User login
If you don’t ask (about memory), they probably won’t tell
- Ask elderly patients whether they’re having any memory problems, since they are unlikely to volunteer this information on their own. Doing so may help to identify potentially frail patients (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Objectives To investigate the prevalence and potential clinical implications of self-reported memory impairment among elderly patients in general practice.
Methods This was a cross-sectional study in 17 general practices serving 40,865 patients, of whom 2934 were 65 years of age or older. Outcome measures were self-reported memory impairment, health-related quality of life, and cognition.
Results In total, 177 (23.4%) out of 758 elderly patients consulting their physician reported impaired memory. Only 33 (18.6%) had consulted their physician for memory problems. The only independent predictor for impaired memory was a lower quality-of-life score: scores on the EuroQol-5D-VAS of 0 to 49 and 50–74 points both correlated with memory complaints (odds ratios=4.8 and 4.1, respectively).
Conclusions Memory impairment is a common complaint among elderly patients in general practice, but many patients will not present with these symptoms. It may be useful for general practitioners (GPs) to ask about memory problems in order to identify potentially frail patients. Prospective trials are warranted.
In studies of older patients, the prevalence of subjective memory complaints in community-based populations varies from 11% to 56%,1,2 depending on sample selection and on how the complaints are assessed.1 Subjective memory complaints may be associated with psychiatric symptoms—in particular, depression3,4 and anxiety—as well as older age, lower education, and female gender.1 In these studies, some association has been found between memory complaints and cognitive impairment on testing, even after adjustment for depressive symptoms.4,5
Researchers have suggested that subjective memory complaints may be an early indicator for dementia,1 and could therefore be considered as a marker for identification of dementia in general practice. However, these complaints may be the result of a wide range of conditions; longitudinal studies assessing the value of memory complaints in predicting dementia or cognitive decline have shown varying results.6-8
The prevalence of subjective memory complaints among elderly patients consulting their GP is not known, and the clinical implication of these complaints is not well established. We conducted this study in order to investigate the prevalence and potential clinical implications of subjective memory complaints among elderly patients in general practice.
Methods
Recruiting the subjects
Seventeen general medical practices with 24 GPs located in the central district of Copenhagen, Denmark, participated in this study. These practices served a total of 40,865 patients, 2934 of whom were 65 years of age or older.
We asked all patients 65 years of age or older who consulted their GP in October and November 2002 to participate in the study, regardless of the reason for the encounter. We excluded patients who were not able to read Danish or not able to sign an informed consent form. We also excluded those with severe acute or terminal illness or a diagnosis of dementia.
Assessment of the patients
Participant questionnaire. Before the visit with their GP, we asked all qualifying patients to complete a questionnaire with items about self-reported health and memory status, as well as demographic questions. The item regarding memory status was phrased: “How would you evaluate your memory?” The categories were “excellent,” “good,” “less good,” “poor,” and “miserable.” Patients rating their memory as “less good,” “poor,” or “miserable” were classified as patients with subjective memory complaints, whereas patients rating their memory as “excellent” or “good” were defined as patients without subjective memory complaints.
Quality-of-life assessment. During their visit, the patients also completed the Danish Validated Version of EuroQoL-5D, which includes a visual analogue scale (VAS). EuroQoL-5D is a standardized instrument for use as a measure of health outcomes.9 Patients are asked to assess their health—in regards to mobility, self-care, everyday activities, pain, and anxiety—by checking 1 of 3 boxes. They are then asked to assess their general state of health on a VAS ranging from 0 to 100.
GP questionnaire. A questionnaire dealing with the GP’s clinical impression of dementia was developed together with 2 of the GPs and tested in a pilot survey. This questionnaire was completed by the GP for each patient before they administered the Mini Mental State Examination (MMSE), with no information from the completed participant questionnaire. The GPs could complete the questionnaire before or during the office visit.
MMSE. The MMSE, recommended in GP guidelines as a cognitive screening test, was given to the patients after the GPs completed their own questionnaires.10 The test is a 30-point questionnaire that assesses cognition; it includes simple questions and problems in a number of areas: time and place of the test, repeating lists of words, math problems, language use and comprehension, and copying a drawing. An MMSE score <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.
Registry data and ethics
The Danish National Health Register provided the information regarding the physicians and their practices.11 The municipality of Copenhagen provided information regarding the nursing home status of patients.
The Scientific Ethical Committee for Copenhagen and Frederiksberg Municipalities evaluated the project. The Danish Data Protection Agency and the Danish College of General Practitioners Study Committee approved the project.
How we analyzed the data
All statistical analyses were performed using SAS, version 9.1 (SAS Institute Inc, Cary, NC). To avoid a possible cluster effect between the 17 practices, probabilities and corresponding 95% confidence intervals were estimated using a Generalized Estimating Equation (GEE) regression model. We used this method so that we could compare participants to nonparticipants, as well as to patients with subjective memory complaints and those without them. A backward elimination and a significance level of 5% to stay in model were used. Pearson’s chi-square was used to evaluate Goodness of Fit for the reduced model.
In the hypothesis-generating analysis, the following variables were included: age, gender, living with partner, receiving home care, school education, MMSE score, and EuroQoL-5D-VAS score. The EuroQoL-5D results were categorized into 3 groups: severe impairment (0 to 49 points), mild to moderate impairment (50 to 74), and normal (75 to 100). The MMSE was adjusted for age and education.
Results
Only quality-of-life scores predicted memory complaints
A total of 1180 patients 65 years of age and older consulted their GPs in the study period. From this group, we excluded 133 patients. Of the eligible 1047 patients, 775 (74.0%) patients agreed to participate in the study. These patents had a mean age of 74.8 years (standard deviation [SD], 7.1), and an average relationship with their GP of 11 years. Those who refused to participate in the study were more likely to be female and were less likely to complain about memory problems, according to the GP surveys.
The average MMSE score for these 775 patients was 28.2 (SD, 2.0), and the average EuroQoL-5D-VAS score was 70.9 (SD, 18.9). A total of 758 patients responded to the patient questionnaire regarding memory. Of these 758 patients, 177 (23.4%) reported memory complaints (that is, indicated their memory was “less good,” “poor,” or “miserable”). Only 33 (18.6%) of these 177 patients had previously consulted their GP regarding memory problems. The TABLE shows the characteristics of participants based on self-reported memory complaints.
In a hypothesis-generating analysis, we found that the only predictor for subjective memory complaints, as compared with those patients with good memory (stated as “excellent” or “good”), was an impairment of EuroQoL-5D-VAS: for a score of 0–49 points, the odds ratio (OR) for subjective memory complaints was 4.8; for a score of 50–74 points, the OR was 4.1. The patients’ gender, education, MMSE score, whether they lived alone or with a partner, and whether they were receiving home care did not seem to be independent predictors.
TABLE
Quality-of-life score was the only predictor of self-reported memory problems
| SELF-RATED MEMORY (n=758)* | ||
|---|---|---|
| EXCELLENT OR GOOD (N=581) | LESS GOOD, POOR, OR MISERABLE (N=177) | |
| Age, years (95% CI) | 74.5 (73.9–75.1) | 75.7 (74.6–76.8) |
| Female, n (%) | 348/581 (59.9%) | 116/177 (65.5%) |
| 8 years or less schooling, n (%) | 203/558 (36.4%) | 60/168 (35.7%) |
| Living without partner, n (%) | 340/580 (58.6%) | 115/175 (65.7%) |
| Receiving home care, n (%) | 106/579 (18.3%) | 49/175 (28.0%) |
| Cognition | ||
| Participant had previously complained about memory (per GP survey), n (%) | 16/567 (2.8%) | 33/175 (18.9%) |
| MMSE score (95% CI) | 28.3 (28.2–28.5) | 27.8 (27.3–28.0) |
| Quality of life: EuroQol-5D-VAS score (95% CI) | 73.8 (72.3–75.4) | 61.4 (58.5–64.2) |
| *We did not obtain self-rated memory status from 17 participants. Of the 758 subjects who took the survey, not everyone answered every question. | ||
Discussion
Other predictors of memory problems remain to be discovered
Depression,12 other psychiatric conditions,3,4 as well as certain medications may be associated with self-reported memory problems in elderly patients. These associations may explain why we found a correlation between reports of a lower quality of life and subjective memory complaints. Advanced age, female gender, and a low level of education have also been associated with a higher prevalence of memory complaints in other studies, but our study did not confirm any of these findings.
Limitations of this study
This study had several limitations. It had some selection bias, which may decrease its generalizability. In addition, this study was not designed to clarify whether memory complaints could be an early indicator for onset of dementia, or whether these complaints are associated with mild cognitive impairment or existing dementia.
The collection of data was monitored on a weekly basis by site visits from a study nurse. However, we did not monitor the actual exams.
An MMSE score of <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.12 However, research has shown that MMSE scores are affected by age, education, and cultural background; this may explain why the MMSE by itself is not sufficient to diagnose dementia.12
Future studies should focus on clinically relevant outcomes
Further prospective studies in GP settings are needed to examine the potential implications of subjective memory complaints. We suggest that in future studies, clinically relevant outcomes—such as death, nursing home placement, medication usage, or health care usage—be used as possible correlating factors.
Correspondence
Frans Boch Waldorff, MD, PhD, Research Unit of General Practice, Kommunehospitalet, Øster Farimagsgade 5, DK-1014 Copenhagen, Denmark; fbw@gpract.ku.dk
1. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000;15:983-991.
2. Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc 2004;52:263-268.
3. Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Demen 2004;19:353-360.
4. Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology 1994;13:145-154.
5. O’Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry 1990;47:224-227.
6. Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging Study. Int J Geriatr Psychiatry 2006;21:432-441.
7. Geerlings MI, Jonker C, Bouter lM, Ader HJ, Schmand B. Association between memory complaints and incident alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999;156:531-537.
8. Schmand B, Jonker C, Hooijer C, lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996;46:121-125.
9. Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-343.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-198.
11. Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Register. A tool for primary health care research. Dan Med Bull 1997;44:449-453.
12. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-935.
- Ask elderly patients whether they’re having any memory problems, since they are unlikely to volunteer this information on their own. Doing so may help to identify potentially frail patients (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Objectives To investigate the prevalence and potential clinical implications of self-reported memory impairment among elderly patients in general practice.
Methods This was a cross-sectional study in 17 general practices serving 40,865 patients, of whom 2934 were 65 years of age or older. Outcome measures were self-reported memory impairment, health-related quality of life, and cognition.
Results In total, 177 (23.4%) out of 758 elderly patients consulting their physician reported impaired memory. Only 33 (18.6%) had consulted their physician for memory problems. The only independent predictor for impaired memory was a lower quality-of-life score: scores on the EuroQol-5D-VAS of 0 to 49 and 50–74 points both correlated with memory complaints (odds ratios=4.8 and 4.1, respectively).
Conclusions Memory impairment is a common complaint among elderly patients in general practice, but many patients will not present with these symptoms. It may be useful for general practitioners (GPs) to ask about memory problems in order to identify potentially frail patients. Prospective trials are warranted.
In studies of older patients, the prevalence of subjective memory complaints in community-based populations varies from 11% to 56%,1,2 depending on sample selection and on how the complaints are assessed.1 Subjective memory complaints may be associated with psychiatric symptoms—in particular, depression3,4 and anxiety—as well as older age, lower education, and female gender.1 In these studies, some association has been found between memory complaints and cognitive impairment on testing, even after adjustment for depressive symptoms.4,5
Researchers have suggested that subjective memory complaints may be an early indicator for dementia,1 and could therefore be considered as a marker for identification of dementia in general practice. However, these complaints may be the result of a wide range of conditions; longitudinal studies assessing the value of memory complaints in predicting dementia or cognitive decline have shown varying results.6-8
The prevalence of subjective memory complaints among elderly patients consulting their GP is not known, and the clinical implication of these complaints is not well established. We conducted this study in order to investigate the prevalence and potential clinical implications of subjective memory complaints among elderly patients in general practice.
Methods
Recruiting the subjects
Seventeen general medical practices with 24 GPs located in the central district of Copenhagen, Denmark, participated in this study. These practices served a total of 40,865 patients, 2934 of whom were 65 years of age or older.
We asked all patients 65 years of age or older who consulted their GP in October and November 2002 to participate in the study, regardless of the reason for the encounter. We excluded patients who were not able to read Danish or not able to sign an informed consent form. We also excluded those with severe acute or terminal illness or a diagnosis of dementia.
Assessment of the patients
Participant questionnaire. Before the visit with their GP, we asked all qualifying patients to complete a questionnaire with items about self-reported health and memory status, as well as demographic questions. The item regarding memory status was phrased: “How would you evaluate your memory?” The categories were “excellent,” “good,” “less good,” “poor,” and “miserable.” Patients rating their memory as “less good,” “poor,” or “miserable” were classified as patients with subjective memory complaints, whereas patients rating their memory as “excellent” or “good” were defined as patients without subjective memory complaints.
Quality-of-life assessment. During their visit, the patients also completed the Danish Validated Version of EuroQoL-5D, which includes a visual analogue scale (VAS). EuroQoL-5D is a standardized instrument for use as a measure of health outcomes.9 Patients are asked to assess their health—in regards to mobility, self-care, everyday activities, pain, and anxiety—by checking 1 of 3 boxes. They are then asked to assess their general state of health on a VAS ranging from 0 to 100.
GP questionnaire. A questionnaire dealing with the GP’s clinical impression of dementia was developed together with 2 of the GPs and tested in a pilot survey. This questionnaire was completed by the GP for each patient before they administered the Mini Mental State Examination (MMSE), with no information from the completed participant questionnaire. The GPs could complete the questionnaire before or during the office visit.
MMSE. The MMSE, recommended in GP guidelines as a cognitive screening test, was given to the patients after the GPs completed their own questionnaires.10 The test is a 30-point questionnaire that assesses cognition; it includes simple questions and problems in a number of areas: time and place of the test, repeating lists of words, math problems, language use and comprehension, and copying a drawing. An MMSE score <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.
Registry data and ethics
The Danish National Health Register provided the information regarding the physicians and their practices.11 The municipality of Copenhagen provided information regarding the nursing home status of patients.
The Scientific Ethical Committee for Copenhagen and Frederiksberg Municipalities evaluated the project. The Danish Data Protection Agency and the Danish College of General Practitioners Study Committee approved the project.
How we analyzed the data
All statistical analyses were performed using SAS, version 9.1 (SAS Institute Inc, Cary, NC). To avoid a possible cluster effect between the 17 practices, probabilities and corresponding 95% confidence intervals were estimated using a Generalized Estimating Equation (GEE) regression model. We used this method so that we could compare participants to nonparticipants, as well as to patients with subjective memory complaints and those without them. A backward elimination and a significance level of 5% to stay in model were used. Pearson’s chi-square was used to evaluate Goodness of Fit for the reduced model.
In the hypothesis-generating analysis, the following variables were included: age, gender, living with partner, receiving home care, school education, MMSE score, and EuroQoL-5D-VAS score. The EuroQoL-5D results were categorized into 3 groups: severe impairment (0 to 49 points), mild to moderate impairment (50 to 74), and normal (75 to 100). The MMSE was adjusted for age and education.
Results
Only quality-of-life scores predicted memory complaints
A total of 1180 patients 65 years of age and older consulted their GPs in the study period. From this group, we excluded 133 patients. Of the eligible 1047 patients, 775 (74.0%) patients agreed to participate in the study. These patents had a mean age of 74.8 years (standard deviation [SD], 7.1), and an average relationship with their GP of 11 years. Those who refused to participate in the study were more likely to be female and were less likely to complain about memory problems, according to the GP surveys.
The average MMSE score for these 775 patients was 28.2 (SD, 2.0), and the average EuroQoL-5D-VAS score was 70.9 (SD, 18.9). A total of 758 patients responded to the patient questionnaire regarding memory. Of these 758 patients, 177 (23.4%) reported memory complaints (that is, indicated their memory was “less good,” “poor,” or “miserable”). Only 33 (18.6%) of these 177 patients had previously consulted their GP regarding memory problems. The TABLE shows the characteristics of participants based on self-reported memory complaints.
In a hypothesis-generating analysis, we found that the only predictor for subjective memory complaints, as compared with those patients with good memory (stated as “excellent” or “good”), was an impairment of EuroQoL-5D-VAS: for a score of 0–49 points, the odds ratio (OR) for subjective memory complaints was 4.8; for a score of 50–74 points, the OR was 4.1. The patients’ gender, education, MMSE score, whether they lived alone or with a partner, and whether they were receiving home care did not seem to be independent predictors.
TABLE
Quality-of-life score was the only predictor of self-reported memory problems
| SELF-RATED MEMORY (n=758)* | ||
|---|---|---|
| EXCELLENT OR GOOD (N=581) | LESS GOOD, POOR, OR MISERABLE (N=177) | |
| Age, years (95% CI) | 74.5 (73.9–75.1) | 75.7 (74.6–76.8) |
| Female, n (%) | 348/581 (59.9%) | 116/177 (65.5%) |
| 8 years or less schooling, n (%) | 203/558 (36.4%) | 60/168 (35.7%) |
| Living without partner, n (%) | 340/580 (58.6%) | 115/175 (65.7%) |
| Receiving home care, n (%) | 106/579 (18.3%) | 49/175 (28.0%) |
| Cognition | ||
| Participant had previously complained about memory (per GP survey), n (%) | 16/567 (2.8%) | 33/175 (18.9%) |
| MMSE score (95% CI) | 28.3 (28.2–28.5) | 27.8 (27.3–28.0) |
| Quality of life: EuroQol-5D-VAS score (95% CI) | 73.8 (72.3–75.4) | 61.4 (58.5–64.2) |
| *We did not obtain self-rated memory status from 17 participants. Of the 758 subjects who took the survey, not everyone answered every question. | ||
Discussion
Other predictors of memory problems remain to be discovered
Depression,12 other psychiatric conditions,3,4 as well as certain medications may be associated with self-reported memory problems in elderly patients. These associations may explain why we found a correlation between reports of a lower quality of life and subjective memory complaints. Advanced age, female gender, and a low level of education have also been associated with a higher prevalence of memory complaints in other studies, but our study did not confirm any of these findings.
Limitations of this study
This study had several limitations. It had some selection bias, which may decrease its generalizability. In addition, this study was not designed to clarify whether memory complaints could be an early indicator for onset of dementia, or whether these complaints are associated with mild cognitive impairment or existing dementia.
The collection of data was monitored on a weekly basis by site visits from a study nurse. However, we did not monitor the actual exams.
An MMSE score of <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.12 However, research has shown that MMSE scores are affected by age, education, and cultural background; this may explain why the MMSE by itself is not sufficient to diagnose dementia.12
Future studies should focus on clinically relevant outcomes
Further prospective studies in GP settings are needed to examine the potential implications of subjective memory complaints. We suggest that in future studies, clinically relevant outcomes—such as death, nursing home placement, medication usage, or health care usage—be used as possible correlating factors.
Correspondence
Frans Boch Waldorff, MD, PhD, Research Unit of General Practice, Kommunehospitalet, Øster Farimagsgade 5, DK-1014 Copenhagen, Denmark; fbw@gpract.ku.dk
- Ask elderly patients whether they’re having any memory problems, since they are unlikely to volunteer this information on their own. Doing so may help to identify potentially frail patients (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Objectives To investigate the prevalence and potential clinical implications of self-reported memory impairment among elderly patients in general practice.
Methods This was a cross-sectional study in 17 general practices serving 40,865 patients, of whom 2934 were 65 years of age or older. Outcome measures were self-reported memory impairment, health-related quality of life, and cognition.
Results In total, 177 (23.4%) out of 758 elderly patients consulting their physician reported impaired memory. Only 33 (18.6%) had consulted their physician for memory problems. The only independent predictor for impaired memory was a lower quality-of-life score: scores on the EuroQol-5D-VAS of 0 to 49 and 50–74 points both correlated with memory complaints (odds ratios=4.8 and 4.1, respectively).
Conclusions Memory impairment is a common complaint among elderly patients in general practice, but many patients will not present with these symptoms. It may be useful for general practitioners (GPs) to ask about memory problems in order to identify potentially frail patients. Prospective trials are warranted.
In studies of older patients, the prevalence of subjective memory complaints in community-based populations varies from 11% to 56%,1,2 depending on sample selection and on how the complaints are assessed.1 Subjective memory complaints may be associated with psychiatric symptoms—in particular, depression3,4 and anxiety—as well as older age, lower education, and female gender.1 In these studies, some association has been found between memory complaints and cognitive impairment on testing, even after adjustment for depressive symptoms.4,5
Researchers have suggested that subjective memory complaints may be an early indicator for dementia,1 and could therefore be considered as a marker for identification of dementia in general practice. However, these complaints may be the result of a wide range of conditions; longitudinal studies assessing the value of memory complaints in predicting dementia or cognitive decline have shown varying results.6-8
The prevalence of subjective memory complaints among elderly patients consulting their GP is not known, and the clinical implication of these complaints is not well established. We conducted this study in order to investigate the prevalence and potential clinical implications of subjective memory complaints among elderly patients in general practice.
Methods
Recruiting the subjects
Seventeen general medical practices with 24 GPs located in the central district of Copenhagen, Denmark, participated in this study. These practices served a total of 40,865 patients, 2934 of whom were 65 years of age or older.
We asked all patients 65 years of age or older who consulted their GP in October and November 2002 to participate in the study, regardless of the reason for the encounter. We excluded patients who were not able to read Danish or not able to sign an informed consent form. We also excluded those with severe acute or terminal illness or a diagnosis of dementia.
Assessment of the patients
Participant questionnaire. Before the visit with their GP, we asked all qualifying patients to complete a questionnaire with items about self-reported health and memory status, as well as demographic questions. The item regarding memory status was phrased: “How would you evaluate your memory?” The categories were “excellent,” “good,” “less good,” “poor,” and “miserable.” Patients rating their memory as “less good,” “poor,” or “miserable” were classified as patients with subjective memory complaints, whereas patients rating their memory as “excellent” or “good” were defined as patients without subjective memory complaints.
Quality-of-life assessment. During their visit, the patients also completed the Danish Validated Version of EuroQoL-5D, which includes a visual analogue scale (VAS). EuroQoL-5D is a standardized instrument for use as a measure of health outcomes.9 Patients are asked to assess their health—in regards to mobility, self-care, everyday activities, pain, and anxiety—by checking 1 of 3 boxes. They are then asked to assess their general state of health on a VAS ranging from 0 to 100.
GP questionnaire. A questionnaire dealing with the GP’s clinical impression of dementia was developed together with 2 of the GPs and tested in a pilot survey. This questionnaire was completed by the GP for each patient before they administered the Mini Mental State Examination (MMSE), with no information from the completed participant questionnaire. The GPs could complete the questionnaire before or during the office visit.
MMSE. The MMSE, recommended in GP guidelines as a cognitive screening test, was given to the patients after the GPs completed their own questionnaires.10 The test is a 30-point questionnaire that assesses cognition; it includes simple questions and problems in a number of areas: time and place of the test, repeating lists of words, math problems, language use and comprehension, and copying a drawing. An MMSE score <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.
Registry data and ethics
The Danish National Health Register provided the information regarding the physicians and their practices.11 The municipality of Copenhagen provided information regarding the nursing home status of patients.
The Scientific Ethical Committee for Copenhagen and Frederiksberg Municipalities evaluated the project. The Danish Data Protection Agency and the Danish College of General Practitioners Study Committee approved the project.
How we analyzed the data
All statistical analyses were performed using SAS, version 9.1 (SAS Institute Inc, Cary, NC). To avoid a possible cluster effect between the 17 practices, probabilities and corresponding 95% confidence intervals were estimated using a Generalized Estimating Equation (GEE) regression model. We used this method so that we could compare participants to nonparticipants, as well as to patients with subjective memory complaints and those without them. A backward elimination and a significance level of 5% to stay in model were used. Pearson’s chi-square was used to evaluate Goodness of Fit for the reduced model.
In the hypothesis-generating analysis, the following variables were included: age, gender, living with partner, receiving home care, school education, MMSE score, and EuroQoL-5D-VAS score. The EuroQoL-5D results were categorized into 3 groups: severe impairment (0 to 49 points), mild to moderate impairment (50 to 74), and normal (75 to 100). The MMSE was adjusted for age and education.
Results
Only quality-of-life scores predicted memory complaints
A total of 1180 patients 65 years of age and older consulted their GPs in the study period. From this group, we excluded 133 patients. Of the eligible 1047 patients, 775 (74.0%) patients agreed to participate in the study. These patents had a mean age of 74.8 years (standard deviation [SD], 7.1), and an average relationship with their GP of 11 years. Those who refused to participate in the study were more likely to be female and were less likely to complain about memory problems, according to the GP surveys.
The average MMSE score for these 775 patients was 28.2 (SD, 2.0), and the average EuroQoL-5D-VAS score was 70.9 (SD, 18.9). A total of 758 patients responded to the patient questionnaire regarding memory. Of these 758 patients, 177 (23.4%) reported memory complaints (that is, indicated their memory was “less good,” “poor,” or “miserable”). Only 33 (18.6%) of these 177 patients had previously consulted their GP regarding memory problems. The TABLE shows the characteristics of participants based on self-reported memory complaints.
In a hypothesis-generating analysis, we found that the only predictor for subjective memory complaints, as compared with those patients with good memory (stated as “excellent” or “good”), was an impairment of EuroQoL-5D-VAS: for a score of 0–49 points, the odds ratio (OR) for subjective memory complaints was 4.8; for a score of 50–74 points, the OR was 4.1. The patients’ gender, education, MMSE score, whether they lived alone or with a partner, and whether they were receiving home care did not seem to be independent predictors.
TABLE
Quality-of-life score was the only predictor of self-reported memory problems
| SELF-RATED MEMORY (n=758)* | ||
|---|---|---|
| EXCELLENT OR GOOD (N=581) | LESS GOOD, POOR, OR MISERABLE (N=177) | |
| Age, years (95% CI) | 74.5 (73.9–75.1) | 75.7 (74.6–76.8) |
| Female, n (%) | 348/581 (59.9%) | 116/177 (65.5%) |
| 8 years or less schooling, n (%) | 203/558 (36.4%) | 60/168 (35.7%) |
| Living without partner, n (%) | 340/580 (58.6%) | 115/175 (65.7%) |
| Receiving home care, n (%) | 106/579 (18.3%) | 49/175 (28.0%) |
| Cognition | ||
| Participant had previously complained about memory (per GP survey), n (%) | 16/567 (2.8%) | 33/175 (18.9%) |
| MMSE score (95% CI) | 28.3 (28.2–28.5) | 27.8 (27.3–28.0) |
| Quality of life: EuroQol-5D-VAS score (95% CI) | 73.8 (72.3–75.4) | 61.4 (58.5–64.2) |
| *We did not obtain self-rated memory status from 17 participants. Of the 758 subjects who took the survey, not everyone answered every question. | ||
Discussion
Other predictors of memory problems remain to be discovered
Depression,12 other psychiatric conditions,3,4 as well as certain medications may be associated with self-reported memory problems in elderly patients. These associations may explain why we found a correlation between reports of a lower quality of life and subjective memory complaints. Advanced age, female gender, and a low level of education have also been associated with a higher prevalence of memory complaints in other studies, but our study did not confirm any of these findings.
Limitations of this study
This study had several limitations. It had some selection bias, which may decrease its generalizability. In addition, this study was not designed to clarify whether memory complaints could be an early indicator for onset of dementia, or whether these complaints are associated with mild cognitive impairment or existing dementia.
The collection of data was monitored on a weekly basis by site visits from a study nurse. However, we did not monitor the actual exams.
An MMSE score of <24 has been widely used as an indication of the presence of cognitive impairment in population-based studies.12 However, research has shown that MMSE scores are affected by age, education, and cultural background; this may explain why the MMSE by itself is not sufficient to diagnose dementia.12
Future studies should focus on clinically relevant outcomes
Further prospective studies in GP settings are needed to examine the potential implications of subjective memory complaints. We suggest that in future studies, clinically relevant outcomes—such as death, nursing home placement, medication usage, or health care usage—be used as possible correlating factors.
Correspondence
Frans Boch Waldorff, MD, PhD, Research Unit of General Practice, Kommunehospitalet, Øster Farimagsgade 5, DK-1014 Copenhagen, Denmark; fbw@gpract.ku.dk
1. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000;15:983-991.
2. Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc 2004;52:263-268.
3. Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Demen 2004;19:353-360.
4. Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology 1994;13:145-154.
5. O’Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry 1990;47:224-227.
6. Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging Study. Int J Geriatr Psychiatry 2006;21:432-441.
7. Geerlings MI, Jonker C, Bouter lM, Ader HJ, Schmand B. Association between memory complaints and incident alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999;156:531-537.
8. Schmand B, Jonker C, Hooijer C, lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996;46:121-125.
9. Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-343.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-198.
11. Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Register. A tool for primary health care research. Dan Med Bull 1997;44:449-453.
12. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-935.
1. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000;15:983-991.
2. Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc 2004;52:263-268.
3. Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Demen 2004;19:353-360.
4. Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology 1994;13:145-154.
5. O’Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry 1990;47:224-227.
6. Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging Study. Int J Geriatr Psychiatry 2006;21:432-441.
7. Geerlings MI, Jonker C, Bouter lM, Ader HJ, Schmand B. Association between memory complaints and incident alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999;156:531-537.
8. Schmand B, Jonker C, Hooijer C, lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996;46:121-125.
9. Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-343.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-198.
11. Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Register. A tool for primary health care research. Dan Med Bull 1997;44:449-453.
12. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-935.
Varicella vaccination: 2 doses now the standard
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
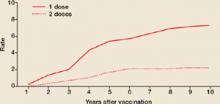
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
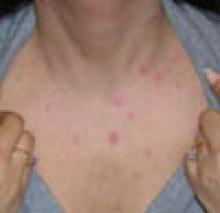
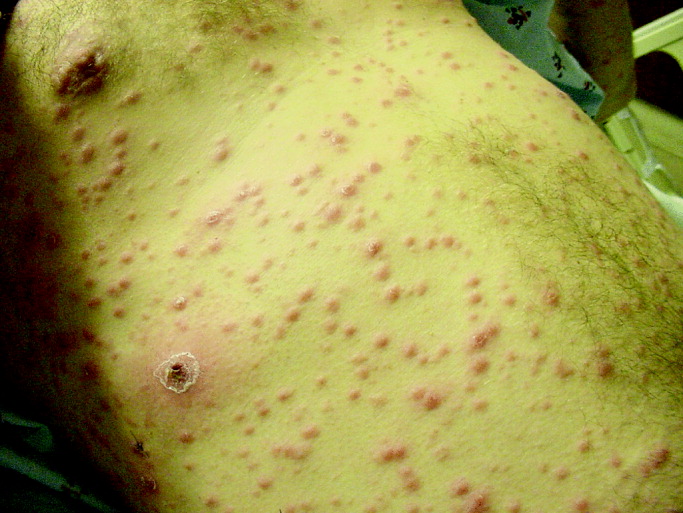
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
Geriatric Syndromes in Older Cardiology Patients
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
Copyright © 2007 Society of Hospital Medicine
Systematic Review of Rapid Response Systems
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.
Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).
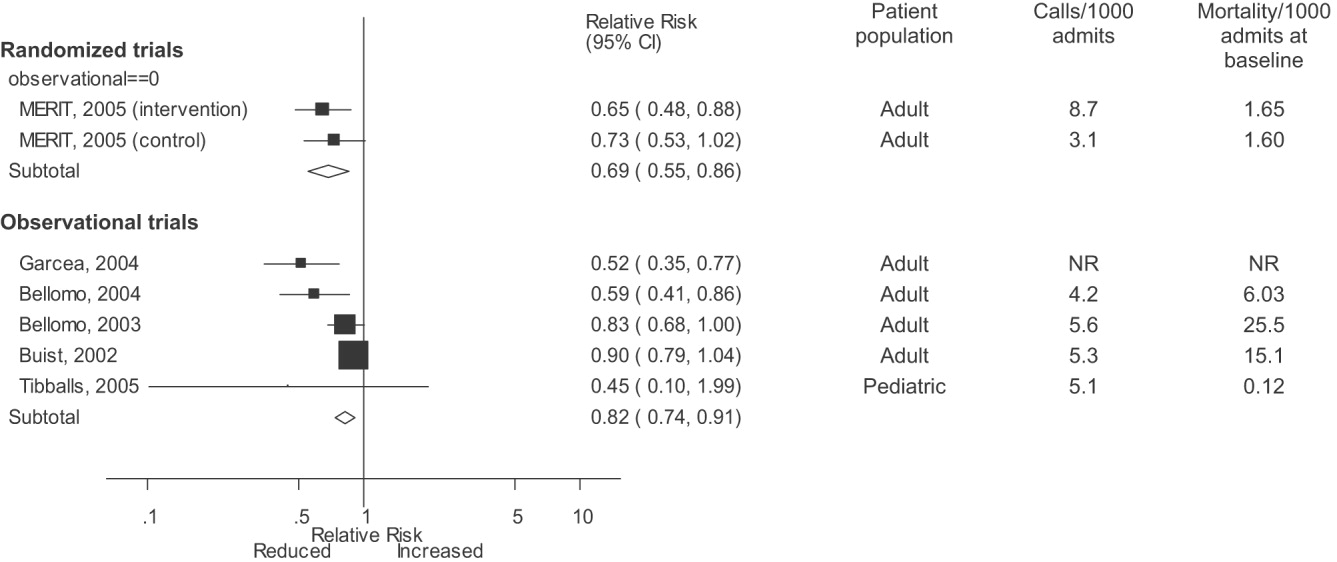
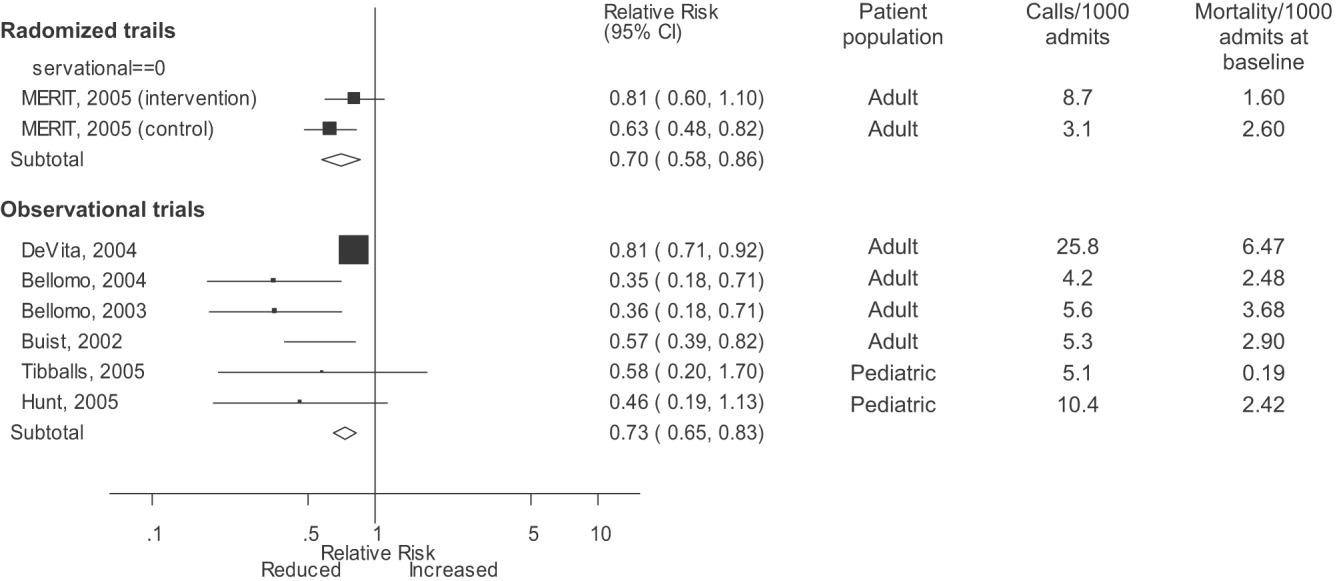
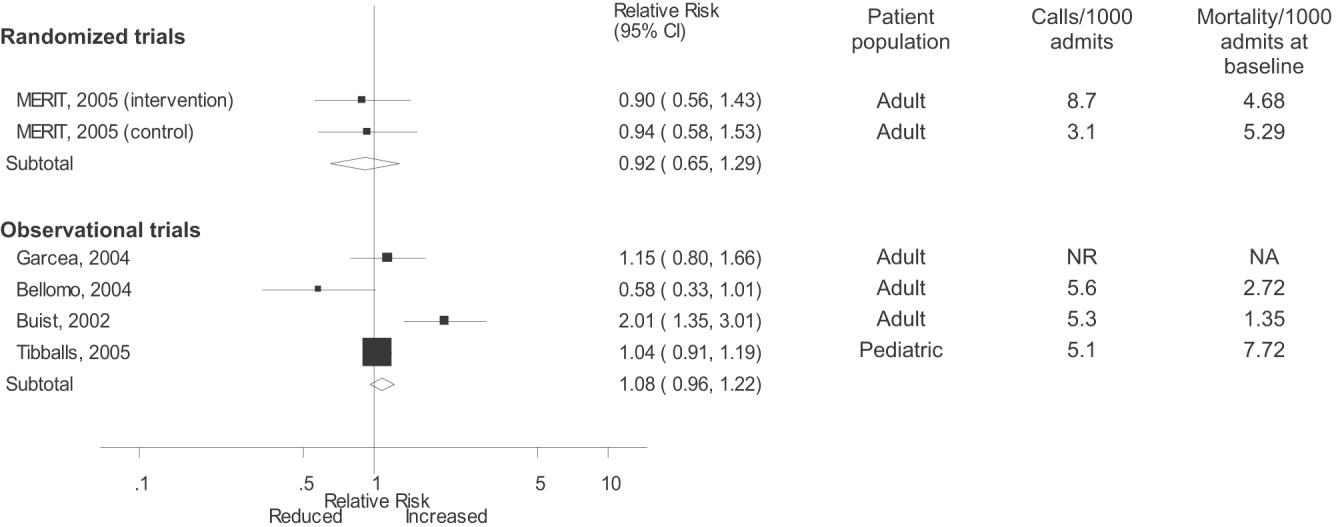
DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
Identifying Hospitalized Heart Failure Patients / Halasyamani et al.
There has been increasing emphasis on the development and successful execution of disease management strategies to improve the delivery of evidence‐based care for hospitalized patients with heart failure.14 Current care is woefully suboptimal for heart failure patients. Fonorow et al. describe the significant gap in performance on the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) heart failure core measures, with the median rate of conformity with all measures at only 24% nationally.5 For a variety of clinical and external factors, such as publicly reported quality measures and pay‐for‐performance incentives, institutions are increasingly motivated to identify patients who will make up the denominator of the heart failure metrics. At first glance, system‐level identification of heart failure patients may not seem critical to the delivery of evidence‐based care, but given that the management of patients with heart failure is multidisciplinary, it is critical that patients who have heart failure be clearly identified for all members of the care team. The capability of prospectively identifying inpatients with the principal diagnosis of heart failure is an essential first step in the implementation of performance improvement programs.
Successful interventions have included a multidisciplinary intervention with postdischarge follow‐up.6 However, the interventions described do not fully indicate how patients with heart failure are identified while in the hospital, so those interventions may be difficult to replicate in other settings. It has not been easy to identify these patients in a timely fashion given that a chief complaint of shortness of breath can indicate other clinical conditions in addition to heart failure. Previous studies have used an admission diagnosis of heart failure or suggestive chest X‐ray findings to trigger a clinical evaluation.7, 8 However, the sensitivity and specificity of these case‐finding methods have not been reported. Furthermore, patients presenting with shortness of breath may not have a diagnosis established until a series of diagnostic and therapeutic maneuvers have been performed. The challenge of promoting physician provision and documentation of evidence‐based care is compounded by these patients usually not being housed in a single geographical unit, possibly being attended by any number of medical specialties, and often having short lengths of stay. Given the multiple factors contributing to the complexity of identifying patients hospitalized with heart failure, it is important to delineate case‐finding strategies that efficiently and effectively identify heart failure patients so that clinical care and self‐management interventions are optimized.
With this goal in mind, we hypothesized that the receipt of intravenous loop diuretics may be an important trigger for identifying patients with heart failure. Receipt of intravenous loop diuretics is ubiquitous in the management of decompensated systolic and diastolic heart failure. We compare 2 electronic pharmacy‐based strategies in a tertiary‐care community teaching hospital to identify hospitalized patients early in their stay who were likely to be discharged with a principal diagnosis of heart failure (HF).
METHODS
Study Setting
The study was conducted in a 487‐bed not‐for‐profit community hospital in southeastern Michigan. The organization's institutional review board for all studies involving human subjects approved the study. In this hospital, heart failure patients are geographically dispersed throughout the institution, but all patient care orders are entered in a computerized provider order entry system. Approximately 70% of heart failure patients are admitted to the general medicine service, where care is directed by 3 types of attending physicians (academic hospitalists, private‐practice hospitalists, and community physicians, as previously described),9 14% are on the cardiology service, and the remainder are distributed among the surgical and intensive care unit services. The accuracy of 2 case‐finding strategies was tested using data from a 2‐year period. The institution had 28,005 adult hospitalizations during the prediction development period, July 1, 2003, to June 30, 2004, and 28,297 adult hospitalizations during the prediction testing period, July 1, 2004, to June 30, 2005. Receipt of intravenous loop diuretics had been used previously as a marker by the hospital's disease management program, but the accuracy of this strategy had not been tested.
Development of Prediction Algorithms
The outcome of interest was a principal diagnosis of HF, as assigned by medical records personnel after hospital discharge. This population corresponds to the denominator used to construct various performance measures. We evaluated 2 strategies for identifying targeted patients using information available prior to discharge. The first was the receipt of an intravenous loop diuretic at any point during the hospitalization (yes or no) as a single indicator. The second strategy used additional information to construct a multivariable predictor. Explanatory variables were considered for inclusion if they were available electronically, did not require additional manual retrieval or data entry, and had a clinical relationship with a diagnosis of HF. The variables selected were patient age, sex, receipt of intravenous loop diuretic, spironolactone, B‐type natriuretic peptide (BNP) level, serum creatinine, serum sodium, number of previous hospitalizations in the last 180 days with a principal discharge diagnosis of heart failure, and attending physician specialty. Cardiac ejection fraction was not included because the data were not available electronically.
Statistical Analysis
All analyses were performed using SAS version 9 (SAS Institute Inc., Cary NC). The data set was split chronologically into 2 sets each covering a 1‐year period in order to test the stability of the case‐finding strategies from one year to the next.
Initial model building for the multivariable strategy was done through logistic regression. Individual variables associated with heart failure (P < .05) were entered into a multivariable derivation model and retained if the main effect had a P value < .05. Sex and serum sodium were not included in the final model because of their high P values. To account for circumstances in which patients could have combinations of risk factors, interaction terms were also considered and were retained in the multivariable model at the same level of significance. The final parameter estimates for the derivation model were obtained from a generalized estimating equation (GEE) with an exchangeable working correlation structure to account for the possibility of multiple hospitalizations per year for a given patient. The z scores for the variables in the model provided insight into the relative importance of the factors associated with a heart failure diagnosis.
Laboratory values for the potential prediction variables were not available for every patient in our study; for example, a BNP level was obtained for only 7.6% of the study population. A simple strategy for addressing missing laboratory information was needed in order to derive a multivariable prediction model that could be used on a daily basis in a real‐world setting. We found that patients who had a BNP test drawn, regardless of the result, had a 27% chance of heart failure compared with those for whom BNP results were not available, whose chance of HF was 1%. Therefore, we could not simply impute the average BNP level for patients missing data on this parameter. Instead, we assumed the BNP levels of those not tested would be very low, and so gave these patients a BNP level of 1. Serum creatinine was not included in the multivariable model, despite its having a significant bivariate relationship with HF diagnosis, because valid imputation strategies for creatinine would be too complicated to implement in daily clinical practice.
The sensitivity and specificity of the single loop diuretic indicator was determined from a 2‐by‐2 table using data from the second study year. For both the multivariable and single loop diuretic approaches, test discrimination was evaluated by the c statistic from logistic regression.10 The calibration and overall performance of the multivariable derivation GEE model was assessed by a second GEE model run with the second‐year data set. For the testing model, the sole explanatory variable was a linear predictor derived from the covariate values of year 2 patients with the corresponding parameter estimates from the year 1 GEE. A well‐calibrated model with this configuration would be expected to have an intercept of 0 and a beta coefficient of the linear predictor of 1.11 Sensitivity, specificity, and positive predictive value were determined for 2 thresholds of predicted probability of heart failure, as derived from the linear predictor. If a subject's predicted probability at least equaled the threshold, then he or she would be considered to have tested positive for heart failure.
RESULTS
Salient features of the study population in the first and second study years are shown in Table 1. Mean age was approximately 59 years, and women made up 60% of the patients. The percentage of patients with a principal diagnosis of heart failure was 3% each year. Serum creatinine levels were available for 78% of patients in year 1 and 80% of patients in year 2. Serum BNP levels were available for 7.6% of patients in year 1 and 9% of patients in year 2.
| Variable | Year 1 (n = 28,005) | Year 2 (n = 28, 297) | |||
|---|---|---|---|---|---|
| No. with information | Mean or percent | No. with information | Mean or percent | P value | |
| |||||
| Age (years) | 28,005 | 58.7 | 28,297 | 58.9 | .36 |
| HF principal diagnosis (%) | 28,005 | 3.0% | 28,297 | 3.1% | .41* |
| Female (%) | 28,005 | 60.6% | 28,297 | 60.3% | .48* |
| First BNP level obtained (pg/mL) | 2132 | 813.7 | 2578 | 766.5 | .83 |
| First serum creatinine level obtained (mg/dL) | 21,839 | 1.4 | 22,596 | 1.4 | .54 |
| Patient received IV loop diuretic (%) | 28,005 | 16.3% | 28,297 | 15.8% | .07* |
| Patient received spironolactone (%) | 28,005 | 2.8% | 28,297 | 3.0% | .08* |
| Number of previous hospitalizations with HF in preceding 180 days | 28,005 | 2.4 | 28,297 | 2.9 | .09 |
The parameter estimates and 95% confidence intervals of the main effects of the final prediction model are shown in Table 2, with interaction terms noted in the footnote. Examination of the z scores (available from the authors) indicated that by far the most influential risk factor in the multivariable model was receipt of intravenous diuretics, with receipt of spironolactone a very distant second. The probability that a given patient had heart failure increased with the number of risk factors present and the magnitude of their parameter estimates. For example, an older patient who had been hospitalized with heart failure previously and who was currently receiving intravenous diuretics and spironolactone would be more likely to have a principal diagnosis of heart failure than would an older patient receiving intravenous diuretics who had no other risk factors. However, the interaction terms with negative values (see the footnote in Table 2) indicate that certain combinations of risk factors convey a level of risk somewhat less than the sum of their parts.
| Estimate | Standard error | Lower 95% CL | Upper 95% CL | |
|---|---|---|---|---|
| ||||
| Intercept | 8.28 | 0.40 | 9.06 | 7.50 |
| Centered age in 10‐year increments | 0.31 | 0.05 | 0.22 | 0.40 |
| Receipt of IV loop diuretic | 2.72 | 0.15 | 2.42 | 3.01 |
| Receipt of spironolactone | 1.53 | 0.19 | 1.16 | 1.90 |
| Centered logged BNP | 0.68 | 0.11 | 0.47 | 0.89 |
| Attending physician specialty | 2.46 | 0.41 | 1.66 | 3.26 |
| Count of hospitalizations for heart failure in previous 180 days | 2.43 | 0.48 | 1.48 | 3.37 |
The identification strategies performed well from one year to the next, as summarized in Table 3. Receipt of intravenous loop diuretics had a strong association with diagnosis of heart failure (OR 51.6, 95% CI 41.7, 63.7, P < .0001), with a c statistic of 0.88, a sensitivity of 0.89, and a specificity of 0.87.
| Strategy | TPs of possible 890 HF cases (n) | Sensitivity (# TPs/890) | FPs (n) | TNs of possible 27,407 hospitalizations without HF principal diagnosis (n) | Specificity (# TNs/27,40) | Positive predictive value (# TPs/all positives) | Likelihood ratio (TP/FP) |
|---|---|---|---|---|---|---|---|
| |||||||
| Use receipt of IV loop diuretic | 791 | 0.89 | 3676 | 23,731 | 0.87 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.02 | 833 | 0.94 | 3859 | 23,548 | 0.86 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.04 | 808 | 0.91 | 3045 | 24,362 | 0.89 | 0.21 | 8.2 |
The linear predictor of the multivariable prediction model as described in the Methods section also performed well in year 2 with excellent discrimination (c statistic of 0.96). Calibration was also excellent, as demonstrated by an intercept of 0.03 (standard error 0.05) and a beta coefficient of 1.02 (SE 0.03). If the threshold for identifying potential heart failure cases was defined as a predicted probability of at least 0.02, then the sensitivity of the multivariable predictor was 0.94 and the specificity was 0.86. If the positivity threshold was raised to 0.04, then the predictor's sensitivity dropped slightly, to 0.91, but specificity increased to 0.89.
The principal diagnoses of the 3045 patients in year 2 who were incorrectly predicted as having a principal diagnosis of heart failure (ie, false positives) were cardiac related (1026 of 3045; 34%), pulmonary related (685 of 3045; 22%), and renal‐ or fluid electrolyte related (117 of 3045; 4%), as determined using the multivariable approach with a 0.04 positivity threshold.
DISCUSSION
Identification of patients with heart failure early in their hospitalization is critical for successfully implementing disease management programs targeted at optimizing evidenced‐based care. Furthermore, public reporting of performance measures has increased the scrutiny of care delivered to those having this principal diagnosis. We developed a strategy that used the receipt of intravenous diuretics as a trigger of further clinical evaluation. We subsequently tested the value of other electronically available indicators to improve the sensitivity and specificity of the case‐finding strategy.
The receipt of an intravenous loop diuretic alone had a sensitivity of .89 and a specificity of .87. Incorporation of the additional information available to us electronically improved the sensitivity to .91 and the specificity to .89 (using a positivity threshold of 0.04), although these might be slightly different if BNP levels had been available for more patients. As with all diagnostic testing, there is a trade‐off between improved sensitivity and decreased specificity. At first glance, the resulting number of false positives generated by either prediction strategy may appear problematic. Although a formal cost‐effectiveness analysis of our case‐finding strategies is beyond the scope of this article, the cost of a false positive in this scenario is likely to be small.
For example in our hospital, clinical pharmacists place a reminder on the charts of patients who appear to have heart failure in order to prompt the clinical team to provide the recommended care processes. A list of inpatients treated with an IV diuretic is generated daily. A clinical pharmacist then reviews identified patient medical records to determine whether the diuretic was ordered for heart failure management or for some other purpose. This review consists of reading the completed history and physical and/or progress notes. On average, each medical record review takes 60 seconds to complete, with a range of 30‐90 seconds. At this speed, roughly 3000 minutes per year (or approximately 1 hour per week) would be spent reviewing the medical records of patients who would not have a principal diagnosis of heart failure. Nevertheless, we found that at least one‐third of the nominal false positives (multivariable rule, threshold of 0.04) still had a cardiac‐related diagnosis. Many of these had heart failure as a secondary diagnosis, but other diagnoses such as acute myocardial infarction took precedence in coding algorithms that assigned the principal diagnosis at discharge. Such patients might still benefit from the interventions and so are not truly false positives.
Patients with heart failure missed by this strategy included patients admitted for placement of pacemakers and/or defibrillators. Patients in this specialized population always had a single team managing their care, so the clinical and educational interventions were integrated into that team's daily work flow. Patients on dialysis with volume overload were not identified using this algorithm and constituted a very small number of patients in our heart failure population. Patients with stable heart failure on oral diuretics were not the focus of this case‐finding strategy and became a target for further intervention only if their heart failure worsened and required intravenous diuretics while they were hospitalized.
The identification of predictors for heart failure has allowed us not only to more effectively identify and risk‐stratify patients with heart failure but also to integrate the case‐finding strategy into clinical care delivery. This approach may also be relevant in hospitals that do not have computerized provider order entry (CPOE) systems but may be able to implement this case‐finding strategy by simply requesting a daily report of patients prescribed intravenous diuretics. As more institutions move to adopt CPOE platforms and clinical information such as ejection fractions become available, the predictors studied here may be augmented to form more sophisticated clinical rules and alerts.
Our study had several limitations. Although we validated our predictors in a separate cohort of patients, this is a single‐site study and may not be representative of the diverse institutions that care for patients with heart failure. There may also be interinstitutional differences in how a principal diagnosis of heart failure is assigned. We have demonstrated the stability of locally derived predictors from one year to the next but cannot make claims about how well our parameter estimates would perform in other settings. Finally, the complexity of the multivariable predictor requires an integrated database and computer application of a formula that may not be commonly available elsewhere at this time.
If disease management strategies are to be successful, early identification of at‐risk patients is crucial for both clinical care delivery and patient education regarding self‐management. The strategies tested here may be useful for other community‐based institutions whose care of heart failure patients is decentralized and involves multiple clinicians.
- ,,, et al.Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure.Congest Heart Fail.2005;11:315–321.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 Suppl):S1–S15.
- .Heart failure disease management: implementation and outcomes.Cardiol Rev.2005;13:231–239.
- ,,, et al.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;173(1):40–45.
- ,,, et al.Adherence to heart failure quality‐of‐care indicators in US hospitals: analysis of the ADHERE Registry.Arch Intern Med.2005;165:1469–1477.
- ,,, et al.Comprehensive discharge planning with post‐discharge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:2080–2081.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141:254–258.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- ,,, et al.Evaluating the yield of medical tests.JAMA.1982;247:2543–2546.
- .Two further applications of a model for binary regression.Biometrika.1958;45:562–565.
There has been increasing emphasis on the development and successful execution of disease management strategies to improve the delivery of evidence‐based care for hospitalized patients with heart failure.14 Current care is woefully suboptimal for heart failure patients. Fonorow et al. describe the significant gap in performance on the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) heart failure core measures, with the median rate of conformity with all measures at only 24% nationally.5 For a variety of clinical and external factors, such as publicly reported quality measures and pay‐for‐performance incentives, institutions are increasingly motivated to identify patients who will make up the denominator of the heart failure metrics. At first glance, system‐level identification of heart failure patients may not seem critical to the delivery of evidence‐based care, but given that the management of patients with heart failure is multidisciplinary, it is critical that patients who have heart failure be clearly identified for all members of the care team. The capability of prospectively identifying inpatients with the principal diagnosis of heart failure is an essential first step in the implementation of performance improvement programs.
Successful interventions have included a multidisciplinary intervention with postdischarge follow‐up.6 However, the interventions described do not fully indicate how patients with heart failure are identified while in the hospital, so those interventions may be difficult to replicate in other settings. It has not been easy to identify these patients in a timely fashion given that a chief complaint of shortness of breath can indicate other clinical conditions in addition to heart failure. Previous studies have used an admission diagnosis of heart failure or suggestive chest X‐ray findings to trigger a clinical evaluation.7, 8 However, the sensitivity and specificity of these case‐finding methods have not been reported. Furthermore, patients presenting with shortness of breath may not have a diagnosis established until a series of diagnostic and therapeutic maneuvers have been performed. The challenge of promoting physician provision and documentation of evidence‐based care is compounded by these patients usually not being housed in a single geographical unit, possibly being attended by any number of medical specialties, and often having short lengths of stay. Given the multiple factors contributing to the complexity of identifying patients hospitalized with heart failure, it is important to delineate case‐finding strategies that efficiently and effectively identify heart failure patients so that clinical care and self‐management interventions are optimized.
With this goal in mind, we hypothesized that the receipt of intravenous loop diuretics may be an important trigger for identifying patients with heart failure. Receipt of intravenous loop diuretics is ubiquitous in the management of decompensated systolic and diastolic heart failure. We compare 2 electronic pharmacy‐based strategies in a tertiary‐care community teaching hospital to identify hospitalized patients early in their stay who were likely to be discharged with a principal diagnosis of heart failure (HF).
METHODS
Study Setting
The study was conducted in a 487‐bed not‐for‐profit community hospital in southeastern Michigan. The organization's institutional review board for all studies involving human subjects approved the study. In this hospital, heart failure patients are geographically dispersed throughout the institution, but all patient care orders are entered in a computerized provider order entry system. Approximately 70% of heart failure patients are admitted to the general medicine service, where care is directed by 3 types of attending physicians (academic hospitalists, private‐practice hospitalists, and community physicians, as previously described),9 14% are on the cardiology service, and the remainder are distributed among the surgical and intensive care unit services. The accuracy of 2 case‐finding strategies was tested using data from a 2‐year period. The institution had 28,005 adult hospitalizations during the prediction development period, July 1, 2003, to June 30, 2004, and 28,297 adult hospitalizations during the prediction testing period, July 1, 2004, to June 30, 2005. Receipt of intravenous loop diuretics had been used previously as a marker by the hospital's disease management program, but the accuracy of this strategy had not been tested.
Development of Prediction Algorithms
The outcome of interest was a principal diagnosis of HF, as assigned by medical records personnel after hospital discharge. This population corresponds to the denominator used to construct various performance measures. We evaluated 2 strategies for identifying targeted patients using information available prior to discharge. The first was the receipt of an intravenous loop diuretic at any point during the hospitalization (yes or no) as a single indicator. The second strategy used additional information to construct a multivariable predictor. Explanatory variables were considered for inclusion if they were available electronically, did not require additional manual retrieval or data entry, and had a clinical relationship with a diagnosis of HF. The variables selected were patient age, sex, receipt of intravenous loop diuretic, spironolactone, B‐type natriuretic peptide (BNP) level, serum creatinine, serum sodium, number of previous hospitalizations in the last 180 days with a principal discharge diagnosis of heart failure, and attending physician specialty. Cardiac ejection fraction was not included because the data were not available electronically.
Statistical Analysis
All analyses were performed using SAS version 9 (SAS Institute Inc., Cary NC). The data set was split chronologically into 2 sets each covering a 1‐year period in order to test the stability of the case‐finding strategies from one year to the next.
Initial model building for the multivariable strategy was done through logistic regression. Individual variables associated with heart failure (P < .05) were entered into a multivariable derivation model and retained if the main effect had a P value < .05. Sex and serum sodium were not included in the final model because of their high P values. To account for circumstances in which patients could have combinations of risk factors, interaction terms were also considered and were retained in the multivariable model at the same level of significance. The final parameter estimates for the derivation model were obtained from a generalized estimating equation (GEE) with an exchangeable working correlation structure to account for the possibility of multiple hospitalizations per year for a given patient. The z scores for the variables in the model provided insight into the relative importance of the factors associated with a heart failure diagnosis.
Laboratory values for the potential prediction variables were not available for every patient in our study; for example, a BNP level was obtained for only 7.6% of the study population. A simple strategy for addressing missing laboratory information was needed in order to derive a multivariable prediction model that could be used on a daily basis in a real‐world setting. We found that patients who had a BNP test drawn, regardless of the result, had a 27% chance of heart failure compared with those for whom BNP results were not available, whose chance of HF was 1%. Therefore, we could not simply impute the average BNP level for patients missing data on this parameter. Instead, we assumed the BNP levels of those not tested would be very low, and so gave these patients a BNP level of 1. Serum creatinine was not included in the multivariable model, despite its having a significant bivariate relationship with HF diagnosis, because valid imputation strategies for creatinine would be too complicated to implement in daily clinical practice.
The sensitivity and specificity of the single loop diuretic indicator was determined from a 2‐by‐2 table using data from the second study year. For both the multivariable and single loop diuretic approaches, test discrimination was evaluated by the c statistic from logistic regression.10 The calibration and overall performance of the multivariable derivation GEE model was assessed by a second GEE model run with the second‐year data set. For the testing model, the sole explanatory variable was a linear predictor derived from the covariate values of year 2 patients with the corresponding parameter estimates from the year 1 GEE. A well‐calibrated model with this configuration would be expected to have an intercept of 0 and a beta coefficient of the linear predictor of 1.11 Sensitivity, specificity, and positive predictive value were determined for 2 thresholds of predicted probability of heart failure, as derived from the linear predictor. If a subject's predicted probability at least equaled the threshold, then he or she would be considered to have tested positive for heart failure.
RESULTS
Salient features of the study population in the first and second study years are shown in Table 1. Mean age was approximately 59 years, and women made up 60% of the patients. The percentage of patients with a principal diagnosis of heart failure was 3% each year. Serum creatinine levels were available for 78% of patients in year 1 and 80% of patients in year 2. Serum BNP levels were available for 7.6% of patients in year 1 and 9% of patients in year 2.
| Variable | Year 1 (n = 28,005) | Year 2 (n = 28, 297) | |||
|---|---|---|---|---|---|
| No. with information | Mean or percent | No. with information | Mean or percent | P value | |
| |||||
| Age (years) | 28,005 | 58.7 | 28,297 | 58.9 | .36 |
| HF principal diagnosis (%) | 28,005 | 3.0% | 28,297 | 3.1% | .41* |
| Female (%) | 28,005 | 60.6% | 28,297 | 60.3% | .48* |
| First BNP level obtained (pg/mL) | 2132 | 813.7 | 2578 | 766.5 | .83 |
| First serum creatinine level obtained (mg/dL) | 21,839 | 1.4 | 22,596 | 1.4 | .54 |
| Patient received IV loop diuretic (%) | 28,005 | 16.3% | 28,297 | 15.8% | .07* |
| Patient received spironolactone (%) | 28,005 | 2.8% | 28,297 | 3.0% | .08* |
| Number of previous hospitalizations with HF in preceding 180 days | 28,005 | 2.4 | 28,297 | 2.9 | .09 |
The parameter estimates and 95% confidence intervals of the main effects of the final prediction model are shown in Table 2, with interaction terms noted in the footnote. Examination of the z scores (available from the authors) indicated that by far the most influential risk factor in the multivariable model was receipt of intravenous diuretics, with receipt of spironolactone a very distant second. The probability that a given patient had heart failure increased with the number of risk factors present and the magnitude of their parameter estimates. For example, an older patient who had been hospitalized with heart failure previously and who was currently receiving intravenous diuretics and spironolactone would be more likely to have a principal diagnosis of heart failure than would an older patient receiving intravenous diuretics who had no other risk factors. However, the interaction terms with negative values (see the footnote in Table 2) indicate that certain combinations of risk factors convey a level of risk somewhat less than the sum of their parts.
| Estimate | Standard error | Lower 95% CL | Upper 95% CL | |
|---|---|---|---|---|
| ||||
| Intercept | 8.28 | 0.40 | 9.06 | 7.50 |
| Centered age in 10‐year increments | 0.31 | 0.05 | 0.22 | 0.40 |
| Receipt of IV loop diuretic | 2.72 | 0.15 | 2.42 | 3.01 |
| Receipt of spironolactone | 1.53 | 0.19 | 1.16 | 1.90 |
| Centered logged BNP | 0.68 | 0.11 | 0.47 | 0.89 |
| Attending physician specialty | 2.46 | 0.41 | 1.66 | 3.26 |
| Count of hospitalizations for heart failure in previous 180 days | 2.43 | 0.48 | 1.48 | 3.37 |
The identification strategies performed well from one year to the next, as summarized in Table 3. Receipt of intravenous loop diuretics had a strong association with diagnosis of heart failure (OR 51.6, 95% CI 41.7, 63.7, P < .0001), with a c statistic of 0.88, a sensitivity of 0.89, and a specificity of 0.87.
| Strategy | TPs of possible 890 HF cases (n) | Sensitivity (# TPs/890) | FPs (n) | TNs of possible 27,407 hospitalizations without HF principal diagnosis (n) | Specificity (# TNs/27,40) | Positive predictive value (# TPs/all positives) | Likelihood ratio (TP/FP) |
|---|---|---|---|---|---|---|---|
| |||||||
| Use receipt of IV loop diuretic | 791 | 0.89 | 3676 | 23,731 | 0.87 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.02 | 833 | 0.94 | 3859 | 23,548 | 0.86 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.04 | 808 | 0.91 | 3045 | 24,362 | 0.89 | 0.21 | 8.2 |
The linear predictor of the multivariable prediction model as described in the Methods section also performed well in year 2 with excellent discrimination (c statistic of 0.96). Calibration was also excellent, as demonstrated by an intercept of 0.03 (standard error 0.05) and a beta coefficient of 1.02 (SE 0.03). If the threshold for identifying potential heart failure cases was defined as a predicted probability of at least 0.02, then the sensitivity of the multivariable predictor was 0.94 and the specificity was 0.86. If the positivity threshold was raised to 0.04, then the predictor's sensitivity dropped slightly, to 0.91, but specificity increased to 0.89.
The principal diagnoses of the 3045 patients in year 2 who were incorrectly predicted as having a principal diagnosis of heart failure (ie, false positives) were cardiac related (1026 of 3045; 34%), pulmonary related (685 of 3045; 22%), and renal‐ or fluid electrolyte related (117 of 3045; 4%), as determined using the multivariable approach with a 0.04 positivity threshold.
DISCUSSION
Identification of patients with heart failure early in their hospitalization is critical for successfully implementing disease management programs targeted at optimizing evidenced‐based care. Furthermore, public reporting of performance measures has increased the scrutiny of care delivered to those having this principal diagnosis. We developed a strategy that used the receipt of intravenous diuretics as a trigger of further clinical evaluation. We subsequently tested the value of other electronically available indicators to improve the sensitivity and specificity of the case‐finding strategy.
The receipt of an intravenous loop diuretic alone had a sensitivity of .89 and a specificity of .87. Incorporation of the additional information available to us electronically improved the sensitivity to .91 and the specificity to .89 (using a positivity threshold of 0.04), although these might be slightly different if BNP levels had been available for more patients. As with all diagnostic testing, there is a trade‐off between improved sensitivity and decreased specificity. At first glance, the resulting number of false positives generated by either prediction strategy may appear problematic. Although a formal cost‐effectiveness analysis of our case‐finding strategies is beyond the scope of this article, the cost of a false positive in this scenario is likely to be small.
For example in our hospital, clinical pharmacists place a reminder on the charts of patients who appear to have heart failure in order to prompt the clinical team to provide the recommended care processes. A list of inpatients treated with an IV diuretic is generated daily. A clinical pharmacist then reviews identified patient medical records to determine whether the diuretic was ordered for heart failure management or for some other purpose. This review consists of reading the completed history and physical and/or progress notes. On average, each medical record review takes 60 seconds to complete, with a range of 30‐90 seconds. At this speed, roughly 3000 minutes per year (or approximately 1 hour per week) would be spent reviewing the medical records of patients who would not have a principal diagnosis of heart failure. Nevertheless, we found that at least one‐third of the nominal false positives (multivariable rule, threshold of 0.04) still had a cardiac‐related diagnosis. Many of these had heart failure as a secondary diagnosis, but other diagnoses such as acute myocardial infarction took precedence in coding algorithms that assigned the principal diagnosis at discharge. Such patients might still benefit from the interventions and so are not truly false positives.
Patients with heart failure missed by this strategy included patients admitted for placement of pacemakers and/or defibrillators. Patients in this specialized population always had a single team managing their care, so the clinical and educational interventions were integrated into that team's daily work flow. Patients on dialysis with volume overload were not identified using this algorithm and constituted a very small number of patients in our heart failure population. Patients with stable heart failure on oral diuretics were not the focus of this case‐finding strategy and became a target for further intervention only if their heart failure worsened and required intravenous diuretics while they were hospitalized.
The identification of predictors for heart failure has allowed us not only to more effectively identify and risk‐stratify patients with heart failure but also to integrate the case‐finding strategy into clinical care delivery. This approach may also be relevant in hospitals that do not have computerized provider order entry (CPOE) systems but may be able to implement this case‐finding strategy by simply requesting a daily report of patients prescribed intravenous diuretics. As more institutions move to adopt CPOE platforms and clinical information such as ejection fractions become available, the predictors studied here may be augmented to form more sophisticated clinical rules and alerts.
Our study had several limitations. Although we validated our predictors in a separate cohort of patients, this is a single‐site study and may not be representative of the diverse institutions that care for patients with heart failure. There may also be interinstitutional differences in how a principal diagnosis of heart failure is assigned. We have demonstrated the stability of locally derived predictors from one year to the next but cannot make claims about how well our parameter estimates would perform in other settings. Finally, the complexity of the multivariable predictor requires an integrated database and computer application of a formula that may not be commonly available elsewhere at this time.
If disease management strategies are to be successful, early identification of at‐risk patients is crucial for both clinical care delivery and patient education regarding self‐management. The strategies tested here may be useful for other community‐based institutions whose care of heart failure patients is decentralized and involves multiple clinicians.
There has been increasing emphasis on the development and successful execution of disease management strategies to improve the delivery of evidence‐based care for hospitalized patients with heart failure.14 Current care is woefully suboptimal for heart failure patients. Fonorow et al. describe the significant gap in performance on the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) heart failure core measures, with the median rate of conformity with all measures at only 24% nationally.5 For a variety of clinical and external factors, such as publicly reported quality measures and pay‐for‐performance incentives, institutions are increasingly motivated to identify patients who will make up the denominator of the heart failure metrics. At first glance, system‐level identification of heart failure patients may not seem critical to the delivery of evidence‐based care, but given that the management of patients with heart failure is multidisciplinary, it is critical that patients who have heart failure be clearly identified for all members of the care team. The capability of prospectively identifying inpatients with the principal diagnosis of heart failure is an essential first step in the implementation of performance improvement programs.
Successful interventions have included a multidisciplinary intervention with postdischarge follow‐up.6 However, the interventions described do not fully indicate how patients with heart failure are identified while in the hospital, so those interventions may be difficult to replicate in other settings. It has not been easy to identify these patients in a timely fashion given that a chief complaint of shortness of breath can indicate other clinical conditions in addition to heart failure. Previous studies have used an admission diagnosis of heart failure or suggestive chest X‐ray findings to trigger a clinical evaluation.7, 8 However, the sensitivity and specificity of these case‐finding methods have not been reported. Furthermore, patients presenting with shortness of breath may not have a diagnosis established until a series of diagnostic and therapeutic maneuvers have been performed. The challenge of promoting physician provision and documentation of evidence‐based care is compounded by these patients usually not being housed in a single geographical unit, possibly being attended by any number of medical specialties, and often having short lengths of stay. Given the multiple factors contributing to the complexity of identifying patients hospitalized with heart failure, it is important to delineate case‐finding strategies that efficiently and effectively identify heart failure patients so that clinical care and self‐management interventions are optimized.
With this goal in mind, we hypothesized that the receipt of intravenous loop diuretics may be an important trigger for identifying patients with heart failure. Receipt of intravenous loop diuretics is ubiquitous in the management of decompensated systolic and diastolic heart failure. We compare 2 electronic pharmacy‐based strategies in a tertiary‐care community teaching hospital to identify hospitalized patients early in their stay who were likely to be discharged with a principal diagnosis of heart failure (HF).
METHODS
Study Setting
The study was conducted in a 487‐bed not‐for‐profit community hospital in southeastern Michigan. The organization's institutional review board for all studies involving human subjects approved the study. In this hospital, heart failure patients are geographically dispersed throughout the institution, but all patient care orders are entered in a computerized provider order entry system. Approximately 70% of heart failure patients are admitted to the general medicine service, where care is directed by 3 types of attending physicians (academic hospitalists, private‐practice hospitalists, and community physicians, as previously described),9 14% are on the cardiology service, and the remainder are distributed among the surgical and intensive care unit services. The accuracy of 2 case‐finding strategies was tested using data from a 2‐year period. The institution had 28,005 adult hospitalizations during the prediction development period, July 1, 2003, to June 30, 2004, and 28,297 adult hospitalizations during the prediction testing period, July 1, 2004, to June 30, 2005. Receipt of intravenous loop diuretics had been used previously as a marker by the hospital's disease management program, but the accuracy of this strategy had not been tested.
Development of Prediction Algorithms
The outcome of interest was a principal diagnosis of HF, as assigned by medical records personnel after hospital discharge. This population corresponds to the denominator used to construct various performance measures. We evaluated 2 strategies for identifying targeted patients using information available prior to discharge. The first was the receipt of an intravenous loop diuretic at any point during the hospitalization (yes or no) as a single indicator. The second strategy used additional information to construct a multivariable predictor. Explanatory variables were considered for inclusion if they were available electronically, did not require additional manual retrieval or data entry, and had a clinical relationship with a diagnosis of HF. The variables selected were patient age, sex, receipt of intravenous loop diuretic, spironolactone, B‐type natriuretic peptide (BNP) level, serum creatinine, serum sodium, number of previous hospitalizations in the last 180 days with a principal discharge diagnosis of heart failure, and attending physician specialty. Cardiac ejection fraction was not included because the data were not available electronically.
Statistical Analysis
All analyses were performed using SAS version 9 (SAS Institute Inc., Cary NC). The data set was split chronologically into 2 sets each covering a 1‐year period in order to test the stability of the case‐finding strategies from one year to the next.
Initial model building for the multivariable strategy was done through logistic regression. Individual variables associated with heart failure (P < .05) were entered into a multivariable derivation model and retained if the main effect had a P value < .05. Sex and serum sodium were not included in the final model because of their high P values. To account for circumstances in which patients could have combinations of risk factors, interaction terms were also considered and were retained in the multivariable model at the same level of significance. The final parameter estimates for the derivation model were obtained from a generalized estimating equation (GEE) with an exchangeable working correlation structure to account for the possibility of multiple hospitalizations per year for a given patient. The z scores for the variables in the model provided insight into the relative importance of the factors associated with a heart failure diagnosis.
Laboratory values for the potential prediction variables were not available for every patient in our study; for example, a BNP level was obtained for only 7.6% of the study population. A simple strategy for addressing missing laboratory information was needed in order to derive a multivariable prediction model that could be used on a daily basis in a real‐world setting. We found that patients who had a BNP test drawn, regardless of the result, had a 27% chance of heart failure compared with those for whom BNP results were not available, whose chance of HF was 1%. Therefore, we could not simply impute the average BNP level for patients missing data on this parameter. Instead, we assumed the BNP levels of those not tested would be very low, and so gave these patients a BNP level of 1. Serum creatinine was not included in the multivariable model, despite its having a significant bivariate relationship with HF diagnosis, because valid imputation strategies for creatinine would be too complicated to implement in daily clinical practice.
The sensitivity and specificity of the single loop diuretic indicator was determined from a 2‐by‐2 table using data from the second study year. For both the multivariable and single loop diuretic approaches, test discrimination was evaluated by the c statistic from logistic regression.10 The calibration and overall performance of the multivariable derivation GEE model was assessed by a second GEE model run with the second‐year data set. For the testing model, the sole explanatory variable was a linear predictor derived from the covariate values of year 2 patients with the corresponding parameter estimates from the year 1 GEE. A well‐calibrated model with this configuration would be expected to have an intercept of 0 and a beta coefficient of the linear predictor of 1.11 Sensitivity, specificity, and positive predictive value were determined for 2 thresholds of predicted probability of heart failure, as derived from the linear predictor. If a subject's predicted probability at least equaled the threshold, then he or she would be considered to have tested positive for heart failure.
RESULTS
Salient features of the study population in the first and second study years are shown in Table 1. Mean age was approximately 59 years, and women made up 60% of the patients. The percentage of patients with a principal diagnosis of heart failure was 3% each year. Serum creatinine levels were available for 78% of patients in year 1 and 80% of patients in year 2. Serum BNP levels were available for 7.6% of patients in year 1 and 9% of patients in year 2.
| Variable | Year 1 (n = 28,005) | Year 2 (n = 28, 297) | |||
|---|---|---|---|---|---|
| No. with information | Mean or percent | No. with information | Mean or percent | P value | |
| |||||
| Age (years) | 28,005 | 58.7 | 28,297 | 58.9 | .36 |
| HF principal diagnosis (%) | 28,005 | 3.0% | 28,297 | 3.1% | .41* |
| Female (%) | 28,005 | 60.6% | 28,297 | 60.3% | .48* |
| First BNP level obtained (pg/mL) | 2132 | 813.7 | 2578 | 766.5 | .83 |
| First serum creatinine level obtained (mg/dL) | 21,839 | 1.4 | 22,596 | 1.4 | .54 |
| Patient received IV loop diuretic (%) | 28,005 | 16.3% | 28,297 | 15.8% | .07* |
| Patient received spironolactone (%) | 28,005 | 2.8% | 28,297 | 3.0% | .08* |
| Number of previous hospitalizations with HF in preceding 180 days | 28,005 | 2.4 | 28,297 | 2.9 | .09 |
The parameter estimates and 95% confidence intervals of the main effects of the final prediction model are shown in Table 2, with interaction terms noted in the footnote. Examination of the z scores (available from the authors) indicated that by far the most influential risk factor in the multivariable model was receipt of intravenous diuretics, with receipt of spironolactone a very distant second. The probability that a given patient had heart failure increased with the number of risk factors present and the magnitude of their parameter estimates. For example, an older patient who had been hospitalized with heart failure previously and who was currently receiving intravenous diuretics and spironolactone would be more likely to have a principal diagnosis of heart failure than would an older patient receiving intravenous diuretics who had no other risk factors. However, the interaction terms with negative values (see the footnote in Table 2) indicate that certain combinations of risk factors convey a level of risk somewhat less than the sum of their parts.
| Estimate | Standard error | Lower 95% CL | Upper 95% CL | |
|---|---|---|---|---|
| ||||
| Intercept | 8.28 | 0.40 | 9.06 | 7.50 |
| Centered age in 10‐year increments | 0.31 | 0.05 | 0.22 | 0.40 |
| Receipt of IV loop diuretic | 2.72 | 0.15 | 2.42 | 3.01 |
| Receipt of spironolactone | 1.53 | 0.19 | 1.16 | 1.90 |
| Centered logged BNP | 0.68 | 0.11 | 0.47 | 0.89 |
| Attending physician specialty | 2.46 | 0.41 | 1.66 | 3.26 |
| Count of hospitalizations for heart failure in previous 180 days | 2.43 | 0.48 | 1.48 | 3.37 |
The identification strategies performed well from one year to the next, as summarized in Table 3. Receipt of intravenous loop diuretics had a strong association with diagnosis of heart failure (OR 51.6, 95% CI 41.7, 63.7, P < .0001), with a c statistic of 0.88, a sensitivity of 0.89, and a specificity of 0.87.
| Strategy | TPs of possible 890 HF cases (n) | Sensitivity (# TPs/890) | FPs (n) | TNs of possible 27,407 hospitalizations without HF principal diagnosis (n) | Specificity (# TNs/27,40) | Positive predictive value (# TPs/all positives) | Likelihood ratio (TP/FP) |
|---|---|---|---|---|---|---|---|
| |||||||
| Use receipt of IV loop diuretic | 791 | 0.89 | 3676 | 23,731 | 0.87 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.02 | 833 | 0.94 | 3859 | 23,548 | 0.86 | 0.18 | 6.6 |
| Use predicted probability of heart failure (per multivariable model) 0.04 | 808 | 0.91 | 3045 | 24,362 | 0.89 | 0.21 | 8.2 |
The linear predictor of the multivariable prediction model as described in the Methods section also performed well in year 2 with excellent discrimination (c statistic of 0.96). Calibration was also excellent, as demonstrated by an intercept of 0.03 (standard error 0.05) and a beta coefficient of 1.02 (SE 0.03). If the threshold for identifying potential heart failure cases was defined as a predicted probability of at least 0.02, then the sensitivity of the multivariable predictor was 0.94 and the specificity was 0.86. If the positivity threshold was raised to 0.04, then the predictor's sensitivity dropped slightly, to 0.91, but specificity increased to 0.89.
The principal diagnoses of the 3045 patients in year 2 who were incorrectly predicted as having a principal diagnosis of heart failure (ie, false positives) were cardiac related (1026 of 3045; 34%), pulmonary related (685 of 3045; 22%), and renal‐ or fluid electrolyte related (117 of 3045; 4%), as determined using the multivariable approach with a 0.04 positivity threshold.
DISCUSSION
Identification of patients with heart failure early in their hospitalization is critical for successfully implementing disease management programs targeted at optimizing evidenced‐based care. Furthermore, public reporting of performance measures has increased the scrutiny of care delivered to those having this principal diagnosis. We developed a strategy that used the receipt of intravenous diuretics as a trigger of further clinical evaluation. We subsequently tested the value of other electronically available indicators to improve the sensitivity and specificity of the case‐finding strategy.
The receipt of an intravenous loop diuretic alone had a sensitivity of .89 and a specificity of .87. Incorporation of the additional information available to us electronically improved the sensitivity to .91 and the specificity to .89 (using a positivity threshold of 0.04), although these might be slightly different if BNP levels had been available for more patients. As with all diagnostic testing, there is a trade‐off between improved sensitivity and decreased specificity. At first glance, the resulting number of false positives generated by either prediction strategy may appear problematic. Although a formal cost‐effectiveness analysis of our case‐finding strategies is beyond the scope of this article, the cost of a false positive in this scenario is likely to be small.
For example in our hospital, clinical pharmacists place a reminder on the charts of patients who appear to have heart failure in order to prompt the clinical team to provide the recommended care processes. A list of inpatients treated with an IV diuretic is generated daily. A clinical pharmacist then reviews identified patient medical records to determine whether the diuretic was ordered for heart failure management or for some other purpose. This review consists of reading the completed history and physical and/or progress notes. On average, each medical record review takes 60 seconds to complete, with a range of 30‐90 seconds. At this speed, roughly 3000 minutes per year (or approximately 1 hour per week) would be spent reviewing the medical records of patients who would not have a principal diagnosis of heart failure. Nevertheless, we found that at least one‐third of the nominal false positives (multivariable rule, threshold of 0.04) still had a cardiac‐related diagnosis. Many of these had heart failure as a secondary diagnosis, but other diagnoses such as acute myocardial infarction took precedence in coding algorithms that assigned the principal diagnosis at discharge. Such patients might still benefit from the interventions and so are not truly false positives.
Patients with heart failure missed by this strategy included patients admitted for placement of pacemakers and/or defibrillators. Patients in this specialized population always had a single team managing their care, so the clinical and educational interventions were integrated into that team's daily work flow. Patients on dialysis with volume overload were not identified using this algorithm and constituted a very small number of patients in our heart failure population. Patients with stable heart failure on oral diuretics were not the focus of this case‐finding strategy and became a target for further intervention only if their heart failure worsened and required intravenous diuretics while they were hospitalized.
The identification of predictors for heart failure has allowed us not only to more effectively identify and risk‐stratify patients with heart failure but also to integrate the case‐finding strategy into clinical care delivery. This approach may also be relevant in hospitals that do not have computerized provider order entry (CPOE) systems but may be able to implement this case‐finding strategy by simply requesting a daily report of patients prescribed intravenous diuretics. As more institutions move to adopt CPOE platforms and clinical information such as ejection fractions become available, the predictors studied here may be augmented to form more sophisticated clinical rules and alerts.
Our study had several limitations. Although we validated our predictors in a separate cohort of patients, this is a single‐site study and may not be representative of the diverse institutions that care for patients with heart failure. There may also be interinstitutional differences in how a principal diagnosis of heart failure is assigned. We have demonstrated the stability of locally derived predictors from one year to the next but cannot make claims about how well our parameter estimates would perform in other settings. Finally, the complexity of the multivariable predictor requires an integrated database and computer application of a formula that may not be commonly available elsewhere at this time.
If disease management strategies are to be successful, early identification of at‐risk patients is crucial for both clinical care delivery and patient education regarding self‐management. The strategies tested here may be useful for other community‐based institutions whose care of heart failure patients is decentralized and involves multiple clinicians.
- ,,, et al.Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure.Congest Heart Fail.2005;11:315–321.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 Suppl):S1–S15.
- .Heart failure disease management: implementation and outcomes.Cardiol Rev.2005;13:231–239.
- ,,, et al.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;173(1):40–45.
- ,,, et al.Adherence to heart failure quality‐of‐care indicators in US hospitals: analysis of the ADHERE Registry.Arch Intern Med.2005;165:1469–1477.
- ,,, et al.Comprehensive discharge planning with post‐discharge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:2080–2081.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141:254–258.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- ,,, et al.Evaluating the yield of medical tests.JAMA.1982;247:2543–2546.
- .Two further applications of a model for binary regression.Biometrika.1958;45:562–565.
- ,,, et al.Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure.Congest Heart Fail.2005;11:315–321.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 Suppl):S1–S15.
- .Heart failure disease management: implementation and outcomes.Cardiol Rev.2005;13:231–239.
- ,,, et al.Systematic review of multidisciplinary interventions in heart failure.Heart.2005;173(1):40–45.
- ,,, et al.Adherence to heart failure quality‐of‐care indicators in US hospitals: analysis of the ADHERE Registry.Arch Intern Med.2005;165:1469–1477.
- ,,, et al.Comprehensive discharge planning with post‐discharge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39:2080–2081.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141:254–258.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- ,,, et al.Evaluating the yield of medical tests.JAMA.1982;247:2543–2546.
- .Two further applications of a model for binary regression.Biometrika.1958;45:562–565.
Copyright © 2007 Society of Hospital Medicine
In the Eye of the Storm
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.
Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
Liver Abscess and Metastatic Endophthalmitis
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.
Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.

Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.

Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
A Rash Decision
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.
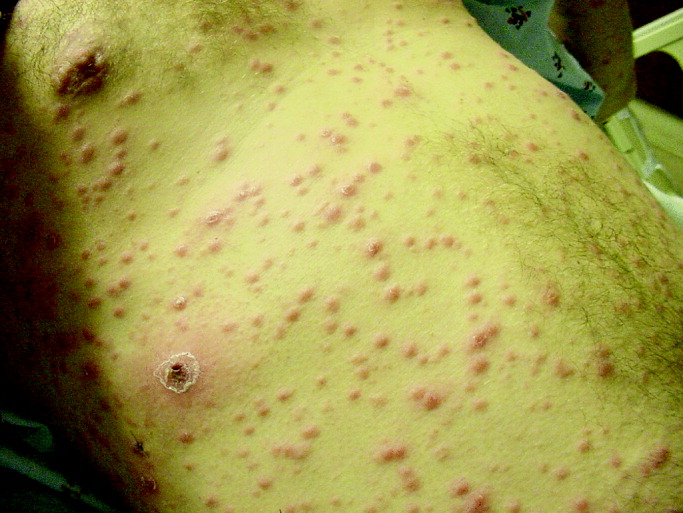
The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
Asymmetric muscle atrophy from childhood polio
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.
Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.


Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.


Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
Handoffs