0001 | .6409 |
| Ace inhibitor | 1250 (41) | 712 (53) | 538 (31) | <.0001 | .6075 | |
| Beta‐blocker | 1220 (40) | 767 (57) | 453 (26) | <.0001 | .4058 | |
| Insulin | 478 (16) | 254 (19) | 224 (13) | <.0001 | .7919 | |
| Clonidine | 115 (4) | 61 (5) | 54 (3) | .0454 | .6141 | |
| Type of surgery | Aorta | 232 (8) | 106 (8) | 126 (7) | <.0001 | .9899 |
| Carotid | 875 (29) | 510 (38) | 365 (21) | |||
| Amputation | 867 (28) | 274 (20) | 593 (35) | |||
| Bypass | 1088 (36) | 456 (34) | 632 (37) | |||
| RCRI | 0 | 1223 (40) | 389 (29) | 834 (49) | <.0001 | .9831 |
| 1 | 1005 (33) | 507 (38) | 498 (29) | |||
| 2 | 598 (20) | 318 (24) | 280 (16) | |||
| 3 | 200 (7) | 109 (8) | 91 (5) | |||
| 4 | 36 (1) | 23 (1) | 13 (0.76) | |||
| Year of surgery | 1998 | 544 (18) | 134 (10) | 410 (24) | <.0001 | 1 |
| 1999 | 463 (15) | 163 (12) | 300 (17) | |||
| 2000 | 420 (13) | 178 (13) | 242 (14) | |||
| 2001 | 407 (13) | 188 (14) | 219 (13) | |||
| 2002 | 374 (12) | 194 (14) | 180 (10) | |||
| 2003 | 371 (12) | 209 (16) | 162 (9) | |||
| 2004 | 407 (13) | 229 (17) | 178 (10) | |||
| 2005 | 76 (3) | 51 (4) | 25 (1.5) | |||
| Tobacco user | Yes | 971 (32) | 494 (37) | 477 (28) | <.0001 | .9809 |
| No | 649 (21) | 335 (25) | 314 (18) | |||
| Null | 1442 (47) | 517 (38) | 925 (54) | |||
| Ethnicity | White | 563 (18) | 263 (20) | 300 (17) | .1544 | .9475 |
| Other | 39 (1) | 13 (1) | 26 (1.5) | |||
| Unknown | 2460 (80) | 1070 (79) | 1390 (81) | |||
| Variable, N (%) | Level | Overall N = 3062 | BB users N = 1617 (53) | Non‐BB users N = 1445 (47) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|
| ||||||
| Age in years, median (IQR) | 67 (5974) | 67 (5975) | 68 (6076) | .0526 | .7671 | |
| Sex | Female | 45 (1) | 12 (1) | 33 (2) | .0004 | .585 |
| Male | 3017 (99) | 1605 (99) | 1412 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 1398 (86) | 1017 (70) | <.0001 | .1837 |
| CVA/TIA | 589 (19) | 364 (23) | 225 (16) | <.0001 | .3206 | |
| CA | 679 (22) | 359 (22) | 320 (22) | .9701 | .4288 | |
| DM | 1474 (48) | 739 (46) | 735 (51) | .0043 | .6329 | |
| Lipid | 872 (28) | 555 (34) | 317 (22) | <.0001 | .7180 | |
| COPD | 913 (30) | 487 (30) | 426 (29) | .7007 | .8022 | |
| CAD | 1491 (49) | 975 (60) | 516 (36) | <.0001 | .3496 | |
| CHF | 747 (24) | 439 (27) | 308 (21) | .0002 | .6509 | |
| CKD | 443 (14) | 248 (15) | 195 (13) | .1480 | .8544 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 132 (8) | 97 (7) | .1277 | .5867 |
| Albumin 3.5 | 596 (23) | 252 (18) | 344 (30) | <.0001 | .5347 | |
| Medication use | Aspirin | 1789 (58) | 1046 (65) | 743 (51) | <.0001 | .4942 |
| Ace inhibitor | 1250 (41) | 760 (47) | 490 (34) | <.0001 | .4727 | |
| Statin | 1220 (40) | 932 (58) | 414 (29) | <.0001 | .3706 | |
| Insulin | 478 (16) | 255 (16) | 223 (15) | .7973 | .5991 | |
| Clonidine | 115 (4) | 77 (5) | 38 (3) | .0019 | .8241 | |
| Type of surgery | Aorta | 232 (8) | 176 (11) | 56 (4) | <.0001 | .5664 |
| Carotid | 875 (29) | 515 (32) | 360 (25) | |||
| Amputation | 867 (28) | 339 (21) | 528 (37) | |||
| Bypass | 1088 (36) | 587 (36) | 501 (35) | |||
| RCRI | 0 | 1223 (40) | 518 (32) | 705 (49) | <.0001 | .5489 |
| 1 | 1005 (33) | 583 (36) | 422 (29) | |||
| 2 | 598 (20) | 358 (22) | 240 (17) | |||
| 3 | 200 (7) | 130 (8) | 70 (5) | |||
| 4 | 36 (1) | 28 (2) | 8 (1) | |||
| Year of surgery | 1998 | 544 (18) | 200 (12) | 344 (24) | <.0001 | .3832 |
| 1999 | 463 (15) | 211 (13) | 252 (17) | |||
| 2000 | 420 (13) | 210 (13) | 210 (15) | |||
| 2001 | 407 (13) | 209 (13) | 198 (14) | |||
| 2002 | 374 (12) | 220 (14) | 154 (11) | |||
| 2003 | 371 (12) | 238 (15) | 133 (9) | |||
| 2004 | 407 (13) | 279 (17) | 128 (9) | |||
| 2005 | 76 (3) | 50 (3) | 26 (2) | |||
| Tobacco user | Yes | 971 (32) | 569 (35) | 402 (28) | <.0001 | .9025 |
| No | 649 (21) | 370 (23) | 279 (19) | |||
| Null | 1442 (47) | 678 (42) | 764 (53) | |||
| Ethnicity | White | 563 (18) | 309 (19) | 254 (18) | .4962 | .8762 |
| Other | 39 (1) | 19 (1) | 20 (1) | |||
| Unknown | 2460 (80) | 1289 (80) | 1171 (81) | |||
| N (%) Variable | Level | Overall N = 3062 | BB alone N = 685 (22) | Statin alone N = 414 (14) | Both drugs N = 932 (30) | Neither drug N = 1031 (34) | Unadjusted P value | Propensity‐adjusted P value |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age in years, median (IQR) | 67 (5974) | 68 (6075) | 67 (6075) | 66 (5973) | 69 (6076) | .0029 | .9824 | |
| Sex | Female | 45 (1) | 7 (1) | 10 (2) | 5 (1) | 23 (2) | .0042 | .5815 |
| Male | 3017 (99) | 678 (99) | 404 (98) | 927 (99) | 1008 (98) | |||
| Preoperative medical conditions | HTN | 2415 (79) | 560 (82) | 338 (82) | 838 (90) | 679 (66) | <.0001 | .0251 |
| CVA/TIA | 589 (19) | 127 (19) | 91 (22) | 237 (25) | 134 (13) | <.0001 | .4543 | |
| CA | 679 (22) | 150 (22) | 98 (24) | 209 (22) | 222 (22) | .8379 | .9749 | |
| DM | 1474 (48) | 291 (43) | 218 (53) | 448 (48) | 517 (50) | .0031 | .3943 | |
| Lipid | 872 (28) | 125 (18) | 199 (48) | 430 (46) | 118 (11) | <.0001 | <.0001 | |
| COPD | 913 (30) | 199 (29) | 123 (30) | 288 (9) | 303 (29) | .8475. | .9769 | |
| CAD | 1491 (49) | 327 (48) | 189 (46) | 648 (70) | 327 (32) | <.0001 | <.0001 | |
| CHF | 747 (24) | 163 (24) | 94 (23) | 276 (30) | 214 (21) | <.0001 | .7031 | |
| CKD | 443 (14) | 92 (13) | 52 (13) | 156 (17) | 143 (14) | .1120 | .8364 | |
| Blood chemistry | Creatinine > 2 | 229 (7) | 52 (8) | 21 (5) | 80 (9) | 76 (7) | .1619 | .7184 |
| Albumin 3.5 | 596 (23) | 134 (20) | 73 (20) | 118 (14) | 271 (34) | <.0001 | .2846 | |
| Medication use | Aspirin | 1789 (58) | 398 (58) | 256 (62) | 648 (70) | 487 (47) | <.0001 | .2334 |
| Ace inhibitor | 1250 (41) | 264 (39) | 216 (52) | 496 (53) | 274 (27) | <.0001 | .0216 | |
| Insulin | 478 (16) | 93 (14) | 92 (22) | 162 (17) | 131 (13) | <.0001 | .2952 | |
| Clonidine | 115 (4) | 28 (4) | 12 (3) | 49 (5) | 26 (3) | .0107 | .8035 | |
| Type of surgery | Aorta | 232 (8) | 78 (11) | 8 (2) | 98 (11) | 48 (5) | <.0001 | .008 |
| Carotid | 875 (29) | 165 (24) | 160 (39) | 350 (38) | 200 (19) | |||
| Amputation | 867 (28) | 164 (24) | 99 (24) | 175 (19) | 429 (42) | |||
| Bypass | 1088 (36) | 278 (41) | 147 (36) | 309 (33) | 354 (34) | |||
| RCRI | 0 | 1223 (40) | 288 (42) | 159 (38) | 230 (25) | 546 (53) | <.0001 | .5392 |
| 1 | 1005 (33) | 219 (32) | 143 (35) | 364 (39) | 279 (27) | |||
| 2 | 598 (20) | 125 (18) | 85 (21) | 233 (25) | 155 (15) | |||
| 3 | 200 (7) | 46 (7) | 25 (6) | 84 (9) | 45 (4) | |||
| 4 | 36 (1) | 7 (1) | 2 (0) | 21 (2) | 6 (1) | |||
| Year of surgery | 1998 | 544 (18) | 126 (18) | 60 (14) | 74 (8) | 284 (28) | <.0001 | .3105 |
| 1999 | 463 (15) | 111 (16) | 63 (15) | 100 (11) | 189 (18) | |||
| 2000 | 420 (13) | 87 (13) | 55 (13) | 123 (13) | 155 (15) | |||
| 2001 | 407 (13) | 84 (12) | 63 (15) | 125 (13) | 135 (13) | |||
| 2002 | 374 (12) | 81 (12) | 55 (13) | 139 (15) | 99 (10 | |||
| 2003 | 371 (12) | 85 (13) | 56 (14) | 153 (16) | 77 (7) | |||
| 2004 | 407 (13) | 96 (14) | 46 (11) | 183 (20) | 82 (8) | |||
| 2005 | 76 (3) | 15 (2) | 16 (4) | 35 (4) | 10 (1) | |||
| Tobacco user | Yes | 971 (32) | 227 (33) | 152 (37) | 342 (37) | 250 (24) | <.0001 | .3914 |
| No | 649 (21) | 134 (20) | 99 (24) | 236 (25) | 180 (17) | |||
| Null | 1442 (47) | 324 (47) | 163 (39) | 354 (38) | 601 (58) | |||
| Ethnicity | White | 563 (18) | 115 (17) | 69 (17) | 194 (21) | 185 (18) | .2821 | .9771 |
| Other | 39 (1) | 10 (1) | 4 (1) | 9 (1) | 16 (2) | |||
| Unknown | 2460 (80) | 560 (82) | 341 (82) | 729 (78) | 830 (81) | |||
Univariate Survival Analysis
Univariate Cox regression analysis revealed a strong effect of the composite RCRI, which was predictive of mortality in a linear fashion over the course of the study compared with an RCRI of 0 (Table 1). Univariate analysis showed significant associations with decreased mortality for statins (hazard ratio [HR] = 0.66 [95% CI 0.580.75], P < .0001) and beta‐blockers (HR = 0.74 [95% CI 0.660.84], P = .0001); see Table 1. Of note, compared with that in 1998, mortality did not change for all the years for which data were complete. In addition, compared with taking neither study drug, taking a statin only, a beta‐blocker only, or both was associated with decreased mortality (P = .0002, P = .0079, and P < .0001, respectively; Fig. 1).

Propensity Score Analysis for Single Study Drug
There were significant differences in demographic and clinical characteristics between statin‐users versus statin nonusers, and between beta‐blocker users versus beta‐blocker nonusers. These differences became insignificant after the propensity score adjustment, with the exception of hyperlipidemia for statins, P = .02, which was added to the model as a confounder (Table 2). The distribution of the propensity scores was similar for study drug users and nonusers within each stratum. The association with decreased mortality remained significant after adjusting for propensity score (for statins, HR = 0.78 [95% CI 0.670.92, P = .0050], number needed to treat [NNT] = 22; for beta‐blockers HR = 0.84 [95% CI 0.730.96, P = .0103], NNT = 30; Fig. 2).

Combination Study Drugs and Revised Cardiac Risk Index: Univariate Analysis
We wanted our results to closely model those of combination use of the study drugs by patients in a clinical situation. Therefore, we first examined the effects of ambulatory statins alone, beta‐blockers alone, and a combination of statins and beta‐blockers by univariate analysis. Grouping patients by study drug use has not commonly been reported in the literature. We also examined the statistical interaction between the study drugs and the RCRI. The main‐effects model adequately explained all‐cause mortality, and the statistical interaction between the study drugs and the RCRI was not significant.
The final univariate Cox regression model, which compared use of a statin alone, a beta‐blocker alone, and a statin and beta‐blocker in combination with using neither study drug, demonstrated that the combination of statins and beta‐blockers had an HR over the whole study period of 0.43 (95% CI 0.360.51, P < .0001), statins alone had an HR of 0.59 (95% CI 0.480.72, P < .0001), and beta‐blockers alone had an HR of 0.71 (95% CI 0.610.83, P < .0001).
To clarify the effects of the study drugs on patients at different levels of risk, we stratified patients by the RCRI and evaluated the effects of the study drugs on mortality at 2 years, comparing the results to a referent of taking no study drugs. The use of both a statin and a beta‐blocker consistently produced a relative risk reduction (RRR) of approximately 50% with an NNT of 410, with highly statistically significant results for patients at all levels of risk (Table 5). As patient risk level increased, the NNT decreased, consistent with higher‐risk patients benefiting most from combination therapy with statins and beta‐blockers.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.19 | |||
| BB | 288 (73) | 0.14 | 20 | 0.27 | .0023 | |
| Statin | 159 (30) | 0.12 | 14 | 0.39 | <.0001 | |
| Statin+BB | 230 (23) | 0.09 | 10 | 0.54 | <.0001 | |
| 1 | None | 279 (130) | 0.28 | |||
| BB | 219 (71) | 0.21 | 14 | 0.26 | .0028 | |
| Statin | 143 (41) | 0.17 | 10 | 0.37 | <.0001 | |
| Statin+BB | 364 (73) | 0.13 | 7 | 0.53 | <.0001 | |
| 2 | None | 155 (100) | 0.43 | |||
| BB | 125 (60) | 0.33 | 10 | 0.23 | .0045 | |
| Statin | 85 (42) | 0.28 | 7 | 0.35 | <.0001 | |
| Statin+BB | 233 (72) | 0.22 | 5 | 0.50 | <.0001 | |
| 3 | None | 51 (39) | 0.59 | |||
| BB | 53 (29) | 0.47 | 9 | 0.20 | .0296 | |
| Statin | 27 (14) | 0.41 | 6 | 0.31 | .0014 | |
| Statin+BB | 105 (52) | 0.32 | 4 | 0.46 | <.0001 | |
In addition, the range of outcomes can be clearly seen for both patient‐specific risk level and study drug use. For example, overall mortality at 2 years for all patients was 22%. For the study drugs, mortality ranged from 16% for patient who used both a statin and a beta‐blocker to 27% for those patients who used neither study drug. The use of the RCRI showed that the healthiest patients who were taking both a statin and a beta‐blocker did the best, with a 2‐year mortality of 9%, compared with the sickest patients who were taking neither study drug, whose 2‐year mortality was 59%. Use of both study drugs by the sickest patients was associated with a reduction in 2‐year mortality to 32% (P < .0001; Table 5).
Propensity Score Analysis of Use of Combination Study Drugs
Because there was very limited literature to guide us in the use of propensity score analysis of multiple treatment groups, we performed these analyses in an exploratory manner. There were significant differences between combination statin and beta‐blocker users and nonusers. These differences became insignificant after adjusting for propensity score, except for the 5 variables previously mentioned, which were added to the model as potential confounders (Table 4). The propensity‐adjusted Cox regression model comparing use of each study drug alone and in combination with taking neither over the whole study period still showed an association with decreased mortality. The combination of statins and beta‐blockers had an HR of 0.56 (95% CI 0.420.74), P < .0001; statins alone had an HR of 0.79 (95% CI 0.620.99), P = .0472; and beta‐blockers alone had an HR of 0.80 (95% CI 0.670.94), P = .0183.
Combination Study Drugs and Revised Cardiac Risk Index: Propensity Analysis
We performed the stratified Cox regression adjusted for the propensity scores for each level of RCRI and estimated 2‐year mortality. The use of both a statin and a beta‐blocker compared with using none was still consistently statistically significant, with an RRR of approximately 36% and an NNT of 820 for all levels of patient risk (Table 6). Possibly because of the reduced number of patients in each RCRI category, neither single‐agent study drug compared with none showed a statistically significant decrease in mortality at any level of patient‐specific risk (Table 6). Again, higher‐risk patients benefited most from combination therapy.
| RCRI | Drug | N (Deaths) | Mortality | NNT | RRR | P value |
|---|---|---|---|---|---|---|
| ||||||
| 0 | None | 546 (176) | 0.14 | |||
| BB | 288 (73) | 0.11 | 47 | 0.16 | .3778 | |
| Statin | 159 (30) | 0.11 | 40 | 0.19 | .2902 | |
| Statin+BB | 230 (23) | 0.08 | 20 | 0.38 | .0184 | |
| 1 | None | 279 (130) | 0.21 | |||
| BB | 219 (71) | 0.17 | 32 | 0.15 | .2837 | |
| Statin | 143 (41) | 0.17 | 27 | 0.18 | .1969 | |
| Statin+BB | 364 (73) | 0.13 | 14 | 0.37 | .0038 | |
| 2 | None | 155 (100) | 0.29 | |||
| BB | 125 (60) | 0.25 | 24 | 0.15 | .3295 | |
| Statin | 85 (42) | 0.24 | 20 | 0.17 | .2396 | |
| Statin+BB | 233 (72) | 0.18 | 10 | 0.36 | .0077 | |
| 3 | None | 51 (39) | 0.42 | |||
| BB | 53 (29) | 0.37 | 19 | 0.13 | .3553 | |
| Statin | 27 (14) | 0.36 | 16 | 0.15 | .2653 | |
| Statin+BB | 105 (52) | 0.28 | 8 | 0.33 | .0106 | |
Study Drug Timing: Subcohort Analysis
A subcohort analysis was performed to clarify the timing of the study drugs. Of the patients taking statins, 69 of 1346 (5.1%) took the drug before surgery only, 119 of 1346 (8.8%) took the drug after surgery only, and 1158 of 1346 (86%) took the drug both before and after surgery. Of the patients taking beta‐blockers, 54 of 1617 (3.3%) took the drug before surgery only, 397 of 1617 (24.6%) took the drug after surgery only, and 1166 of 1617 (72.1%) took the drug both before and after surgery. The use of statins and beta‐blockers had a correlation of 0.29 (contingency coefficient).
DISCUSSION
In this retrospective observational study we found that after vascular surgery the use of propensity‐adjusted statins compared with no use of statins reduced long‐term mortality over the study period by 22%, with a number needed to treat of 22, and the use of propensity‐adjusted beta‐blockers compared with no use also reduced long‐term mortality, by 16%, with a number needed to treat of 30. There were no statistically significant differences between outcomes of statin users and beta‐blocker users. In addition, using a propensity‐adjusted combination of statin and beta‐blockers compared with using neither decreased mortality overall by 44%, with a number needed to treat of 9. We focused on the use of outpatient drugs 30 days before or after surgery, as the timing of potentially beneficial medications has not been clearly established. Over time, more patients originally categorized as not taking a study drug began taking one, so that by 2 years after surgery, 58% of the patients were taking a statin, and 67% were taking a beta‐blocker, compared with 44% and 53%, respectively, of the study cohort initially. This would have made it more difficult to demonstrate a difference between these 2 groups. As more patients ended up taking the study drugs over time than the originally identified study drug users, and a mortality difference was still demonstrated, there may be an increased advantage in taking the study drugs around the time of surgery. As our focus was on long‐term postoperative mortality, which has not commonly been studied according to the literature, we preferred to also focus on long‐term, chronic ambulatory use of the study drugs. We did perform a subcohort analysis of the timing of study drug use. This confirmed that this cohort predominately comprised long‐term users of the study drugs who took the drug both before and after surgery. This study was not powered to comment on 30‐day mortality.
Perioperative beta‐blockers have been shown in retrospective cohort studies, case‐control studies, randomized clinical trials, meta‐analyses, and systematic reviews to decrease mortality and morbidity after noncardiac surgery. Although recent studies have not shown a benefit for more moderate‐ to low‐risk subjects,11, 12 perioperative beta‐blockers are still considered an indicator of health care quality in the United States.25 At present, perioperative beta‐blockers have an ACC/AHA class I indication (should be administered; Evidence level C) for patients undergoing vascular surgery with a positive stress test, and class IIa indication (reasonable to administer; Evidence level B) for vascular surgery patients with coronary heart disease or multiple clinical risk factors.26 A recent observational study in noncardiac surgery patients demonstrated perioperative beta‐blockers may be most helpful to prevent in‐hospital death after surgery of patients with an RCRI 2 and may be unhelpful or harmful for patients with an RCRI 1.27 Our univariate RCRI findings did not agree, as we found all patients whatever their level of risk benefited from perioperative use of beta‐blockers, alone or in combination. Our study population was older, had a higher RCRI, and underwent comparatively higher‐risk surgery, we were investigating longer‐term outcome, and we concentrated on ambulatory use of beta‐blockers, which may have contributed to the divergence in findings. Our propensity‐adjusted RCRI analysis did not show beta‐blockers associated with any change in mortality at any patient risk level. This may be, in part, because of the reduced number of patients in the RCRI strata. RCRI stratum‐specific analysis is limited by the number of patients and deaths in each RCRI stratum. For example, the power to detect a 2‐year difference of 10% (or 5%) between statin users and nonusers is approximately 99% (66%), 99% (59%), 92% (42%), and 61% (23%) for RCRI = 0, 1, 2, and 3, respectively.
Case‐control and retrospective cohort studies and one randomized clinical trial have shown perioperative statins to decrease either short‐term cardiovascular morbidity or mortality up to 30 days after surgery, and a limited number of retrospective cohort studies have shown reduced mortality for longer‐term follow‐up.1418, 28 There was one previous preliminary study of vascular surgery patients that demonstrated an additive benefit of using statins and beta‐blockers up to 30 days after surgery. This additive effect was only observed in patients with an RCRI 3.29 The results of our longer‐term follow‐up study of a larger cohort did not agree. Compared with patients who did not take a statin or a beta‐blocker, those patients who took both study drugs decreased their relative risk of mortality by approximately 36% in propensity‐ adjusted analysis and by about 50% in univariate analysis, regardless of patient‐specific risk level. For example, in the propensity‐adjusted analysis, the healthiest patients with an RCRI of 0 who took both study drugs had lower mortality than patients who took neither study drug, 8% versus 14%, a 38% relative reduction in mortality, with a number needed to treat of 20 (P = .0184).
In addition, the use of the RCRI for the first time highlighted the divergent long‐term mortality rates for patient‐specific risk levels and the striking long‐term associations of the perioperative use of ambulatory statins, beta‐blockers, and both drugs in combination with improved long‐term mortality. The long‐term use of the study drugs may indeed help all patients with atherosclerotic vascular disease, regardless of surgery. However, vascular surgery presents an opportunity for medical intervention, and our results are most applicable for these patients. In addition, the perioperative state has a unique physiology of acute and intense inflammation and thrombosis. Beta‐blockers and statins have antiadrenergic, anti‐inflammatory, and antithrombotic properties that may be beneficial during this high‐risk state.
Our findings should be viewed with some caution. The use of ICD‐9 codes and demographic data is dependent on the documentation and coding of comorbidities in the medical record and database. The use of statins and beta‐blockers was not random, and patients who took statins and beta‐blockers were different than those who did not. We used rigorous propensity and multivariate analysis, including controlling for clonidine, which has been shown to decrease death after vascular surgery.30 We also controlled for serum albumin level, which has been shown to be a leading predictor of postoperative death.31 We further separately stratified patients by RCRI, as this was a powerful predictor of death in the univariate analysis, but because of the retrospective nature of the study, unmeasured confounders may exist. Only 1% of the study patients were women, which is a limitation of the study. This administrative database is also limited by not having information on tobacco use for 47% of the patients and by not knowing ethnicity for 80% of the patients.
The use of perioperative statins and beta‐blockers used alone or in combination was associated with a reduction in long‐term mortality for vascular surgery patients, and combination use benefited patients at all levels of risk. Higher‐risk patients benefited most by taking both study drugs. These findings extend prior data, add to the natural history of long‐term postoperative outcomes, and also support clinical trials that would evaluate the prospective use of both these medications in vascular surgery patients with attention to patient‐specific risk level. Until the results of 2 randomized controlled trials become available, which may further clarify the use of perioperative statins and beta‐blockers in noncardiac, and noncardiac vascular surgery,13, 32 the use of statins and beta‐blockers should be considered for all patients undergoing vascular surgery. In addition, long‐term use of statins and beta‐blockers for all patients with atherosclerotic vascular disease should be considered.33
Acknowledgements
The authors thank LeAnn Snodgrass for assistance with data extraction and management. This work was funded by the Oregon Health & Science University Medical Research Foundation.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
- ,,,,,.The influence of perioperative myocardial infarction on long‐term prognosis following elective vascular surgery.Chest.1998;113:681–686.
- ,,, et al.Postoperative and amputation‐free survival outcomes after femorodistal bypass grafting surgery: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program.J Vasc Surg.2001;34:283–290.
- ,,,.Perioperative‐ and long‐term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population.Anesth Analg1999;89:849–855.
- ,.Very late survival after vascular surgery.J Surg Res.2002;105(2):109–114.
- ,,, et al.Women have increased risk of perioperative myocardial infarction and higher long‐term mortality rates after lower extremity arterial bypass grafting.J Vasc Surg.1999;29:807–812; discussion12–13.
- ,,,,,.Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery.The Study of Perioperative Ischemia Research Group.N Engl J Med.1990;323:1781–1788.
- ,,,,.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:851–857.
- ,,, et al.Perioperative myocardial ischemia in patients undergoing noncardiac surgery—I: Incidence and severity during the 4 day perioperative period.The Study of Perioperative Ischemia (SPI) Research Group.J Am Coll Cardiol.1991;17:843–850.
- ,,,.Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.Multicenter Study of Perioperative Ischemia Research Group.N Engl J Med.1996;335:1713–1720.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.Effect of perioperative beta blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial.BMJ.2006;332:1482.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA2004;291:2092–2099.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion75–6.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,,,,.The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery.Int J Cardiol.2005;104:264–268.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,,.Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration.Ann Epidemiol.1996;6(2):102–109.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- ,.A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use.Stat Med.2006;25:2084–2106.
- ,,,,.Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care.Health Serv Res2005;40(1):253–78.
- ,,.Making Health Care Safer: A Critical Analysis of Patient Safety Practices: Evidence Report/Technology Assessment.Rockville, Md:AHRQ;2001. Report No. 43.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,, et al.Association between long‐term statin use and mortality after successful abdominal aortic aneurysm surgery.Am J Med.2004;116(2):96–103.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,,,,.Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study.Arch Surg.1999;134(1):36–42.
- ,,, et al.Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study.Am Heart J.2004;148:1047–1052.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation.Circulation.2006;113:e463–e654.
Copyright © 2007 Society of Hospital Medicine
Hypoglycemia in Hospitalized Patients / Varghese et al.
Glycemic control in the inpatient setting has received increasing attention in recent years, with the demonstration that appropriate blood glucose (BG) control prevents adverse events in both intensive care unit (ICU) and non‐ICU settings.1 Recent recommendations set target blood glucose levels near euglycemia for most hospitalized patients.1 Unfortunately, the risk of hypoglycemia increases with tighter glycemic control,2 and hypoglycemia may result in catastrophic events.35 Although hyperglycemia is associated with postoperative infection,6 and effective management decreases wound infections,7 few reports have detailed the hypoglycemia rates among surgical patients.8 Hypoglycemia rates on medical services are as high as 28%,9, 10 and efforts to achieve more normal BG levels in hospitalized patients have been associated with more hypoglycemia.11
We undertook a study of hypoglycemia in all adult hospitalized patients receiving hypoglycemic therapy at our institution. The purpose of this study was to determine the incidence, natural history, associations, and consequences of hypoglycemia in this broad inpatient population in order to have a baseline prior to introducing any formal hospital strategies to achieve the newer targets for glycemic control.
Research Design and Methods
Thomas Jefferson University Hospital (TJUH) is a 675‐bed acute care teaching institution in center‐city Philadelphia with more than 30,000 patient admissions each year. We undertook a prospective, consecutive medical record review from August 16, 2004, to November 15, 2004, of hospitalized patients who had experienced at least 1 hypoglycemic episode, defined as at least one blood glucose (BG) 60 mg/dL within 48 hours of administration of an antihyperglycemic agent in the hospital. The definition of hypoglycemia was consistent with our hospital policies and a compromise between the BG 70 mg/dL proposed by the American Diabetes Association (ADA) hypoglycemia workgroup12 and the BG 40 mg/dL used by authors studying glycemic control in the ICU.13, 14
Hypoglycemic episodes were identified by a daily electronic search of the online medication administration record (MAR) where nurses document all point‐of‐care (POC) BG values. Two of the authors (P.V. and V.G.) reviewed the medical record for each episode and excluded pediatric (<18 years), emergency department, and maternity patients. Intensive care, step‐down, and medical/surgical unit patients were all included if the hypoglycemia had occurred within 48 hours of hospital administration of an antihyperglycemic agent. All medication orders at TJUH are placed through the computerized prescriber order entry (CPOE) system (Centricity Enterprise), which links all antihyperglycemic agents to a standardized hypoglycemia treatment protocol. The protocol includes instructions to administer glucose and/or glucagon and check the BG 15 minutes after a hypoglycemic episode. We established operational definitions prior to chart review (Appendix). A symptomatic hypoglycemia‐related adverse event was defined as any documented event occurring at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased length of hospitalization. We did not include following our hypoglycemic protocol with the administration of 50% dextrose or glucagon as a change of care, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
We searched the University Health System Consortium Clinical Database (CDB) to quantify the number of patients at TJUH receiving any antihyperglycemic agent during the study period and to identify the specific agents received. The CDB receives all patient, physician, and pharmacy dose‐ specific information from the hospital clinical and billing information systems. We defined subgroups of patients taking insulin(s) only, taking oral agent(s) only, and taking a combination.
Differences between proportions were evaluated using the chi‐square statistic; differences between means were evaluated using the Student t test. Probabilities of the null hypothesis less than .05 were considered significant.
The project was approved by the Institutional Review Board for Human Subjects at Thomas Jefferson University.
RESULTS
Over the 2‐month study period 8140 patients were admitted, of whom 2174 (27%) received an antihyperglycemic agent. Five hundred and sixty‐eight hypoglycemic episodes (BG 60 mg/dL) occurred in 265 patients. We excluded 84 episodes among 59 patients who did not receive antihyperglycemic agents, resulting in 484 episodes of hypoglycemia occurring within 48 hours of hospital administration of an antihyperglycemic agent in 206 patients, an average of 5.26 episodes per day. Of the 2174 of patients receiving antihyperglycemic agents, 206 (9.5%) experienced 1 or more episodes of hypoglycemia.
Patient ages ranged from 20 to 93 years, with an average of 62 years. Fifty‐seven percent (118 of 206) of participants were female. About one‐fourth of all episodes (23.8%) occurred in the ICU setting. The distribution of patients by decade and their ICU status are presented in Figure 1. Of the 206 patients, 29% (59) had type 1 diabetes mellitus (DM), 49% (102) had type 2 DM, 1% (2) had new‐onset diabetes, and 21% (43) had no diagnosis of DM. Of the 484 hypoglycemic episodes, 37.8% occurred in patients with type 1 DM, 46.9% in patients with type 2 DM, and 0.6% in patients with new‐onset DM. The remaining 14.5% occurred in patients with no documented history of DM, although they were receiving antihyperglycemic agents. More than 1 episode was experienced by 44% of patients, and 12% experienced 5 or more episodes.

The BG was between 51 and 60 mg/dL in 282 of the episodes (58.2%), between 41 and 50 mg/dL in 149 episodes (30.8%) and 40 mg/dL or less in 53 episodes (11%). In 20 episodes (4.1% of episodes, representing fewer than 1% of all patients receiving an antihyperglycemic agent), a symptomatic hypoglycemia‐related adverse event was documented. All but 1 adverse event occurred outside the ICU. Ten of these events (2.1% of all hypoglycemic episodes) in 10 patients involved symptoms including headache, agitation, disorientation, and tremors. Of these patients 9 had type 1 DM, and 1 had type 2 DM. Six events (1.2% of hypoglycemic episodes) in 4 patients involved seizures. Two of these patients had type 1 DM, and 2 had type 2 DM. Four events (0.8% of hypoglycemic episodes) in 4 patients involved an unresponsive or unarousable state, including the sole ICU episode of symptomatic hypoglycemia. Three of these patients had type 1 DM, and 1 had type 2 DM. Patients with hypoglycemia‐related adverse events had a mean BG of 43.0 mg/dL, significantly lower (P = .01) than the mean BG of 50.9 mg/dL for hypoglycemic episodes without such events. However, 35% of these events occurred with a measured BG between 50 and 60 mg/dL. The distributions of BG values associated with symptomatic and asymptomatic events are shown in Figure 2. There is no useful threshold that separates symptomatic from asymptomatic hypoglycemia. No deaths or irreversible consequences were associated with hypoglycemia.
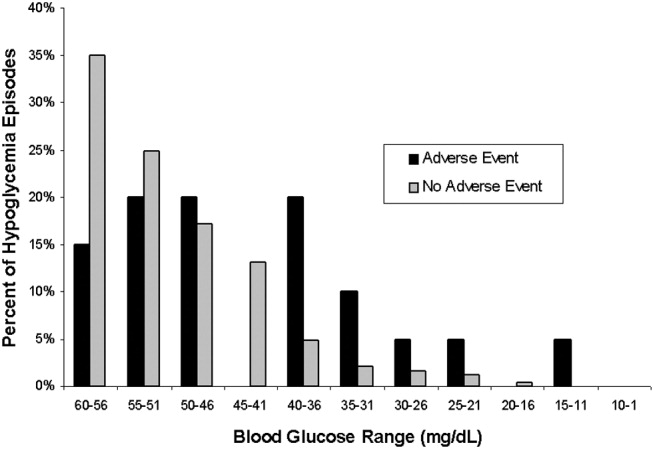
Approximately 40% (195 of 484) of the hypoglycemic episodes were related to decreased enteral intake (Table 1). In addition, 6.1% (30 of 484) of hypoglycemic episodes were related to insulin adjustment and 0.4% (2 of 484) to steroid withdrawal. In 43% (209 of 484) of the episodes the cause of the hypoglycemia was unclear. The remaining 10.4% of episodes were attributed to diverse causes.
| N (%) | |
|---|---|
| |
| NPO for unknown reason | 30 (6.2) |
| NPO for procedure/emntubated | 29 (6) |
| NPO for other documented reason (ie, fever/sepsis) | 10 (2.1) |
| Decreased PO intake (includes missed meal) | 126 (26) |
| No change in PO intake | 289 (59.7) |
One third of patients had a documented BG rechecked within 60 minutes, and fewer than half of the hypoglycemic patients had documented euglycemia within 2 hours of their low blood glucose measurement. The average time to documented resolution of a hypoglycemic episode was 4 hours, 3 minutes, with a median of 2 hours, 25 minutes.
Table 2 delineates the various combinations of antihyperglycemic agents that the 206 patients received in the 48 hours prior to a hypoglycemic episode. Of the 484 hypoglycemic episodes, 362 involved insulin. Of patients receiving insulin, 38 of 362 of episodes of hypoglycemia occurred in patients receiving sliding‐scale insulin (SSI) dosing as the only insulin order. In 163 hypoglycemic episodes, insulin was dosed with a combination of SSI and infusion or SSI with daily long‐acting insulin. The remaining 161 episodes involved administration of insulin to patients without an accompanying sliding‐scale order.
| |||||||
| Insulin Alone | 149 | ||||||
| Without insulin | With insulin | ||||||
| Single | Glimepiride | 1 | 4 | ||||
| Oral | Glipizide | 2 | 11 | ||||
| Agent | Glyburide | 2 | 7 | ||||
| Metformin | 5 | ||||||
| Repaglinide | 2 | ||||||
| Two | Glimepiride | AND | Metformin | 1 | |||
| Oral | Glimepiride | AND | Rosiglitazone | 1 | |||
| Agents | Glimepiride | AND | Pioglitazone | 1 | |||
| Glipizide | AND | Pioglitazone | 1 | ||||
| Glipizide | AND | Metformin | 4 | ||||
| Glyburide | AND | Metformin | 5 | ||||
| Metformin | AND | Rosiglitazone | 3 | 1 | |||
| Rosiglitazone | AND | Repaglinide | 1 | 1 | |||
| Three | Glipizide | AND | Metformin | AND | Rosiglitazone | 1 | |
| Oral | Glyburide | AND | Metformin | AND | Pioglitazone | 1 | |
| Agents | Pioglitazone | AND | Nateglinide | AND | Repaglinide | 1 | |
| TOTAL | 206 | ||||||
The prevalence of hypoglycemia did not significantly differ among patients treated with oral agents alone (9 of 85, 10.6%), patients treated with insulin alone (149 of 1497, 10%), and patients treated with both (47 of 592, 7.9%). However, there was a significant relationship between specific oral agent and probability of hypoglycemia. Glyburide was associated with a higher risk of hypoglycemia (19.1%, P < .01) than were other oral agents (Table 3).
| Oral agent | Patients with hypoglycemia | P value |
|---|---|---|
| ||
| Sulfonylureas | ||
| Glimepiride | 13.6% (8/59) | |
| Glipizide | 10.0% (19/190) | |
| Glyburide | 19.1% (18/94) | < .01 |
| Biguanide | 6.4% (22/344) | |
| Metformin | < .05 | |
| Thiazolidinediones | ||
| Pioglitazone | 5.1% (4/78) | |
| Rosiglitazone | 6.4% (6/94) | |
| Meglitinides | ||
| Nateglinide | 7.1% (1/14) | |
| Repaglinide | 7.0% (4/57) | |
DISCUSSION
Recently, many have called for substantive changes in the management of the hospitalized diabetic.15, 16 Most have recommended replacing sliding‐scale insulin with basal bolus insulin dosing and have challenged the historic tolerance of hyperglycemia during an acute hospital stay.17 However, as hospitals and physicians transform the management of inpatient hyperglycemia, they must assess the frequency of hypoglycemia and evaluate the risk/benefit ratio of strict glycemic control.18 Thus, one study found that eliminating sliding‐scale insulin markedly improved diabetes control but hypoglycemia (BG 60 mg/dL) was more frequent.10 The cost of euglycemia is hypoglycemia.2
We report a 9.5% rate of hypoglycemia among adult hospitalized patients being treated for hyperglycemia, including those in the ICU and those in non‐ICU settings. In widely publicized landmark trials, 5.2% of intensively treated surgical ICU patients14 and 18.7% of intensively treated medical ICU patients13 experienced hypoglycemia with no adverse events. Using those studies' definition of hypoglycemia (BG 40 mg/dL), only 2.4% (53 of 2174) of our patients experienced hypoglycemia. However, our survey included general medical and surgical patients as well as ICU patients treated at their physicians' discretion, reflecting the greater variability in care that exists outside a randomized, ICU trial.
We did not anticipate the duration to documented resolution of hypoglycemic episodes, nor did we anticipate the number of hypoglycemia‐related adverse events. We believe that hospitals will need to develop formal strategies to minimize the hypoglycemic risk from tight glycemic control. The frequency and duration of the time it took to recheck the glucose, coupled with the 4.1% symptomatic event rate, suggests that inpatient hypoglycemia deserves more attention. One potential focus is the interruption of nutrition, as medications may not be readjusted when patients' oral intake declines or when they travel for tests.19
More than 40% of our hypoglycemic patients experienced recurrent episodes. This may reflect a lack of adjustment of medications following hypoglycemia. However, recurrent hypoglycemia may also be explained by hypoglycemia‐associated autonomic failure and the desensitization to hypoglycemia that occurs once a patient has lower blood glucose.2 Thus, hypoglycemic patients are at high risk of repeat episodes and often require more frequent BG monitoring. Of note, patients with hypoglycemia unawareness may not have symptoms despite low BG, and thus unless they develop signs of hypoglycemia, they would not meet criteria for an adverse event in our study, despite a very low BG.
Medical error can precipitate hypoglycemia,4, 20 and the Institute for Safe Medication Practices21 and the Joint Commission on Accreditation of Healthcare Organizations22 consider insulin a high‐risk medication. We found no hypoglycemic episodes associated with a medication error. Our CPOE system eliminates ambiguity from poor penmanship, and hospital policy requires 2 nurses to check all administered insulin. However, despite the apparent lack of dispensing/administration medication errors, nearly 10% of patients receiving hypoglycemic therapy experienced iatrogenic hypoglycemia. Thus, strategies to reduce hypoglycemia must expand beyond the prevention of medication errors.
Contrary to our expectation, we found the prevalence of hypoglycemia was at least as high, if not higher, for patients taking only oral hypoglycemics than for patients taking either insulin alone or insulin in combination with oral antihyperglycemic agents. Glyburide appeared to carry the most risk both in our population and in previous studies,2325 perhaps because of a moderately active hepatic metabolite.26 The risk of hypoglycemia with different oral agents warrants further study.
The study stimulated several actions. First, we augmented the online nursing flow sheet to permit documentation of hypoglycemic episodes, including the administration of orange juice or food. Second, our CPOE system now prevents a physician from inadvertently deselecting the hypoglycemia protocol. Third, the CPOE system prompts the nurse to recheck the BG as specified in the hypoglycemia protocol. Finally, the CPOE system warns physicians to adjust antihyperglycemic agents when they institute nutritional changes. We propose that monitoring hypoglycemia rates must become a necessary component of inpatient diabetes care that is both effective and safe and plan to monitor these rates to determine the impact of interventions designed to reduce the frequency of hypoglycemia‐related adverse events.
Our study had several limitations. We only included episodes of hypoglycemia that were identified with a POC BG. This excluded patients treated for symptomatic hypoglycemia without a measured POC BG, potentially underestimating the event rate. Moreover, defining time to resolution of a hypoglycemic episode as that documented with a serum BG but not a POC BG may have resulted in overestimating the duration, and nurses may have documented POC BGs in the MAR after a substantial delay, also artificially lengthening the time to resolution. Capillary BG may underestimate the true degree of hypoglycemia,27 thus confounding the relationship between BG and adverse events. Our study was not designed to evaluate subtle suboptimal management of hyperglycemia as a cause of hypoglycemia, although expansion of the types and combinations of insulin has increased the possibility of prescribing errors. Nor were we able to assess the preventability of hypoglycemic episodes or the independent risk factors for hypoglycemia. Finally, this study originated from a single academic hospital and thus may reflect its unique idiosyncrasies.
We have reported a comprehensive survey of hypoglycemia in patients treated with antihyperglycemic agents at a single hospital. At the time the study took place, we had not instituted hospitalwide strategies to maintain BG near euglycemic targets, although such strategies have since begun. To detect untoward events that may follow from our efforts to better control hyperglycemia, we believe it is important to establish baseline measurements. Even without aiming for tighter glucose control, we identified the need to aim for and possible strategies to achieve, better prevention of hypoglycemia.
APPENDIX
SUMMARY OF DEFINITIONS FOR CHART REVIEW
New onset diabetes was defined as diabetes diagnosed during the current hospital admission when there was no previous history of diabetes.
No diabetes was defined as no history of diabetes and no diagnosis of diabetes during the index hospital stay.
Documentation was defined as any notation in the record by the physician or nurse acknowledging the hypoglycemic episode, other than the BG value itself.
Time to recheck BG was defined as the time in the MAR between a recorded BG 60 mg/dL and the next recorded BG.
Resolution was defined as the time in the MAR between a recorded BG 60 mg/dL and the first recorded BG 80 mg/dL (if, following a BG > 80 mg/dL, the next BG was 60 mg/dL, the 2 BG 60 mg/dL were defined as belonging to the same episode and that no resolution had yet occurred).
Decline in enteral intake was defined as any new NPO order on the day of the episode or missed meal within 3 hours of the episode.
Hypoglycemia‐related symptomatic adverse event was defined as any documented event at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased hospitalization. Change of care did not include following the hypoglycemic protocol and administering 50% dextrose or glucagon, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,,.Drug‐induced hypoglycemic coma in 102 diabetic patients.Arch Intern Med.1999;159:281–284.
- .Unexpected hypoglycemia in a critically ill patient.Ann Intern Med.2002;137:110–116.
- ,.Hypoglycemia: causes, neurological manifestations and outcome.Ann Neurol.1985;17:421–430.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77–81.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thor Surg.1999;67:352–362.
- ,,, et al.Inpatient management of diabetes: survey in a tertiary care center.Postgrad Med J.2003;79:585–587.
- ,.Incidence of hypoglycemia and nutritional intake in patients on a general medical unit.Nursingconnections.1989;2:33–40.
- .Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,.Eliminating sliding‐scale insulin.Diabetes Care.2005;28:1008–1011.
- American Diabetes Association Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes. A report from the American Diabetes Association workgroup on hypoglycemia.Diabetes Care.2005;28:245–1249.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ;,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.American College of Endocrinology Position Statement on inpatient diabetes and metabolicControl Endo Pract.2004;10:77–82.
- ,,.Inpatient diabetology, the new frontier.J Gen Intern Med.2004;19:466–471.
- ,.Counterpoint: inpatient glucose management, a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,,.Hypoglycemia in hospitalized patients: causes and outcomes.N Engl J Med.1986;315:245–1250.
- Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed July 27,2006.
- Joint Commission on Accreditation of Healthcare Organizations. High alert medications and patient safety. Sentinel Event Alert Issue 11, November 19, 1999 Available at: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_11.htm. Accessed July 27,2006.
- .Comparative tolerability of sulfonylureas in diabetes mellitus.Drug Saf.2000;22:313–320.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older people.J Am Geriatr Soc.1996;44:751–755
- ,.Sulfonylureas. In:DeFronzo RA, ed.Current Management of Diabetes Mellitus.St. Louis, MO:Mosby;1998:96–101.
- ,,.Diabetes Mellitus in Pharmacotherapy: A Pathophysiologic Approach.6th ed.Dipiro JT, ed.New York:McGraw Hill;2005.
- ,,, et al.Reliability of point‐of‐care testing for glucose measurement in critically ill adults.Crit Care Med.2005;33:2778–2785.
Glycemic control in the inpatient setting has received increasing attention in recent years, with the demonstration that appropriate blood glucose (BG) control prevents adverse events in both intensive care unit (ICU) and non‐ICU settings.1 Recent recommendations set target blood glucose levels near euglycemia for most hospitalized patients.1 Unfortunately, the risk of hypoglycemia increases with tighter glycemic control,2 and hypoglycemia may result in catastrophic events.35 Although hyperglycemia is associated with postoperative infection,6 and effective management decreases wound infections,7 few reports have detailed the hypoglycemia rates among surgical patients.8 Hypoglycemia rates on medical services are as high as 28%,9, 10 and efforts to achieve more normal BG levels in hospitalized patients have been associated with more hypoglycemia.11
We undertook a study of hypoglycemia in all adult hospitalized patients receiving hypoglycemic therapy at our institution. The purpose of this study was to determine the incidence, natural history, associations, and consequences of hypoglycemia in this broad inpatient population in order to have a baseline prior to introducing any formal hospital strategies to achieve the newer targets for glycemic control.
Research Design and Methods
Thomas Jefferson University Hospital (TJUH) is a 675‐bed acute care teaching institution in center‐city Philadelphia with more than 30,000 patient admissions each year. We undertook a prospective, consecutive medical record review from August 16, 2004, to November 15, 2004, of hospitalized patients who had experienced at least 1 hypoglycemic episode, defined as at least one blood glucose (BG) 60 mg/dL within 48 hours of administration of an antihyperglycemic agent in the hospital. The definition of hypoglycemia was consistent with our hospital policies and a compromise between the BG 70 mg/dL proposed by the American Diabetes Association (ADA) hypoglycemia workgroup12 and the BG 40 mg/dL used by authors studying glycemic control in the ICU.13, 14
Hypoglycemic episodes were identified by a daily electronic search of the online medication administration record (MAR) where nurses document all point‐of‐care (POC) BG values. Two of the authors (P.V. and V.G.) reviewed the medical record for each episode and excluded pediatric (<18 years), emergency department, and maternity patients. Intensive care, step‐down, and medical/surgical unit patients were all included if the hypoglycemia had occurred within 48 hours of hospital administration of an antihyperglycemic agent. All medication orders at TJUH are placed through the computerized prescriber order entry (CPOE) system (Centricity Enterprise), which links all antihyperglycemic agents to a standardized hypoglycemia treatment protocol. The protocol includes instructions to administer glucose and/or glucagon and check the BG 15 minutes after a hypoglycemic episode. We established operational definitions prior to chart review (Appendix). A symptomatic hypoglycemia‐related adverse event was defined as any documented event occurring at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased length of hospitalization. We did not include following our hypoglycemic protocol with the administration of 50% dextrose or glucagon as a change of care, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
We searched the University Health System Consortium Clinical Database (CDB) to quantify the number of patients at TJUH receiving any antihyperglycemic agent during the study period and to identify the specific agents received. The CDB receives all patient, physician, and pharmacy dose‐ specific information from the hospital clinical and billing information systems. We defined subgroups of patients taking insulin(s) only, taking oral agent(s) only, and taking a combination.
Differences between proportions were evaluated using the chi‐square statistic; differences between means were evaluated using the Student t test. Probabilities of the null hypothesis less than .05 were considered significant.
The project was approved by the Institutional Review Board for Human Subjects at Thomas Jefferson University.
RESULTS
Over the 2‐month study period 8140 patients were admitted, of whom 2174 (27%) received an antihyperglycemic agent. Five hundred and sixty‐eight hypoglycemic episodes (BG 60 mg/dL) occurred in 265 patients. We excluded 84 episodes among 59 patients who did not receive antihyperglycemic agents, resulting in 484 episodes of hypoglycemia occurring within 48 hours of hospital administration of an antihyperglycemic agent in 206 patients, an average of 5.26 episodes per day. Of the 2174 of patients receiving antihyperglycemic agents, 206 (9.5%) experienced 1 or more episodes of hypoglycemia.
Patient ages ranged from 20 to 93 years, with an average of 62 years. Fifty‐seven percent (118 of 206) of participants were female. About one‐fourth of all episodes (23.8%) occurred in the ICU setting. The distribution of patients by decade and their ICU status are presented in Figure 1. Of the 206 patients, 29% (59) had type 1 diabetes mellitus (DM), 49% (102) had type 2 DM, 1% (2) had new‐onset diabetes, and 21% (43) had no diagnosis of DM. Of the 484 hypoglycemic episodes, 37.8% occurred in patients with type 1 DM, 46.9% in patients with type 2 DM, and 0.6% in patients with new‐onset DM. The remaining 14.5% occurred in patients with no documented history of DM, although they were receiving antihyperglycemic agents. More than 1 episode was experienced by 44% of patients, and 12% experienced 5 or more episodes.

The BG was between 51 and 60 mg/dL in 282 of the episodes (58.2%), between 41 and 50 mg/dL in 149 episodes (30.8%) and 40 mg/dL or less in 53 episodes (11%). In 20 episodes (4.1% of episodes, representing fewer than 1% of all patients receiving an antihyperglycemic agent), a symptomatic hypoglycemia‐related adverse event was documented. All but 1 adverse event occurred outside the ICU. Ten of these events (2.1% of all hypoglycemic episodes) in 10 patients involved symptoms including headache, agitation, disorientation, and tremors. Of these patients 9 had type 1 DM, and 1 had type 2 DM. Six events (1.2% of hypoglycemic episodes) in 4 patients involved seizures. Two of these patients had type 1 DM, and 2 had type 2 DM. Four events (0.8% of hypoglycemic episodes) in 4 patients involved an unresponsive or unarousable state, including the sole ICU episode of symptomatic hypoglycemia. Three of these patients had type 1 DM, and 1 had type 2 DM. Patients with hypoglycemia‐related adverse events had a mean BG of 43.0 mg/dL, significantly lower (P = .01) than the mean BG of 50.9 mg/dL for hypoglycemic episodes without such events. However, 35% of these events occurred with a measured BG between 50 and 60 mg/dL. The distributions of BG values associated with symptomatic and asymptomatic events are shown in Figure 2. There is no useful threshold that separates symptomatic from asymptomatic hypoglycemia. No deaths or irreversible consequences were associated with hypoglycemia.

Approximately 40% (195 of 484) of the hypoglycemic episodes were related to decreased enteral intake (Table 1). In addition, 6.1% (30 of 484) of hypoglycemic episodes were related to insulin adjustment and 0.4% (2 of 484) to steroid withdrawal. In 43% (209 of 484) of the episodes the cause of the hypoglycemia was unclear. The remaining 10.4% of episodes were attributed to diverse causes.
| N (%) | |
|---|---|
| |
| NPO for unknown reason | 30 (6.2) |
| NPO for procedure/emntubated | 29 (6) |
| NPO for other documented reason (ie, fever/sepsis) | 10 (2.1) |
| Decreased PO intake (includes missed meal) | 126 (26) |
| No change in PO intake | 289 (59.7) |
One third of patients had a documented BG rechecked within 60 minutes, and fewer than half of the hypoglycemic patients had documented euglycemia within 2 hours of their low blood glucose measurement. The average time to documented resolution of a hypoglycemic episode was 4 hours, 3 minutes, with a median of 2 hours, 25 minutes.
Table 2 delineates the various combinations of antihyperglycemic agents that the 206 patients received in the 48 hours prior to a hypoglycemic episode. Of the 484 hypoglycemic episodes, 362 involved insulin. Of patients receiving insulin, 38 of 362 of episodes of hypoglycemia occurred in patients receiving sliding‐scale insulin (SSI) dosing as the only insulin order. In 163 hypoglycemic episodes, insulin was dosed with a combination of SSI and infusion or SSI with daily long‐acting insulin. The remaining 161 episodes involved administration of insulin to patients without an accompanying sliding‐scale order.
| |||||||
| Insulin Alone | 149 | ||||||
| Without insulin | With insulin | ||||||
| Single | Glimepiride | 1 | 4 | ||||
| Oral | Glipizide | 2 | 11 | ||||
| Agent | Glyburide | 2 | 7 | ||||
| Metformin | 5 | ||||||
| Repaglinide | 2 | ||||||
| Two | Glimepiride | AND | Metformin | 1 | |||
| Oral | Glimepiride | AND | Rosiglitazone | 1 | |||
| Agents | Glimepiride | AND | Pioglitazone | 1 | |||
| Glipizide | AND | Pioglitazone | 1 | ||||
| Glipizide | AND | Metformin | 4 | ||||
| Glyburide | AND | Metformin | 5 | ||||
| Metformin | AND | Rosiglitazone | 3 | 1 | |||
| Rosiglitazone | AND | Repaglinide | 1 | 1 | |||
| Three | Glipizide | AND | Metformin | AND | Rosiglitazone | 1 | |
| Oral | Glyburide | AND | Metformin | AND | Pioglitazone | 1 | |
| Agents | Pioglitazone | AND | Nateglinide | AND | Repaglinide | 1 | |
| TOTAL | 206 | ||||||
The prevalence of hypoglycemia did not significantly differ among patients treated with oral agents alone (9 of 85, 10.6%), patients treated with insulin alone (149 of 1497, 10%), and patients treated with both (47 of 592, 7.9%). However, there was a significant relationship between specific oral agent and probability of hypoglycemia. Glyburide was associated with a higher risk of hypoglycemia (19.1%, P < .01) than were other oral agents (Table 3).
| Oral agent | Patients with hypoglycemia | P value |
|---|---|---|
| ||
| Sulfonylureas | ||
| Glimepiride | 13.6% (8/59) | |
| Glipizide | 10.0% (19/190) | |
| Glyburide | 19.1% (18/94) | < .01 |
| Biguanide | 6.4% (22/344) | |
| Metformin | < .05 | |
| Thiazolidinediones | ||
| Pioglitazone | 5.1% (4/78) | |
| Rosiglitazone | 6.4% (6/94) | |
| Meglitinides | ||
| Nateglinide | 7.1% (1/14) | |
| Repaglinide | 7.0% (4/57) | |
DISCUSSION
Recently, many have called for substantive changes in the management of the hospitalized diabetic.15, 16 Most have recommended replacing sliding‐scale insulin with basal bolus insulin dosing and have challenged the historic tolerance of hyperglycemia during an acute hospital stay.17 However, as hospitals and physicians transform the management of inpatient hyperglycemia, they must assess the frequency of hypoglycemia and evaluate the risk/benefit ratio of strict glycemic control.18 Thus, one study found that eliminating sliding‐scale insulin markedly improved diabetes control but hypoglycemia (BG 60 mg/dL) was more frequent.10 The cost of euglycemia is hypoglycemia.2
We report a 9.5% rate of hypoglycemia among adult hospitalized patients being treated for hyperglycemia, including those in the ICU and those in non‐ICU settings. In widely publicized landmark trials, 5.2% of intensively treated surgical ICU patients14 and 18.7% of intensively treated medical ICU patients13 experienced hypoglycemia with no adverse events. Using those studies' definition of hypoglycemia (BG 40 mg/dL), only 2.4% (53 of 2174) of our patients experienced hypoglycemia. However, our survey included general medical and surgical patients as well as ICU patients treated at their physicians' discretion, reflecting the greater variability in care that exists outside a randomized, ICU trial.
We did not anticipate the duration to documented resolution of hypoglycemic episodes, nor did we anticipate the number of hypoglycemia‐related adverse events. We believe that hospitals will need to develop formal strategies to minimize the hypoglycemic risk from tight glycemic control. The frequency and duration of the time it took to recheck the glucose, coupled with the 4.1% symptomatic event rate, suggests that inpatient hypoglycemia deserves more attention. One potential focus is the interruption of nutrition, as medications may not be readjusted when patients' oral intake declines or when they travel for tests.19
More than 40% of our hypoglycemic patients experienced recurrent episodes. This may reflect a lack of adjustment of medications following hypoglycemia. However, recurrent hypoglycemia may also be explained by hypoglycemia‐associated autonomic failure and the desensitization to hypoglycemia that occurs once a patient has lower blood glucose.2 Thus, hypoglycemic patients are at high risk of repeat episodes and often require more frequent BG monitoring. Of note, patients with hypoglycemia unawareness may not have symptoms despite low BG, and thus unless they develop signs of hypoglycemia, they would not meet criteria for an adverse event in our study, despite a very low BG.
Medical error can precipitate hypoglycemia,4, 20 and the Institute for Safe Medication Practices21 and the Joint Commission on Accreditation of Healthcare Organizations22 consider insulin a high‐risk medication. We found no hypoglycemic episodes associated with a medication error. Our CPOE system eliminates ambiguity from poor penmanship, and hospital policy requires 2 nurses to check all administered insulin. However, despite the apparent lack of dispensing/administration medication errors, nearly 10% of patients receiving hypoglycemic therapy experienced iatrogenic hypoglycemia. Thus, strategies to reduce hypoglycemia must expand beyond the prevention of medication errors.
Contrary to our expectation, we found the prevalence of hypoglycemia was at least as high, if not higher, for patients taking only oral hypoglycemics than for patients taking either insulin alone or insulin in combination with oral antihyperglycemic agents. Glyburide appeared to carry the most risk both in our population and in previous studies,2325 perhaps because of a moderately active hepatic metabolite.26 The risk of hypoglycemia with different oral agents warrants further study.
The study stimulated several actions. First, we augmented the online nursing flow sheet to permit documentation of hypoglycemic episodes, including the administration of orange juice or food. Second, our CPOE system now prevents a physician from inadvertently deselecting the hypoglycemia protocol. Third, the CPOE system prompts the nurse to recheck the BG as specified in the hypoglycemia protocol. Finally, the CPOE system warns physicians to adjust antihyperglycemic agents when they institute nutritional changes. We propose that monitoring hypoglycemia rates must become a necessary component of inpatient diabetes care that is both effective and safe and plan to monitor these rates to determine the impact of interventions designed to reduce the frequency of hypoglycemia‐related adverse events.
Our study had several limitations. We only included episodes of hypoglycemia that were identified with a POC BG. This excluded patients treated for symptomatic hypoglycemia without a measured POC BG, potentially underestimating the event rate. Moreover, defining time to resolution of a hypoglycemic episode as that documented with a serum BG but not a POC BG may have resulted in overestimating the duration, and nurses may have documented POC BGs in the MAR after a substantial delay, also artificially lengthening the time to resolution. Capillary BG may underestimate the true degree of hypoglycemia,27 thus confounding the relationship between BG and adverse events. Our study was not designed to evaluate subtle suboptimal management of hyperglycemia as a cause of hypoglycemia, although expansion of the types and combinations of insulin has increased the possibility of prescribing errors. Nor were we able to assess the preventability of hypoglycemic episodes or the independent risk factors for hypoglycemia. Finally, this study originated from a single academic hospital and thus may reflect its unique idiosyncrasies.
We have reported a comprehensive survey of hypoglycemia in patients treated with antihyperglycemic agents at a single hospital. At the time the study took place, we had not instituted hospitalwide strategies to maintain BG near euglycemic targets, although such strategies have since begun. To detect untoward events that may follow from our efforts to better control hyperglycemia, we believe it is important to establish baseline measurements. Even without aiming for tighter glucose control, we identified the need to aim for and possible strategies to achieve, better prevention of hypoglycemia.
APPENDIX
SUMMARY OF DEFINITIONS FOR CHART REVIEW
New onset diabetes was defined as diabetes diagnosed during the current hospital admission when there was no previous history of diabetes.
No diabetes was defined as no history of diabetes and no diagnosis of diabetes during the index hospital stay.
Documentation was defined as any notation in the record by the physician or nurse acknowledging the hypoglycemic episode, other than the BG value itself.
Time to recheck BG was defined as the time in the MAR between a recorded BG 60 mg/dL and the next recorded BG.
Resolution was defined as the time in the MAR between a recorded BG 60 mg/dL and the first recorded BG 80 mg/dL (if, following a BG > 80 mg/dL, the next BG was 60 mg/dL, the 2 BG 60 mg/dL were defined as belonging to the same episode and that no resolution had yet occurred).
Decline in enteral intake was defined as any new NPO order on the day of the episode or missed meal within 3 hours of the episode.
Hypoglycemia‐related symptomatic adverse event was defined as any documented event at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased hospitalization. Change of care did not include following the hypoglycemic protocol and administering 50% dextrose or glucagon, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
Glycemic control in the inpatient setting has received increasing attention in recent years, with the demonstration that appropriate blood glucose (BG) control prevents adverse events in both intensive care unit (ICU) and non‐ICU settings.1 Recent recommendations set target blood glucose levels near euglycemia for most hospitalized patients.1 Unfortunately, the risk of hypoglycemia increases with tighter glycemic control,2 and hypoglycemia may result in catastrophic events.35 Although hyperglycemia is associated with postoperative infection,6 and effective management decreases wound infections,7 few reports have detailed the hypoglycemia rates among surgical patients.8 Hypoglycemia rates on medical services are as high as 28%,9, 10 and efforts to achieve more normal BG levels in hospitalized patients have been associated with more hypoglycemia.11
We undertook a study of hypoglycemia in all adult hospitalized patients receiving hypoglycemic therapy at our institution. The purpose of this study was to determine the incidence, natural history, associations, and consequences of hypoglycemia in this broad inpatient population in order to have a baseline prior to introducing any formal hospital strategies to achieve the newer targets for glycemic control.
Research Design and Methods
Thomas Jefferson University Hospital (TJUH) is a 675‐bed acute care teaching institution in center‐city Philadelphia with more than 30,000 patient admissions each year. We undertook a prospective, consecutive medical record review from August 16, 2004, to November 15, 2004, of hospitalized patients who had experienced at least 1 hypoglycemic episode, defined as at least one blood glucose (BG) 60 mg/dL within 48 hours of administration of an antihyperglycemic agent in the hospital. The definition of hypoglycemia was consistent with our hospital policies and a compromise between the BG 70 mg/dL proposed by the American Diabetes Association (ADA) hypoglycemia workgroup12 and the BG 40 mg/dL used by authors studying glycemic control in the ICU.13, 14
Hypoglycemic episodes were identified by a daily electronic search of the online medication administration record (MAR) where nurses document all point‐of‐care (POC) BG values. Two of the authors (P.V. and V.G.) reviewed the medical record for each episode and excluded pediatric (<18 years), emergency department, and maternity patients. Intensive care, step‐down, and medical/surgical unit patients were all included if the hypoglycemia had occurred within 48 hours of hospital administration of an antihyperglycemic agent. All medication orders at TJUH are placed through the computerized prescriber order entry (CPOE) system (Centricity Enterprise), which links all antihyperglycemic agents to a standardized hypoglycemia treatment protocol. The protocol includes instructions to administer glucose and/or glucagon and check the BG 15 minutes after a hypoglycemic episode. We established operational definitions prior to chart review (Appendix). A symptomatic hypoglycemia‐related adverse event was defined as any documented event occurring at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased length of hospitalization. We did not include following our hypoglycemic protocol with the administration of 50% dextrose or glucagon as a change of care, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
We searched the University Health System Consortium Clinical Database (CDB) to quantify the number of patients at TJUH receiving any antihyperglycemic agent during the study period and to identify the specific agents received. The CDB receives all patient, physician, and pharmacy dose‐ specific information from the hospital clinical and billing information systems. We defined subgroups of patients taking insulin(s) only, taking oral agent(s) only, and taking a combination.
Differences between proportions were evaluated using the chi‐square statistic; differences between means were evaluated using the Student t test. Probabilities of the null hypothesis less than .05 were considered significant.
The project was approved by the Institutional Review Board for Human Subjects at Thomas Jefferson University.
RESULTS
Over the 2‐month study period 8140 patients were admitted, of whom 2174 (27%) received an antihyperglycemic agent. Five hundred and sixty‐eight hypoglycemic episodes (BG 60 mg/dL) occurred in 265 patients. We excluded 84 episodes among 59 patients who did not receive antihyperglycemic agents, resulting in 484 episodes of hypoglycemia occurring within 48 hours of hospital administration of an antihyperglycemic agent in 206 patients, an average of 5.26 episodes per day. Of the 2174 of patients receiving antihyperglycemic agents, 206 (9.5%) experienced 1 or more episodes of hypoglycemia.
Patient ages ranged from 20 to 93 years, with an average of 62 years. Fifty‐seven percent (118 of 206) of participants were female. About one‐fourth of all episodes (23.8%) occurred in the ICU setting. The distribution of patients by decade and their ICU status are presented in Figure 1. Of the 206 patients, 29% (59) had type 1 diabetes mellitus (DM), 49% (102) had type 2 DM, 1% (2) had new‐onset diabetes, and 21% (43) had no diagnosis of DM. Of the 484 hypoglycemic episodes, 37.8% occurred in patients with type 1 DM, 46.9% in patients with type 2 DM, and 0.6% in patients with new‐onset DM. The remaining 14.5% occurred in patients with no documented history of DM, although they were receiving antihyperglycemic agents. More than 1 episode was experienced by 44% of patients, and 12% experienced 5 or more episodes.

The BG was between 51 and 60 mg/dL in 282 of the episodes (58.2%), between 41 and 50 mg/dL in 149 episodes (30.8%) and 40 mg/dL or less in 53 episodes (11%). In 20 episodes (4.1% of episodes, representing fewer than 1% of all patients receiving an antihyperglycemic agent), a symptomatic hypoglycemia‐related adverse event was documented. All but 1 adverse event occurred outside the ICU. Ten of these events (2.1% of all hypoglycemic episodes) in 10 patients involved symptoms including headache, agitation, disorientation, and tremors. Of these patients 9 had type 1 DM, and 1 had type 2 DM. Six events (1.2% of hypoglycemic episodes) in 4 patients involved seizures. Two of these patients had type 1 DM, and 2 had type 2 DM. Four events (0.8% of hypoglycemic episodes) in 4 patients involved an unresponsive or unarousable state, including the sole ICU episode of symptomatic hypoglycemia. Three of these patients had type 1 DM, and 1 had type 2 DM. Patients with hypoglycemia‐related adverse events had a mean BG of 43.0 mg/dL, significantly lower (P = .01) than the mean BG of 50.9 mg/dL for hypoglycemic episodes without such events. However, 35% of these events occurred with a measured BG between 50 and 60 mg/dL. The distributions of BG values associated with symptomatic and asymptomatic events are shown in Figure 2. There is no useful threshold that separates symptomatic from asymptomatic hypoglycemia. No deaths or irreversible consequences were associated with hypoglycemia.

Approximately 40% (195 of 484) of the hypoglycemic episodes were related to decreased enteral intake (Table 1). In addition, 6.1% (30 of 484) of hypoglycemic episodes were related to insulin adjustment and 0.4% (2 of 484) to steroid withdrawal. In 43% (209 of 484) of the episodes the cause of the hypoglycemia was unclear. The remaining 10.4% of episodes were attributed to diverse causes.
| N (%) | |
|---|---|
| |
| NPO for unknown reason | 30 (6.2) |
| NPO for procedure/emntubated | 29 (6) |
| NPO for other documented reason (ie, fever/sepsis) | 10 (2.1) |
| Decreased PO intake (includes missed meal) | 126 (26) |
| No change in PO intake | 289 (59.7) |
One third of patients had a documented BG rechecked within 60 minutes, and fewer than half of the hypoglycemic patients had documented euglycemia within 2 hours of their low blood glucose measurement. The average time to documented resolution of a hypoglycemic episode was 4 hours, 3 minutes, with a median of 2 hours, 25 minutes.
Table 2 delineates the various combinations of antihyperglycemic agents that the 206 patients received in the 48 hours prior to a hypoglycemic episode. Of the 484 hypoglycemic episodes, 362 involved insulin. Of patients receiving insulin, 38 of 362 of episodes of hypoglycemia occurred in patients receiving sliding‐scale insulin (SSI) dosing as the only insulin order. In 163 hypoglycemic episodes, insulin was dosed with a combination of SSI and infusion or SSI with daily long‐acting insulin. The remaining 161 episodes involved administration of insulin to patients without an accompanying sliding‐scale order.
| |||||||
| Insulin Alone | 149 | ||||||
| Without insulin | With insulin | ||||||
| Single | Glimepiride | 1 | 4 | ||||
| Oral | Glipizide | 2 | 11 | ||||
| Agent | Glyburide | 2 | 7 | ||||
| Metformin | 5 | ||||||
| Repaglinide | 2 | ||||||
| Two | Glimepiride | AND | Metformin | 1 | |||
| Oral | Glimepiride | AND | Rosiglitazone | 1 | |||
| Agents | Glimepiride | AND | Pioglitazone | 1 | |||
| Glipizide | AND | Pioglitazone | 1 | ||||
| Glipizide | AND | Metformin | 4 | ||||
| Glyburide | AND | Metformin | 5 | ||||
| Metformin | AND | Rosiglitazone | 3 | 1 | |||
| Rosiglitazone | AND | Repaglinide | 1 | 1 | |||
| Three | Glipizide | AND | Metformin | AND | Rosiglitazone | 1 | |
| Oral | Glyburide | AND | Metformin | AND | Pioglitazone | 1 | |
| Agents | Pioglitazone | AND | Nateglinide | AND | Repaglinide | 1 | |
| TOTAL | 206 | ||||||
The prevalence of hypoglycemia did not significantly differ among patients treated with oral agents alone (9 of 85, 10.6%), patients treated with insulin alone (149 of 1497, 10%), and patients treated with both (47 of 592, 7.9%). However, there was a significant relationship between specific oral agent and probability of hypoglycemia. Glyburide was associated with a higher risk of hypoglycemia (19.1%, P < .01) than were other oral agents (Table 3).
| Oral agent | Patients with hypoglycemia | P value |
|---|---|---|
| ||
| Sulfonylureas | ||
| Glimepiride | 13.6% (8/59) | |
| Glipizide | 10.0% (19/190) | |
| Glyburide | 19.1% (18/94) | < .01 |
| Biguanide | 6.4% (22/344) | |
| Metformin | < .05 | |
| Thiazolidinediones | ||
| Pioglitazone | 5.1% (4/78) | |
| Rosiglitazone | 6.4% (6/94) | |
| Meglitinides | ||
| Nateglinide | 7.1% (1/14) | |
| Repaglinide | 7.0% (4/57) | |
DISCUSSION
Recently, many have called for substantive changes in the management of the hospitalized diabetic.15, 16 Most have recommended replacing sliding‐scale insulin with basal bolus insulin dosing and have challenged the historic tolerance of hyperglycemia during an acute hospital stay.17 However, as hospitals and physicians transform the management of inpatient hyperglycemia, they must assess the frequency of hypoglycemia and evaluate the risk/benefit ratio of strict glycemic control.18 Thus, one study found that eliminating sliding‐scale insulin markedly improved diabetes control but hypoglycemia (BG 60 mg/dL) was more frequent.10 The cost of euglycemia is hypoglycemia.2
We report a 9.5% rate of hypoglycemia among adult hospitalized patients being treated for hyperglycemia, including those in the ICU and those in non‐ICU settings. In widely publicized landmark trials, 5.2% of intensively treated surgical ICU patients14 and 18.7% of intensively treated medical ICU patients13 experienced hypoglycemia with no adverse events. Using those studies' definition of hypoglycemia (BG 40 mg/dL), only 2.4% (53 of 2174) of our patients experienced hypoglycemia. However, our survey included general medical and surgical patients as well as ICU patients treated at their physicians' discretion, reflecting the greater variability in care that exists outside a randomized, ICU trial.
We did not anticipate the duration to documented resolution of hypoglycemic episodes, nor did we anticipate the number of hypoglycemia‐related adverse events. We believe that hospitals will need to develop formal strategies to minimize the hypoglycemic risk from tight glycemic control. The frequency and duration of the time it took to recheck the glucose, coupled with the 4.1% symptomatic event rate, suggests that inpatient hypoglycemia deserves more attention. One potential focus is the interruption of nutrition, as medications may not be readjusted when patients' oral intake declines or when they travel for tests.19
More than 40% of our hypoglycemic patients experienced recurrent episodes. This may reflect a lack of adjustment of medications following hypoglycemia. However, recurrent hypoglycemia may also be explained by hypoglycemia‐associated autonomic failure and the desensitization to hypoglycemia that occurs once a patient has lower blood glucose.2 Thus, hypoglycemic patients are at high risk of repeat episodes and often require more frequent BG monitoring. Of note, patients with hypoglycemia unawareness may not have symptoms despite low BG, and thus unless they develop signs of hypoglycemia, they would not meet criteria for an adverse event in our study, despite a very low BG.
Medical error can precipitate hypoglycemia,4, 20 and the Institute for Safe Medication Practices21 and the Joint Commission on Accreditation of Healthcare Organizations22 consider insulin a high‐risk medication. We found no hypoglycemic episodes associated with a medication error. Our CPOE system eliminates ambiguity from poor penmanship, and hospital policy requires 2 nurses to check all administered insulin. However, despite the apparent lack of dispensing/administration medication errors, nearly 10% of patients receiving hypoglycemic therapy experienced iatrogenic hypoglycemia. Thus, strategies to reduce hypoglycemia must expand beyond the prevention of medication errors.
Contrary to our expectation, we found the prevalence of hypoglycemia was at least as high, if not higher, for patients taking only oral hypoglycemics than for patients taking either insulin alone or insulin in combination with oral antihyperglycemic agents. Glyburide appeared to carry the most risk both in our population and in previous studies,2325 perhaps because of a moderately active hepatic metabolite.26 The risk of hypoglycemia with different oral agents warrants further study.
The study stimulated several actions. First, we augmented the online nursing flow sheet to permit documentation of hypoglycemic episodes, including the administration of orange juice or food. Second, our CPOE system now prevents a physician from inadvertently deselecting the hypoglycemia protocol. Third, the CPOE system prompts the nurse to recheck the BG as specified in the hypoglycemia protocol. Finally, the CPOE system warns physicians to adjust antihyperglycemic agents when they institute nutritional changes. We propose that monitoring hypoglycemia rates must become a necessary component of inpatient diabetes care that is both effective and safe and plan to monitor these rates to determine the impact of interventions designed to reduce the frequency of hypoglycemia‐related adverse events.
Our study had several limitations. We only included episodes of hypoglycemia that were identified with a POC BG. This excluded patients treated for symptomatic hypoglycemia without a measured POC BG, potentially underestimating the event rate. Moreover, defining time to resolution of a hypoglycemic episode as that documented with a serum BG but not a POC BG may have resulted in overestimating the duration, and nurses may have documented POC BGs in the MAR after a substantial delay, also artificially lengthening the time to resolution. Capillary BG may underestimate the true degree of hypoglycemia,27 thus confounding the relationship between BG and adverse events. Our study was not designed to evaluate subtle suboptimal management of hyperglycemia as a cause of hypoglycemia, although expansion of the types and combinations of insulin has increased the possibility of prescribing errors. Nor were we able to assess the preventability of hypoglycemic episodes or the independent risk factors for hypoglycemia. Finally, this study originated from a single academic hospital and thus may reflect its unique idiosyncrasies.
We have reported a comprehensive survey of hypoglycemia in patients treated with antihyperglycemic agents at a single hospital. At the time the study took place, we had not instituted hospitalwide strategies to maintain BG near euglycemic targets, although such strategies have since begun. To detect untoward events that may follow from our efforts to better control hyperglycemia, we believe it is important to establish baseline measurements. Even without aiming for tighter glucose control, we identified the need to aim for and possible strategies to achieve, better prevention of hypoglycemia.
APPENDIX
SUMMARY OF DEFINITIONS FOR CHART REVIEW
New onset diabetes was defined as diabetes diagnosed during the current hospital admission when there was no previous history of diabetes.
No diabetes was defined as no history of diabetes and no diagnosis of diabetes during the index hospital stay.
Documentation was defined as any notation in the record by the physician or nurse acknowledging the hypoglycemic episode, other than the BG value itself.
Time to recheck BG was defined as the time in the MAR between a recorded BG 60 mg/dL and the next recorded BG.
Resolution was defined as the time in the MAR between a recorded BG 60 mg/dL and the first recorded BG 80 mg/dL (if, following a BG > 80 mg/dL, the next BG was 60 mg/dL, the 2 BG 60 mg/dL were defined as belonging to the same episode and that no resolution had yet occurred).
Decline in enteral intake was defined as any new NPO order on the day of the episode or missed meal within 3 hours of the episode.
Hypoglycemia‐related symptomatic adverse event was defined as any documented event at the time of the hypoglycemic episode involving symptoms, change in care, temporary or permanent injury, or increased hospitalization. Change of care did not include following the hypoglycemic protocol and administering 50% dextrose or glucagon, as we considered this usual care, unless symptoms or signs also accompanied the hypoglycemic event.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,,.Drug‐induced hypoglycemic coma in 102 diabetic patients.Arch Intern Med.1999;159:281–284.
- .Unexpected hypoglycemia in a critically ill patient.Ann Intern Med.2002;137:110–116.
- ,.Hypoglycemia: causes, neurological manifestations and outcome.Ann Neurol.1985;17:421–430.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77–81.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thor Surg.1999;67:352–362.
- ,,, et al.Inpatient management of diabetes: survey in a tertiary care center.Postgrad Med J.2003;79:585–587.
- ,.Incidence of hypoglycemia and nutritional intake in patients on a general medical unit.Nursingconnections.1989;2:33–40.
- .Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,.Eliminating sliding‐scale insulin.Diabetes Care.2005;28:1008–1011.
- American Diabetes Association Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes. A report from the American Diabetes Association workgroup on hypoglycemia.Diabetes Care.2005;28:245–1249.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ;,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.American College of Endocrinology Position Statement on inpatient diabetes and metabolicControl Endo Pract.2004;10:77–82.
- ,,.Inpatient diabetology, the new frontier.J Gen Intern Med.2004;19:466–471.
- ,.Counterpoint: inpatient glucose management, a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,,.Hypoglycemia in hospitalized patients: causes and outcomes.N Engl J Med.1986;315:245–1250.
- Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed July 27,2006.
- Joint Commission on Accreditation of Healthcare Organizations. High alert medications and patient safety. Sentinel Event Alert Issue 11, November 19, 1999 Available at: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_11.htm. Accessed July 27,2006.
- .Comparative tolerability of sulfonylureas in diabetes mellitus.Drug Saf.2000;22:313–320.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older people.J Am Geriatr Soc.1996;44:751–755
- ,.Sulfonylureas. In:DeFronzo RA, ed.Current Management of Diabetes Mellitus.St. Louis, MO:Mosby;1998:96–101.
- ,,.Diabetes Mellitus in Pharmacotherapy: A Pathophysiologic Approach.6th ed.Dipiro JT, ed.New York:McGraw Hill;2005.
- ,,, et al.Reliability of point‐of‐care testing for glucose measurement in critically ill adults.Crit Care Med.2005;33:2778–2785.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,,.Drug‐induced hypoglycemic coma in 102 diabetic patients.Arch Intern Med.1999;159:281–284.
- .Unexpected hypoglycemia in a critically ill patient.Ann Intern Med.2002;137:110–116.
- ,.Hypoglycemia: causes, neurological manifestations and outcome.Ann Neurol.1985;17:421–430.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77–81.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thor Surg.1999;67:352–362.
- ,,, et al.Inpatient management of diabetes: survey in a tertiary care center.Postgrad Med J.2003;79:585–587.
- ,.Incidence of hypoglycemia and nutritional intake in patients on a general medical unit.Nursingconnections.1989;2:33–40.
- .Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,.Eliminating sliding‐scale insulin.Diabetes Care.2005;28:1008–1011.
- American Diabetes Association Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes. A report from the American Diabetes Association workgroup on hypoglycemia.Diabetes Care.2005;28:245–1249.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ;,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.American College of Endocrinology Position Statement on inpatient diabetes and metabolicControl Endo Pract.2004;10:77–82.
- ,,.Inpatient diabetology, the new frontier.J Gen Intern Med.2004;19:466–471.
- ,.Counterpoint: inpatient glucose management, a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,,.Hypoglycemia in hospitalized patients: causes and outcomes.N Engl J Med.1986;315:245–1250.
- Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed July 27,2006.
- Joint Commission on Accreditation of Healthcare Organizations. High alert medications and patient safety. Sentinel Event Alert Issue 11, November 19, 1999 Available at: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_11.htm. Accessed July 27,2006.
- .Comparative tolerability of sulfonylureas in diabetes mellitus.Drug Saf.2000;22:313–320.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older people.J Am Geriatr Soc.1996;44:751–755
- ,.Sulfonylureas. In:DeFronzo RA, ed.Current Management of Diabetes Mellitus.St. Louis, MO:Mosby;1998:96–101.
- ,,.Diabetes Mellitus in Pharmacotherapy: A Pathophysiologic Approach.6th ed.Dipiro JT, ed.New York:McGraw Hill;2005.
- ,,, et al.Reliability of point‐of‐care testing for glucose measurement in critically ill adults.Crit Care Med.2005;33:2778–2785.
Copyright © 2007 Society of Hospital Medicine
Adopting NQF Practices
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5
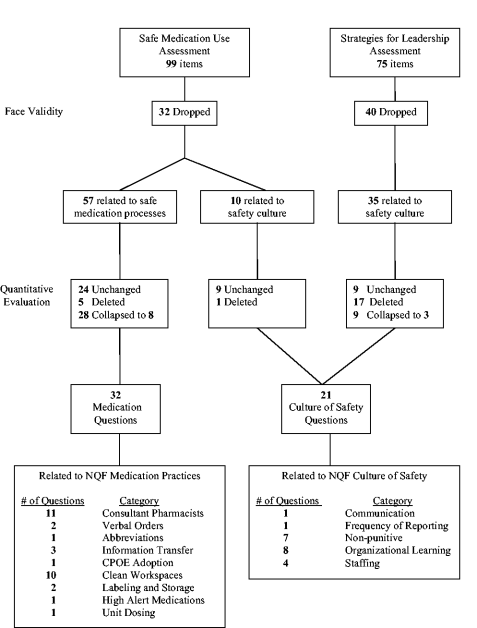
Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5

Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5

Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
Copyright © 2007 Society of Hospital Medicine
Hospitalists and Hip Fractures
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.
| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.

| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.

| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
Copyright © 2007 Society of Hospital Medicine